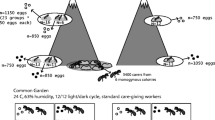
Abstract
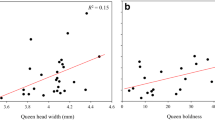
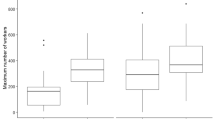
The genetic basis of morphological traits in social insects remains largely unexplored. This is even true for individual body size, a key life-history trait. In the social insects, the size of reproductive individuals affects dispersal decisions, so that small size in queens is often associated with reduced dispersal, and large size with long-range dispersal and independent colony founding. Worker size is connected to division of labour when workers specialize in certain tasks according to their size. In many species, variation in worker size has been shown to increase colony performance. In this study, we present the first evidence of an additive genetic component to queen size in ants, using maternal half sib analysis. We also compared intra-colony size variation in colonies with high (queen doubly mated) versus low (queen singly mated) genetic variability. We found a high and significant heritability (h2=0.51) for queen size in one of the two study years, but not in the other. Size variation among queens was greater in colonies headed by a doubly mated queen in one of the study years, but not in the other. This indicates that genetic factors can influence queen size, but that environmental factors may override these under some circumstances. The heritability for worker size was low (h2=0.09) and non-significant. Increased genetic diversity did not increase worker size variation in the colonies. Worker size appeared largely environmentally determined, potentially allowing colonies to adjust worker size ratios to current conditions.



Similar content being viewed by others
References
Becker WA (1984) Manual of quantitative genetics, 4th edn. Academic, Washington DC
Boomsma JJ, Ratnieks FLW (1996) Paternity in eusocial Hymenoptera. Philos Trans R Soc B 351:947–975
Boomsma JJ, Nielsen J, Sundström L, Oldham NJ, Tentschert J, Petersen HC, Morgan D (2003) Informational constraints on optimal sex allocation in ants. Proc Natl Acad Sci USA 100:8799–8804
Bourke AFG, Franks NR (1995) Social evolution in ants. Princeton University Press, Princeton
Bubli OA, Loeschcke V (2000) High stressful temperature and genetic variation of five quantitative traits in Drosophila melanogaster. Genetica 100:79–85
Cassill DL, Tschinkel WR (1999) Task selection by workers of the fire ant Solenopsis invicta. Behav Ecol Sociobiol 45:301–310
Chapuisat M (1996) Characterization of microsatellite loci in Formica lugubris B and their variability in other ant species. Mol Ecol 5:599–601
Collins AM, Rinderer TE, Harbo JR, Brown MA (1984) Heritabilities and correlations for several characters in the honey bee. J Hered 75:135–140
Cook JM, Comton SG, Herre EA, West SA (1997) Alternative mating tactics and extreme male dimorphism in fig wasps. Proc R Soc Lond B 264:747–754
Crozier R, Page R (1985) On being the right size: male contributions and multiple mating in social Hymenoptera. Behav Ecol Sociobiol 18:105–115
DeHeer CJ, Goodisman MAD, Ross KG (1999) Queen dispersal strategies in the multiple-queen form of the fire ant Solenopsis invicta. Am Nat 153:660–675
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Longman, Harlow
Fraser VS, Kaufmann B, Oldroyd BP, Crozier RH (2000) Genetic influence on caste in the ant Camponotus consobrinus. Behav Ecol Sociobiol 47:188–194
Gonzaga MO, Vasconcellos-Neto J (2001) Female body size, fecundity parameters and foundation of new colonies in Anelosimus jabaquara (Araneae, Theridiidae). Insectes Soc 48:94–100
Goodisman MAD, Mack PD, Pearse DE, Ross KG (1999) Effects of a single gene on worker and male body mass in the fire ant Solenopsis invicta. Ann Ent Soc Am 92:563–570
