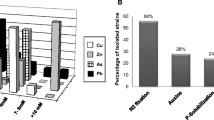
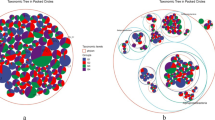
Abstract
Soil heavy metal contamination resulting from mining activities constitutes a major environmental problem worldwide. The spread of heavy metals is often facilitated by scarce vegetation cover, so there is an urgent need to improve plant survival and establishment in these metalliferous areas. This study is aimed at the isolation and analysis of the phylogenetic relationship of culturable bacteria from the rhizosphere of metallophyte plants growing in the Kettara mine, in Marrakech, in order to select plant growth-promoting rhizobacteria (PGPR), which could be used in assisted-phytoremediation. Bacterial isolates were grouped by random amplified polymorphic DNA analysis and identified by 16S rRNA gene sequencing. Strains were further characterized for the production of plant growth-promoting (PGP) substances, such as NH3, siderophores, indol-3-acetic acid (IAA), hydrogen cyanide, and extracellular enzymes, for ACC-deaminase activity, their capacity to solubilize phosphate, and for their tolerance to heavy metals and acidic pH. Rhizosphere soils were highly contaminated with Cu and Zn and presented low fertility. Phylogenetic analysis showed that the rhizobacteria were affiliated to three major groups: γ-Proteobacteria (48 %), β-Proteobacteria (17 %), and Bacilli (17 %). The most represented genera were Pseudomonas (38 %), Bacillus (10 %), Streptomyces (10 %), and Tetrathiobacter (10 %). Overall, rhizobacterial strains showed an ability to produce multiple, important PGP traits, which may be helpful when applied as plant growth promoter agents in contaminated soils. PGPR were also able to withstand high levels of metals (up to 2615.2 mg Zn l−1, 953.29 mg Cu l−1, and 1124.6 mg Cd l−1) and the order of metal toxicity was Cd > Cu > Zn. The rhizobacterial strains isolated in the present study have the potential to be used as efficient bioinoculants in phytoremediation strategies for the recovery of Kettara mine soils.

Similar content being viewed by others
References
Aboudrar W, Schwartz C, Benizri E, Morel JL, Boularbah A (2007) Soil microbial diversity as affected by the rhizosphere of the hyperaccumulator Thlaspicaerulescens under natural conditions. Int J Phytoremediation 9:41–52
Agami RA, Mohamed GF (2013) Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol Environ Saf 94:164–171
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181
Atkins WS (1999) Estratégia de redução dos impactes ambientais associados aos resíduos industriais depositados no CQE, Estudo de Impacte Ambiental (Environmental Impact Study), IPAMB, Lisbon. No. 595.
Baharuddin MFT, Taib S, Hashim R, Abidin MHZ, Rahman NI (2013) Assessment of seawater intrusion to the agricultural sustainability at the coastal area of Carey Island, Selangor, Malaysia. Arab J Geosci 6:3909–3928
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements – review of their distribution, ecology, and phytochemistry. Biorecovery 1:81–126
Banásová V, Ďurišová E, Nadubinská M, Gurinová E, Čiamporová M (2012) Natural vegetation, metal accumulation and tolerance in plants growing on heavy metal rich soils. In: Kothe E, Varma A (eds) Bio-geo interactions in metal-contaminated soils. Springer, Berlin Heidelberg, pp. 233–250
Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR (2005) Cadmium-tolerant plant growth promoting bacteria associated with the roots of Indian mustard (Brassica juncea L, Czern.). Soil Biol Biochem 37:241–250
Bennisse R, Labat M, Elasli A, Brhada F, Chandad F, Liegbott PP, Hibti M, Qatibi AI (2004) Rhizosphere bacterial populations of metallophyte plants in heavy metal-contaminated soils from mining areas in semiarid climate. World J Microbiol Biotechnol 20:759–766
Blakemore LC, Searle PL, Daly BK (1972) Methods for chemical analysis of soils New Zealand soil bureau report 10A. Government printer, Wellington
