Abstract
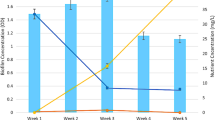
Comparative effects of long-term exposure to Polar Organic Chemical Integrative Sampler (POCIS) extracts (PE) and to a reconstituted mixture based on the major compounds quantified in the PE were evaluated on river biofilm communities. The study aimed to characterize the effects of long-term and low-dose exposure to pesticides on natural biofilm communities and to evaluate if the effects due to PE exposure could be explained solely by the major compounds identified in the extracts. Biofilms from an uncontaminated site were exposed in artificial channels to realistic environmental concentrations using diluted PE, with the 12 major compounds quantified in the extracts (Mix) or with water not containing pesticides (Ctr). Significant differences between biofilms exposed to pesticides or not were observed with regard to diatom density, biomass (dry weight and ash-free dry mass), photosynthetic efficiency (ΦpsII) and antioxidant enzyme activities. After 14 days of exposure to the different treatments, the observed trend towards a decrease of mean diatom cell biovolumes in samples exposed to pesticides was related to the control biofilms’ higher relative abundance of large species like Cocconeis placentula or Amphora copulata and lower relative abundance of small species like Eolimna minima compared to the contaminated ones. Principal component analyses clearly separated contaminated (PE and Mix) from non-contaminated (Ctr) biofilms; on the contrary, the analyses did not reveal separation between biofilms exposed to PE or to the 12 major compounds identified in the extract.






Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 6:105–121
AFNOR (1982) Testing water- determination of the orthophosphate, polyphosphate and total phosphorus content (spectrometric method)- Norme NF T 90-023.
AFNOR (1996) Water quality - determination of nitrite nitrogen and nitrate nitrogen and the sum of both by flow analysis (CFA and FIA) and spectrometric detection- Norme NF EN ISO 13395.
AFNOR (2001) Water quality - determination of soluble silicates - molecular absorption spectrometric method - Norme NF T 90-007 -
AFNOR (2003) Water quality - guidance standard for the routine sampling and pretreatment of benthic diatoms from rivers- Norme NF EN 13946
AFNOR (2005) Water quality - determination of suspended solids - method by filtration through glass fibre filters- Norme EN 872
AFNOR (2009) Water quality—protocol for the initial method performance assessment in a laboratory -Norme NF T90-210.
Arini A, Feurtet-Mazel A, Maury-Brachet R, et al. (2012a) Recovery potential of periphytic biofilms translocated in artificial streams after industrial contamination (Cd and Zn). Ecotoxicology 1–12
Arini A, Feurtet-Mazel A, Maury-Brachet R et al (2012b) Field translocation of diatom biofilms impacted by Cd and Zn to assess decontamination and community restructuring capacities. Ecol Indic 18:520–531
Artigas J, Fund K, Kirchen S et al (2012) Patterns of biofilm formation in two streams from different bioclimatic regions: analysis of microbial community structure and metabolism. Hydrobiologia 695:83–96
Balaam JL, Grover D, Johnson AC et al (2010) The use of modelling to predict levels of estrogens in a river catchment: how does modelled data compare with chemical analysis and in vitro yeast assay results? Sci Total Environ 408:4826–4832
Bonet B, Corcoll N, Guasch H (2012) Antioxidant enzyme activities as biomarkers of Zn pollution in fluvial biofilms. Ecotoxicol Environ Saf 80:172–178
Bonet B, Corcoll N, Acuňa V et al (2013) Seasonal changes in antioxidant enzyme activities of freshwater biofilms in a metal polluted Mediterranean stream. Sci Total Environ 444:1–10
Bonnineau C, Tlili A, Faggiano L et al (2013) The use of antioxidant enzymes in freshwater biofilms: temporal variability vs. toxicological responses. Aquat Toxicol 60–71:136–137
Brack W (2003) Effect-directed analysis: a promising tool for the identification of organic toxicants in complex mixtures? Anal Bioanal Chem 377:397–407
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Corcoll N, Ricart M, Franz S et al (2012a) The use of photosynthetic fluorescence parameters from autotrophic biofilms for monitoring the effect of chemicals in river ecosystems. In: Ginebreda A, Geiszinger A, Guasch H (eds) Handb. Environ. Chem. Springer, Heidelberg, pp 86–114
Corcoll N, Bonet B, Morin S et al (2012b) The effect of metals on photosynthesis processes and diatom metrics of biofilm from a metal-contaminated river: a translocation experiment. Ecol Indic 18:620–631
Debenest T, Silvestre J, Coste M, Pinelli E (2010) Effects of pesticides on freshwater diatoms. Rev Environ Contam Toxicol 203:87–103
Duprat C (2010) PAT Trec Canaule en Lot et Garonne-Suivi de la qualité des rivières-Bilan 2009. Serv. Territ. Chamb. Agric. Lot Garonne
Fang HHP, Xu LC, Chan KY (2002) Effects of toxic metals and chemicals on biofilm and biocorrosion. Water Res 36:4709–4716
Fernández-Alba AR, Hernando MD, Piedra L, Chisti Y (2002) Toxicity evaluation of single and mixed antifouling biocides measured with acute toxicity bioassays. Anal Chim Acta 456:303–312
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Grant DF, Bender DM, Hammock BD (1989) Quantitative kinetic assays for glutathione S-transferase and general esterase in individual mosquitoes using an EIA reader. Insect Biochem 19:741–751
Guillard RRL, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide c. J Phycol 8:10–14