Gyllenstrand N, Gertsch P, Pamilo P (2002) Polymorphic microsatellite DNA markers in the ant Formica exsecta. Mol Ecol Notes 2:67–69
Hannonen M, Sundström L (2003) Worker nepotism among polygynous ants. Nature 421:910
Hannonen M, Sledge M, Turillazzi S, Sundström L (2002) Queen reproduction, chemical signalling and worker behaviour in polygyne colonies of the ant Formica fusca. Anim Behav 64:477–485
Heredia A, Detrain C (2000) Worker size polymorphism and ethological role of sting associated glands in the harvester ant Messor barbarus. Insectes Soc 47:383–389
Hughes WOH, Sumner S, Van Borm S, Boomsma JJ (2003) Worker caste polymorphism has a genetic basis in Acromyrmex leaf-cutting ants. Proc Natl Acad Sci 100:9394–9397
Hölldobler B, Wilson EO (1990) The ants. Belknap, Cambridge, Mass
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev 75:21–64
Keller L (1993) The assessment of reproductive success of queens in ants and other social insects. Oikos 67:177–180
Keller L, Reeve HK (1999) Dynamics of conflicts within insect societies. In: Keller L (ed) Levels of selection in evolution. Princeton University Press, New Jersey, pp 153–175
Keller L, Ross K (1993) Phenotypic plasticity and cultural transmission of alternative social organizations in the fire ant Solenopsis invicta. Behav Ecol Sociobiol 33:121–129
Keller L, Sundström L, Chapuisat M (1997) Male reproductive success: paternity contribution to queens and workers in Formica ants. Behav Ecol Sociobiol 41:11–15
Larsson K, Rattiste K, Lillelecht V (1997) Heritabilities of head size in the common gull Larus canus in relation to environmental conditions during offspring growth. Heredity 79:201–207
Liu FH, Smith SM (2000) Estimating quantitative genetic parameters in haplodiploid organisms. Heredity 85:373–382
McGlynn TP, Owen JP (2002) Food supplementation alters caste allocation in a natural population of Pheidole flavens, a dimorphic leaf-litter dwelling ant. Insectes Soc 49:8–14
Merilä J (1997) Expression of genetic variation in body size of the collared flycatcher under different environmental conditions. Evolution 51:526–536
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13:403–407
Oldroyd B, Moran C (1983) Heritability of worker characteristics in the honeybee (Apis mellifera). Aust J Biol Sci 36:323–332
Oldroyd B, Rinderer T, Buco S (1991) Heritability of morphological characters used to distinguish European and Africanized honeybees. Theor Appl Genet 82:499–504
Oldroyd B, Rinderer T, Buco SM, Beaman LD (1993) Genetic variance in honey bees for preferred foraging distance. Anim Behav 45:323–332
Oster GF, Wilson EO (1978) Caste and ecology in the social insects. Princeton University Press, Princeton
Owen RE, McCorquodale DB (1994) Quantitative variation and heritability of postdiapause development time and body size in the alfalfa leafcutting bee (Hymenoptera: Megachilidae). Ann Ent Soc Am 87:922–927
Pankiw T, Tarpy DR, Page RE Jr (2002) Genotype and rearing environment affect honeybee perception and foraging behaviour. Anim Behav 64:663–672
Passera L, Roncin E, Kaufmann B, Keller L (1996) Increased soldier production in ant colonies exposed to intraspecific competition. Nature 379:630–631
Peeters C, Ito F (2001) Colony dispersal and the evolution of queen morphology in social Hymenoptera. Annu Rev Entomol 46:601–630
Price T, Schluter D (1991) On the low heritability of life history traits. Evolution 45:853–861
Ramalho M, Imperatrix-Fonseca VL, Giannini TC (1998) Within-colony size variation of foragers and pollen load capacity in the stingless bee Melipona quadrifasciata anthidioides Lepeletier (Apidae, Hymenoptera). Apidologie 29:221–228
Roff DA (1992) The evolution of life histories. Chapman & Hall, New York