Boularbah A, Schwartz C, Bitton G, Morel JL (2006a) Heavy metal contamination from mining sites in South Morocco: 1. Use of a biotest to assess metal toxicity of tailings and soils. Chemosphere 63:802–810
Boularbah A, Schwartz C, Bitton G, Aboudrar W, Ouhammou A, Morel JL (2006b) Heavy metal contamination from mining sites in South Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chemosphere 63:811–817
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45:198–207
Cappuccino JC, Sherman N (1992) Negative staining. In: Cappuccino JC, Sherman N (eds) Microbiology: a laboratory manual. Benjamin/Cummings, PubCo, Redwood City, pp. 125–179
Cattelan AJ, Hartel PG, Fuhrmann JJ (1999) Screening for plant growth promoting rhizobacteria to promote early soybean growth. Soil Sci Soc Am J 63:1670–1680
Cervantes-Vega C, Chavez J, Cordova NA, De La Mora P, Amador VJ (1986) Resistance to metals by Pseudomonas aeruginosaclinicalisolates. Microbios 48:159–163
Chauhan H, Bagyaraj DJ, Selvakumar G, Sundaram SP (2015) Novel plant growth promoting rhizobacteria- prospects and potential. Appl Soil Ecol 95:38–53
Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, Lim YW (2007) EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 57:2259–2261
Clemente R, Walker DJ, Roig A, Bernal MP (2003) Heavy metal bioavailability in a soil affected by mineral sulphides contamination following the mine spillage at Aznalcóllar (Spain). Biodegradation 14:199–205
Das SK, Varma A (2011) Role of enzymes in maintaining soil health. In: Shukla G, Varma A (eds) Soil enzymology. Springer, Berlin Heidelberg, pp. 25–42
de-Bashan LE, Hernandez J, Bashana Y, Maier RM (2010) Bacillus pumilus ES4: candidate plant growth-promoting bacterium to enhance establishment of plants in mine tailings. Environ Exp Bot 69:343–352
El Hamiani O, El Khalil H, Lounate K, Sirguey C, Hafidi M, Bitton G, Schwartz C, Boularbah A (2010) Toxicity assessment of garden soils in the vicinity of mining areas in southern Morocco. J Hazard Mater 177:755–761
El Hamiani O, El Khalil H, Sirguey C, Ouhammou A, Bitton G, Schwartz C, Boularbah A (2015) Metal concentrations in plants from mining areas in South Morocco: health risks assessment of consumption of edible and aromatic plants. CLEAN–Soil Air Water 43:399–407
El Khalil H, El Hamiani O, Bitton G, Ouazzani N, Boularbah A (2008) Heavy metal contamination from mining sites in South Morocco: monitoring metal content and toxicity of soil runoff and groundwater. Environ Monit Assess 136:147–160
Euzéby JP (1997) List of bacterial names with standing in nomenclature: a folder available on the internet. Int J Syst Bacteriol 47:590–592
Gadd GM (1992) Metals and microorganisms: a problem of definition. FEMS Microbiol Lett 100:197–204
Gordon SA, Weber RP (1951) Colorimetric estimation of indole acetic acid. Plant Physiol 26:192–195
Grandlic CJ, Mendez MO, Chorover J, Machado B, Maier RM (2008) Plant growth-promoting bacteria for phytostabilization of mine tailings. Environ Sci Technol 42:2079–2084
Grandlic CJ, Palmer MW, Maier RM (2009) Optimization of plant growth-promoting bacteria-assisted phytostabilization of mine tailings. Soil Biol Biochem 41:1734–1740
Gupta P, Samant K, Sahu A (2012) Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int J Microbiol 212:1–5
Hassan W, Bano R, Bashir F, David J (2014) Comparative effectiveness of ACC-deaminase and/or nitrogen-fixing rhizobacteria in promotion of maize (Zea mays L.) growth under lead pollution. Environ Sci Pollut Res 21:10983–10996
He LY, Zhang YF, Ma HY, Su, LN, Chen ZJ, Wang QY, Qian M, Sheng XF (2010) Characterization of copper-resistant bacteria and assessment of bacterial communities in rhizosphere soils of copper-tolerant plants. Appl Soil Ecol 44:49–55
Islam E, Sar P (2011) Culture-dependent and -independent molecular analysis of the bacterial community within uranium ore. J Basic Microbiol 51:372–384