Hamilton PB, Jackson GS, Kaushik NK, Solomon KR (1987) The impact of atrazine on lake periphyton communities, including carbon uptake dynamics using track autoradiography. Environ Pollut 46:83–103
Hecker M, Hollert H (2009) Effect-directed analysis (EDA) in aquatic ecotoxicology: state of the art and future challenges. Environ Sci Pollut Res 16:607–613
Hofmann G, Werum M, Lange-Bertalot H (2011) Diatomeen in Süßwasser-Benthos von Mitteleuropa. A. R. G. Gantner Verlag Kommanditgesellschaft, Rugell
Kim Tiam S, Feurtet-Mazel A, Delmas F et al (2012) Development of q-PCR approaches to assess water quality: effects of cadmium on gene expression of the diatom Eolimna minima. Water Res 46:934–942
Larras F, Bouchez A, Rimet F, Montuelle B (2012) Using bioassays and species sensitivity distributions to assess herbicide toxicity towards benthic diatoms. PLoS One 7:e44458
Lissalde S, Mazzella N, Fauvelle V et al (2011) Liquid chromatography coupled with tandem mass spectrometry method for thirty-three pesticides in natural water and comparison of performance between classical solid phase extraction and passive sampling approaches. J Chromatogr A 1218:1492–1502
Matthiessen P, Arnold D, Johnson AC et al (2006) Contamination of headwater streams in the United Kingdom by oestrogenic hormones from livestock farms. Sci Total Environ 367:616–630
Mazzella N, Lissalde S, Moreira S et al (2010) Evaluation of the use of performance reference compounds in an oasis-HLB adsorbent based passive sampler for improving water concentration estimates of polar herbicides in freshwater. Environ Sci Technol 44:1713–1719
Morin S, Vivas-Nogues M, Duong TT et al (2007) Dynamics of benthic diatom colonization in a cadmium/zinc-polluted river (Riou-Mort, France). Fundam Appl Limnol Arch Für Hydrobiol 168:179–187
Morin S, Bottin M, Mazzella N et al (2009) Linking diatom community structure to pesticide input as evaluated through a spatial contamination potential (Phytopixal): a case study in the Neste river system (South-West France). Aquat Toxicol 94:28–39
Morin S, Proia L, Ricart M et al (2010) Effects of a bactericide on the structure and survival of benthic diatom communities. Vie Milieu Life Environ 60:109–116
Morin S, Pesce S, Kim-Tiam S, et al. (2012) Use of polar organic chemical integrative samplers to assess the effects of chronic pesticide exposure on biofilms. Ecotoxicology 1–11
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Passy SI, Blanchet FG (2007) Algal communities in human-impacted stream ecosystems suffer beta-diversity decline. Divers Distrib 13:670–679
Pérès F, Florin D, Grollier T et al (1996) Effects of the phenylurea herbicide isoproturon on periphytic diatom communities in freshwater indoor microcosm. Environ Pollut 94:141–152
Pesce S, Morin S, Lissalde S et al (2011) Combining polar organic chemical integrative samplers (POCIS) with toxicity testing to evaluate pesticide mixture effects on natural phototrophic biofilms. Environ Pollut 159:735–741
Ricart M, Guasch H, Alberch M et al (2010) Triclosan persistence through wastewater treatment plants and its potential toxic effects on river biofilms. Aquat Toxicol 100:346–353
Roubeix V, Fauvelle V, Tison-Rosebery J et al (2012a) Assessing the impact of chloroacetanilide herbicides and their metabolites on periphyton in the Leyre River (SW France) via short term growth inhibition tests on autochthonous diatoms. J Environ Monit 14:1655–1663
Roubeix V, Pesce S, Mazzella N et al (2012b) Variations in periphytic diatom tolerance to agricultural pesticides in a contaminated river: an analysis at different diversity levels. Fresenius Environ Bull 21:2090–2094
Sabater S, Guasch H, Ricart M et al (2007) Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal Bioanal Chem 387:1425–1434
Schaedle M, Bassham JA (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Schreiber U (1998) Chlorophyll fluorescence: new instruments for special applications. In: Garab G (ed) Photosynthesis: mechanisms and effects, vol V. Kluwer, Dordrecht, pp 4253–4258
Serra A, Corcoll N, Guasch H (2009) Copper accumulation and toxicity in fluvial periphyton: the influence of exposure history. Chemosphere 74:633–641
Tapie N, Devier MH, Soulier C et al (2011) Passive samplers for chemical substance monitoring and associated toxicity assessment in water. Water Sci Technol 63:2418–2426
Tison J, Park YS, Coste M et al (2005) Typology of diatom communities and the influence of hydro-ecoregions: a study on the French hydrosystem scale. Water Res 39:3177–3188
Vermeirssen EL, Hollender J, Bramaz N et al (2010) Linking toxicity in algal and bacterial assays with chemical analysis in passive samplers deployed in 21 treated sewage effluents. Environ Toxicol Chem 29:2575–2582
Acknowledgments
This work was supported by the PoToMAC (Potential Toxicity of pesticides in Continental Aquatic Environments: passive sampling and exposure/impact on biofilms) programme under the reference ANR-11-CESA-022 and the French National Agency for Water and Aquatic Environments (ONEMA). The authors would like to thank S. Moreira, G. Jan, B. Delest, K. Madarassou and A. Moreira from Irstea Bordeaux for their skilful technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 355 kb)
Rights and permissions
About this article
Cite this article
Kim Tiam, S., Morin, S., Bonet, B. et al. Is the toxicity of pesticide mixtures on river biofilm accounted for solely by the major compounds identified?. Environ Sci Pollut Res 22, 4009–4024 (2015). https://doi.org/10.1007/s11356-014-3373-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3373-y