Roff DA (1997) Evolutionary quantitative genetics. Chapman & Hall, New York
Rosengren R, Cherix D, Pamilo P (1985) Insular ecology of the red wood ant Formica truncorum Fabr. I. Polydomous nesting, population size and foraging. Mitt Schweiz Entomol Ges 58:147–175
Rüppell O, Heinze J (1999) Alternative dispersal tactics in females: the case of size polymorphism in winged ant queens. Insectes Soc 46:6–17
Rüppell O, Heinze J, Hölldobler B (1998) Size-dimorphism in the queens of the North American ant Leptothorax rugatulus (Emery). Insectes Soc 45:67–77
Rüppell O, Heinze J, Hölldobler B (2001) Complex determination of queen body size in the queen size dimorphic ant Leptothorax rugatulus (Formicidae: Hymenoptera). Heredity 87:33–40
Searle S, Casella G, McCulloch CG (1992) Variance components. Wiley, New York
Sgrò CM, Hoffmann AA (1998) Effects of stress combinations on the expression of additive genetic variation for fecundity in Drosophila melanogaster. Genet Res 72:13–18
Speight MR, Hunter MD, Watt AD (1999) Ecology of Insects. Blackwell, Oxford
Stearns SC (1992) The evolution of life histories. Oxford University Press, New York
Sundström L (1986) Storleksskillnader kopplade till furageringsradie och arbetsfördelning, samt konsumtion av och sockerhalt i insamlad honungsdagg hos den röda stackmyran (Formica). MSc Thesis, University of Helsinki
Sundström L (1993) Genetic population structure and sociogenetic organization in Formica truncorum (Hymenoptera, Formicidae). Behav Ecol Sociobiol 33:345–354
Sundström L (1994) Sex ratio bias, relatedness asymmetry and queen mating frequency in ants. Nature 367:266–268
Sundström L (1995) Dispersal polymorphism and physiological condition of males and females in the ant, Formica truncorum. Behav Ecol 6:132–139
Sundström L, Boomsma JJ (2000) Reproductive alliances and posthumous fitness enhancement in male ants. Proc R Soc Lond B 267:1439–1444
Tsuji K (1995) Reproductive conflicts and levels of selection in the ant Pristomyrmex pungens: contextual analysis and partitioning of covariance. Am Nat 146:586–607
Wiernasz DC, Cole BJ (2003) Queen size mediates queen survival and colony fitness in harvester ants. Evolution 57:2179–2183
Wolf JB (2003) Genetic architecture and evolutionary constraint when the environment contains genes. Proc Natl Acad Sci 100:4655–4660
Wolf JB, Brodie ED III, Cheverud JM, Moore AJ, Wade MJ (1998) Evolutionary consequences of genetic effects. Trends Ecol Evol 13:64–69
Yamauchi K, Furukawa T, Kinomura K, Takamine H, Tsuji K (1991) Secondary polygyny by inbred sexuals in the dolichoderine ant Technomyrmex albipes. Behav Ecol Sociobiol 29:313–319
Acknowledgements
This work is a continuation of a pilot experiment done by J. Nielsen, which we wish to acknowledge. We also thank H. Helanterä, C. Liautard and three anonymous referees for comments, R. Forsman, P. Gertsch, M. Putkonen, S. Suomensaari and H. Viljanen for help during fieldwork, K. Trontti for assistance in the laboratory, J. Koella for providing video equipment, Tvärminne Zoological station for working facilities, and the Finnish Meteorological Institute for providing weather data. This work was funded by the Academy of Finland (grant nos. 8238 and 42725) (L.S.) and the Finnish School in Wildlife Biology, Conservation and Management, Societas Pro Fauna et Flora Fennica and Entomologiska föreningen i Helsingfors (K.B.). All experiments comply with the laws of Finland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Heinze
Rights and permissions
About this article
Cite this article
Bargum, K., Boomsma, J.J. & Sundström, L. A genetic component to size in queens of the ant, Formica truncorum. Behav Ecol Sociobiol 57, 9–16 (2004). https://doi.org/10.1007/s00265-004-0836-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0836-z