Kabata-Pendias A, Szteke B (2015) Trace elements in abiotic and biotic environments. CrC Press, Taylor and Francis Group
Lenart A, Wolny-Koładka K (2013) The effect of heavy metal concentration and soil pH on the abundance of selected microbial groups within Arcelor Mittal Poland steelworks in Cracow. B Environ Contam Tox 90:85–90
Li K, Ramakrishna W (2011) Effect of multiple metal resistant bacteria from contaminated lake sediments on metal accumulation and plant growth. J Hazard Mater 189:531–539
Lima de Silva AA, Carvalho MAR, Souza SAL, Dias PMT, Filho RGS, Saramago CSM, Bento CAM, Hofer E (2012) Heavy metal tolerance(Cr, Ag and Hg) in bacteria isolated from sewage. Braz J Microbiol 43:1620–1631
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258
Margesin R, Płaza GA, Kasenbacher S (2011) Characterization of bacterial communities at heavy-metal-contaminated sites. Chemosphere 82:1583–1588
Nautiyal V (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. Microbiol Lett 170:265–270
Navarro-Noya YE, Hernández-Mendoza E, Morales-Jiménez J, Jan-Roblero J, Martínez-Romero E, Hernández-Rodríguez C (2012) Isolation and characterization of nitrogen fixing heterotrophic bacteria from the rhizosphere of pioneer plants growing on mine tailings. Appl Soil Ecol 62:52–60
Neethu CS, Mujeeb Rahiman KM, Saramma AV, Mohamed Hatha AA (2015) Heavy-metal resistance in gram-negative bacteria isolated from Kongsfjord, Arctic. Can J Microbiol 61:1–7
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis part 2, 2nd edn. Agronomy Society of America, Madison, pp. 403–430
Pandey A, Trivedi P, Kumar B, Palni LMS (2006) Characterization of a phosphate solubilizing and antagonistic strain of Pseudomonas putida (B0) isolated from a sub-alpine location in the Indian central Himalaya. Curr Microbiol 53:102–107
Peña A, Mingorance MD, Rossini-Oliva S (2015) Soil quality improvement by the establishment of a vegetative cover in a mine soil added with composted municipal sewage sludge. J Geochem Explor 157:178–183
Pereira SIA, Pires C, Henriques I, Correia A, Magan N, Castro PML (2015a) Assessment of rhizospheric culturable bacteria of Phragmites australis and Juncus effusus from polluted sites. J Basic Microbiol 55:1–12
Pereira SIA, Barbosa LV, Castro PML (2015b) Rhizobacteria isolated from a metal polluted area enhance plant growth in zinc and cadmium contaminated soil. Int J Environ Sci Technol 12:2127–2142
Pereira SIA, Lima AIG, Figueira EMAP (2006) Heavy metal toxicity in Rhizobium leguminosarum biovar viciae isolated from soils subjected to different sources of heavy metal contamination: effects on protein expression. Appl Soil Ecol 33:286–293
Pérez-Esteban J, Escolástico C, Masaguer A, Ruiz-Fernández J, Moliner A (2015) Phytoremediation of degraded mine soils using organic amendments and metal-tolerant plants. In: Ansari AA, Gill SS, Gill R, Lanza GR, Newman L (ed) Phytoremediation: management of environmental contaminants, Vol 1. Springer International Publishing. pp 309–321
Piotrowska-Seget Z, Beściak G, Bernaś T, Kozdrój J (2012) GFP-tagged multimetal-tolerant bacteria and their detection in the rhizosphere of white mustard. Ann Microbiol 62:559–567
Rajkumar M, Ae N, Prasad MNV, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28:142–149
Rau N, Mishra V, Sharma M, Das MK, Ahaluwalia K, Sharma RS (2009) Evaluation of functional diversity in rhizobacterial taxa of a wild grass (Saccharumravennae) colonizing abandoned fly ash dumps in Delhi urban ecosystem. Soil Biol Biochem 41:813–821
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 21:1–30
Saleh SS, Glick BR (2001) Involvement of gasS and pros in enhancement of the plant growth-promoting capabilities of Enterobacter cloacae CAL2 and UW4. Can J Microbiol 47:698–705
Schoenholtz SH, Van Miegroet H, Burger JA (2000) A review of chemical and physical properties as indicators of forest soil quality: challenges and opportunities. Forest Ecol Manag 138:335–356
