Abstract
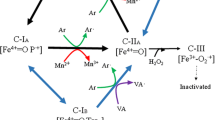
H2O2 has been found to be required for the activity of the main microbial enzymes responsible for lignin oxidative cleavage, peroxidases. Along with other small radicals, it is implicated in the early attack of plant biomass by fungi. Among the few extracellular H2O2-generating enzymes known are the glyoxal oxidases (GLOX). GLOX is a copper-containing enzyme, sharing high similarity at the level of active site structure and chemistry with galactose oxidase. Genes encoding GLOX enzymes are widely distributed among wood-degrading fungi especially white-rot degraders, plant pathogenic and symbiotic fungi. GLOX has also been identified in plants. Although widely distributed, only few examples of characterized GLOX exist. The first characterized fungal GLOX was isolated from Phanerochaete chrysosporium. The GLOX from Utilago maydis has a role in filamentous growth and pathogenicity. More recently, two other glyoxal oxidases from the fungus Pycnoporus cinnabarinus were also characterized. In plants, GLOX from Vitis pseudoreticulata was found to be implicated in grapevine defence mechanisms. Fungal GLOX were found to be activated by peroxidases in vitro suggesting a synergistic and regulatory relationship between these enzymes. The substrates oxidized by GLOX are mainly aldehydes generated during lignin and carbohydrates degradation. The reactions catalysed by this enzyme such as the oxidation of toxic molecules and the production of valuable compounds (organic acids) makes GLOX a promising target for biotechnological applications. This aspect on GLOX remains new and needs to be investigated.
Similar content being viewed by others
References
Aichinger C, Schreier P, Leuthner B, Adamczewski M, Hillebrand S, Kuck KH, Van Kan JAL, Visser J, Stefanato F, Kahmann R (2003) Fungal glyoxal oxidases. US Patent 20030140370
Akinori Y, Miho AG, Kinya F, Hiroyuki U, Takayuki U, Kazuo A (2002) Production of aldehyde oxidases by microorganisms and their enzymatic properties. J Biosci Bioeng 94:124–129
Aylward FO, Burnum-Johnson KE, Tringe SG et al (2013) Leucoagaricus gongylophorus produces diverse enzymes for the degradation of recalcitrant plant polymers in leaf-cutter ant fungus gardens. Appl Environ Microbiol 79:3770–3778
Barrasa JM, Gutiérrez A, Escaso V, Guillén F, Martínez MJ, Martínez AT (1998) Electron and fluorescence microscopy of extracellular glucan and aryl-alcohol oxidase during wheat-straw degradation byPleurotus eryngii. Appl Environ Microbiol 64:325–332
Carro J, Ferreira P, Rodriguez L, Prieto A, Serrano A, Balcells B, Arda A, Jimenez-Barbero J, Gutierrez A, Ullrich R, Hofrichter M, Martinez AT (2015) 5-hydroxymethylfurfural conversion by fungal aryl-alcohol oxidase and unspecific peroxygenase. FEBS J 282:3218–3229
Chaplin AK, Petrus ML, Mangiameli G, Hough MA, Svistunenko DA, Nicholls P, Claessen D, Vijgenboom E, Worrall JA (2015) GlxA is a new structural member of the radical copper oxidase family and is required for glycan deposition at hyphal tips and morphogenesis of Streptomyces lividans. Biochem J 469:433–444
Chaplin AK, Svistunenko DA, Hough M, Wilson MT, Vijgenboom E, Worrall JA (2017) Active site maturation and activity of the copper-radical oxidase GlxA is governed by a tryptophan residue. Biochem J 474:809–825
Ciriminna R, Pina CD, Rossi M, Pagliaro M (2014) Understanding the glycerol market. Eur J Lipid Sci Technol 116:1432–1439
Couturier M, Navarro D, Olivé C, Chevret D, Haon M, Favel A, Lesage-Meessen L, Henrissat B, Coutinho PM, Berrin JG (2012) Post-genomic analyses of fungal lignocellulosic biomass degradation reveal the unexpected potential of the plant pathogen Ustilago maydis. BMC Genom 13:57
Daniel G, Pettersson B, Nllsson T, Volc J (1990) Use of immunogold cytochemistry to detect Mn (II)-dependent and lignin peroxidases in wood degraded by the white rot fungiPhanerochaete chrysosporiumandLentinula edodes. Can J Bot 68:920–933
Daniel G, Volc J, Kubatova E (1994) Pyranose oxidase, a major source of H 2 O 2during wood degradation byPhanerochaete chrysosporium ,Trametes versicolor, andOudemansiella mucida. Appl Environ Microbiol 60:2524–2532
Daou M, Piumi F, Cullen D, Record E, Faulds CB (2016) Heterologous production and characterization of two glyoxal oxidases fromPycnoporus cinnabarinus. Appl Environ Microbiol 82:4867–4875
Dijkman WP, Groothuis DE, Fraaije MW (2014) Enzyme-catalyzed oxidation of 5-hydroxymethylfurfural to furan-2,5-dicarboxylic acid. Angew Chem 53:6515–6518.
Eastwood DC, Floudas D, Binder M, Majcherczyk A, Schneider P, Aerts A, Asiegbu FO, Baker SE, Barry K, Bendiksby M, Blumentritt M (2011) The plant cell wall–decomposing machinery underlies the functional diversity of forest fungi. Science 333:762–765
Evans CS, Dutton MV, Guillén F, Veness RG (1994) Enzymes and small molecular mass agents involved with lignocellulose degradation. FEMS Microbiol Rev 13:235–239.
Fernandez-Fueyo E, Ruiz-Dueñas FJ, Ferreira P et al (2012) Comparative genomics of Ceriporiopsis subvermispora and Phanerochaete chrysosporium provide insight into selective ligninolysis. Proc Nat Acad Sci USA 109:5458–5463.
Floudas D, Binder M, Riley R et al (2012) The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719
Forney LJ, Reddy CA, Tien M, Aust SD (1982) The involvement of hydroxyl radical derived from hydrogen peroxide in lignin degradation by the white rot fungusPhanerochaete chrysosporium. J Biol Chem 257:11455–11462
Gancedo JM, Gancedo C, Asensio C (1967) Widespread occurrence of galactose oxidase and glucose oxidase in fungi. Arch Biochem Biophys 119:588–590
Glenn JK, Gold MH (1985) Purification and characterization of an extracellular Mn (II)-dependent peroxidase from the lignin-degrading basidiomycete,Phanerochaete chrysosporium. Arch Biochem Biophys 242:329–341
Guan X, Zhao H, Xu Y, Wang Y (2011) Transient expression of glyoxal oxidase from the Chinese wild grape Vitis pseudoreticulata can suppress powdery mildew in a susceptible genotype. Protoplasma 248:415–423
Guillén F, Martínez AT, Martínez MJ (1992) Aryl-alcohol oxidase fromPleurotus eryngii : substrate specificity and H2O2—producing system. Biotechnology in Pulp and Paper Industry. UNI Publishing Co., Tokyo, pp 371–376
Gutiérrez R, Urtiaga A, Ortiz I (2010) Separation of phenol and formaldehyde from industrial wastes. Modelling of the phenol extraction equilibrium. J Chem Technol Biotechnol 85:1215–1222
Handa S, Sharma A, Chakraborti K (1986) Natural products and plants as liver protecting drugs. Fitoterapia 57:307–351
Hatakka A (2001) Biodegradation of lignin. In: Hofrichter M, Steinbüchel A (eds) Biopolymers: lignin, humic substances and coal, 1st edn. Wiley, New York, pp 129–180
Hernández-Ortega A, Ferreira P, Martínez AT (2012) Fungal aryl-alcohol oxidase: a peroxide-producing flavoenzyme involved in lignin degradation. Appl Microbiol Biotechnol 93:1395–1410
Hidalgo A, Lopategi A, Prieto M, Serra JL, Llama MJ (2002) Formaldehyde removal in synthetic and industrial wastewater by Rhodococcus erythropolis UPV-1. Appl Microbiol Biotechnol 58:260–263
Hori C, Gaskell J, Igarashi K, Samejima M, Hibbett D, Henrissat B, Cullen D (2013) Genomewide analysis of polysaccharides degrading enzymes in 11 white-and brown-rot Polyporales provides insight into mechanisms of wood decay. Mycologia 105:1412–1427
Ito N, Phillips SE, Stevens C, Ogel ZB, Mcpherson MJ, Keen JN, Yadav KD, Knowles PF (1991) Novel thioether bond revealed by a 1.7 A crystal structure of galactose oxidase. Nature 350:87–90
Ito N, Phillips SEV, Yadav KDS, Knowles PF (1994) Crystal structure of a free-radical enzyme, Galactose-Oxidase. J Mol Biol 238:794–814
Janse BJH, Gaskell J, Akhtar M, Cullen D (1998) Expression of Phanerochaete chrysosporium genes encoding lignin peroxidases, manganese peroxidases, and glyoxal oxidase in wood. Appl Environ Microbiol 64:3536–3538
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112.
Jönsson LJ, Alriksson B, Nilvebrant NO (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:16.
Kelley RL, Reddy CA (1986) Identification of glucose oxidase activity as the primary source of hydrogen peroxide production in ligninolytic cultures of Phanerochaete chrysosporium. Arch Microbiol 144:248–253
Kersten PJ (1990) Glyoxal oxidase of Phanerochaete chrysosporium: its characterization and activation by lignin peroxidase. Proc Nat Acad Sci USA 87:2936–2940
Kersten PJ, Cullen D (1993) Cloning and characterization of cDNA encoding glyoxal oxidase, a H2O2-producing enzyme from the lignin-degrading basidiomycete Phanerochaete chrysosporium. Proc Nat Acad Sci USA 90:7411–7413
Kersten P, Cullen D (2014) Copper radical oxidases and related extracellular oxidoreductases of wood-decay Agaricomycetes. Fungal Genet Biol 72:124–130.