Schwy B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Sheng X, Sun L, Huang Z, He L, Zhang W, Chen Z (2012) Promotion of growth and Cu accumulation of bio-energy crop (Zea mays) by bacteria: implications for energy plant biomass production and phytoremediation. J Environ Manag 103:58–64
Schwartz C, Gérard E, Perronnet K, Morel JL (2001) Measurement of in situ phytoextraction of zinc by spontaneous metallophytes growing on a former smelter site. Sci Total Environ 279:215–221
Siddiqui ZA (2006) PGPR: prospective biocontrol agents of plant pathogens. In: Siddiqui ZA (ed) PGPR: biocontrol and biofertilization. Springer Netherlands pp 111–142
Singh NP, Santal AR (2015) Phytoremediation of heavy metals: the use of green approaches to clean the environment. In: Ansari AA, Gill SS, Gill R, Lanza GR, Newman L (ed) Phytoremediation: management of environmental contaminants, Vol 2. Springer International Publishing pp 115–129
Singh RP, Shelke GM, Kumar A, Jha PN (2015) Biochemistry and genetics of ACC deaminase: a weapon to “stress ethylene” produced in plants. Front Microbiol 6:937
Smibert RM, Krieg NR (1994) Phenotypic characterization. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR (ed) Methods for general and molecular bacteriology American Society for Microbiology. Washington, pp 611–651
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Trivedi P, Pandey A, Palni LMS (2008) In vitro evaluation of antagonistic properties of Pseudomonas corrugata. Microbiol Res 163:329–336
Wang X, Liu Y, Zeng G, Chai L, Xiao X, Song X, Min Z (2008) Pedological characteristics of Mn mine tailings and metal accumulation by native plants. Chemosphere 72:1260–1266
Xu ZY, Tang M, Chen H, Ban YH, Zhang HH (2012) Microbial community structure in the rhizosphere of Sophora viciifolia grown at a lead and zinc mine of northwest China. Sci Total Environ 435:453–464
Yu X, Li Y, Zhang C, Liu H, Liu J, Zheng W, Kang X, Leng X, Zhao K, Gu Y, Zhang X, Xiang Q, Chen Q (2014) Culturable heavy metal-resistant and plant growth promoting bacteria in V-Ti magnetite mine tailing soil from Panzhihua. China. PLoS One 9:1–8
Zhang WH, Huang Z, He LY, Sheng XF (2012) Assessment of bacterial communities and characterization of lead-resistant bacteria in the rhizosphere soils of metal-tolerant Chenopodium ambrosioides grown on lead–zinc mine tailings. Chemosphere 87:1171–1178
Acknowledgments
The authors acknowledge the financial support of the “Convention de coopération CNRST-Morocco/FCT-Portugal.” This work was also supported by Portuguese Funds through Fundação para a Ciência e a Tecnologia (FCT) under the project UID/Multi/50016/2013. S.I.A. Pereira thanks the grant SFRH/BPD/65134/2009 from FCT (Portugal), Fundo Social Europeu and Fundos Nacionais do MEC through the program QREN-POPH-Tipologia 4.1-Formação Avançada. We also thank Dr. A. Pandey for hosting Dr. A. Boularbah during his stay at the GB Paint Institute of the Himalayan Environment and Development, India, under the CV Raman International Fellowship program for African Researchers 2012, giving him the opportunity to work on PGPRs. The authors are grateful to Dr. A. El Gharmali for his constant help in the analysis of the heavy metals in different samples and Dr. R.C. Pullar for proofreading and correcting the English of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yi-ping Chen
Electronic supplementary material
ESM 1
(DOC 77 kb)
Rights and permissions
About this article
Cite this article
Benidire, L., Pereira, S.I.A., Castro, P.M.L. et al. Assessment of plant growth promoting bacterial populations in the rhizosphere of metallophytes from the Kettara mine, Marrakech. Environ Sci Pollut Res 23, 21751–21765 (2016). https://doi.org/10.1007/s11356-016-7378-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7378-6