Kersten PJ, Kirk TK (1987) Involvement of a new enzyme, glyoxal oxidase, in extracellular H 2 O 2production byPhanerochaete chrysosporium. J Bacteriol 169:2195–2201
Kirk TK, Chang HM (1975) Decomposition of lignin by white-rot fungi. II. Characterization of heavily degraded lignins from decayed spruce. Holzforschung 29:56–64
Krystof M, Perez-Sanchez M, Dominguez De Maria P (2013) Lipase-mediated selective oxidation of furfural and 5 hydroxymethylfurfural. ChemSusChem 6:826–830
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Ann Rev Plant Physiol Plant Mol Biol 48:251–275
Leuthner B, Aichinger C, Oehmen E, Koopmann E, Müller O, Müller P, Kahmann R, Bölker M, Schreier PH (2005) A H2O2—producing glyoxal oxidase is required for filamentous growth and pathogenicity inUstilago maydis.Mol Gen Genom 272:639–650
Levasseur A, Drula E, Lombard V, Coutinho PM, Henrissat B (2013) Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol Biofuels 6:1–14
Levasseur A, Lomascolo A, Chabrol O, Ruiz-Dueñas FJ, Boukhris-Uzan E, Piumi F, Kües U, Ram AF, Murat C, Haon M, Benoit I (2014) The genome of the white-rot fungusPycnoporus cinnabarinus: a basidiomycete model with a versatile arsenal for lignocellulosic biomass breakdown. BMC Genom 15:1
Lobos S, Larraín J, Salas L, Cullen D, Vicuña R (1994) Isoenzymes of manganese-dependent peroxidase and laccase produced by the lignin-degrading basidiomyceteCeriporiopsis subvermispora. Microbiology 140:2691–2698
Martinez D, Larrondo LF, Putnam N, Gelpke MD, Huang K, Chapman J, Helfenbein KG, Ramaiya P, Detter JC, Larimer F (2004) Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat Biotechnol 22:695–700
Martinez D, Challacombe J, Morgenstern I et al (2009) Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc Nat Acad Sci USA 106:1954–1959
Mussatto SI, Roberto IC (2004) Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Biores Technol 93:1–10
Ohm RA, De Jong JF, Lugones LG, Aerts A, Kothe E, Stajich JE, De Vries RP, Record E, Levasseur A, Baker SE (2010) Genome sequence of the model mushroom Schizophyllum commune. Nat Biotechnol 28:957–U10
Olson Å, Aerts A, Asiegbu F et al (2012) Insight into trade-off between wood decay and parasitism from the genome of a fungal forest pathogen. New Phytol 194:1001–1013
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Biores Technol 74:25–33.
Parawira W, Tekere M (2011) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Crit Rev Biotechnol 31:20–31
Parish RW, Li SF (2010) Death of a tapetum: a programme of developmental altruism. Plant Sci 178:73–89
Paszczyński A, Huynh VB, Crawford R (1985) Enzymatic activities of an extracellular, manganese-dependent peroxidase from Phanerochaete chrysosporium. FEMS Microbiol Letts 29:37–41.
Phan HA, Iacuone S, Li SF, Parish RW (2011) The MYB80 transcription factor is required for pollen development and the regulation of tapetal programmed cell death in Arabidopsis thaliana. Plant Cell 23:2209–2224
Qin YZ, Li YM, Zong MH, Wu H, Li N (2015) Enzyme-catalyzed selective oxidation of 5-hydroxymethylfurfural (HMF) and separation of HMF and 2,5-diformylfuran using deep eutectic solvents. Green Chem 17:3718–3722
Rahman MA, Humphreys RWR, Wu SR (1995) Method of conditioning fabrics with glyceric acid based biodegradable molecules. Canadian Patent 2151319.
Rea G, Metoui O, Infantino A, Federico R, Angelini R (2002) Copper amine oxidase expression in defense responses to wounding and Ascochyta rabiei invasion. Plant Physiol 128:865–875
Reape TJ, Mccabe PF (2010) Apoptotic-like regulation of programmed cell death in plants. Apoptosis 15:249–256
Roncal T, Muñoz C, Lorenzo L, Maestro B, Díaz De Guereñu MEM (2012) Two-step oxidation of glycerol to glyceric acid catalyzed by the Phanerochaete chrysosporium glyoxal oxidase. Enzyme Microb Technol 50:143–150
Ruiz - Dueñas FJ, Martínez MJ, Martínez AT (1999) Molecular characterization of a novel peroxidase isolated from the ligninolytic fungusPleurotus eryngii. Mol Microbiol 31:223–235
Sasaki Y, Isobe K, Kataoka M, Ogawa J, Iwasaki A, Hasegawa J, Shimizu S (2008) Purification and characterization of a new aldehyde oxidase fromPseudomonas sp. AIU 362. J Biosci Bioeng 106:297–302
Sauer M, Porro D, Mattanovich D, Branduardi P (2008) Microbial production of organic acids: expanding the markets. Trends Biotechnol 26:100–108
Shi J, Sharma-Shivappa RR, Chinn MS (2009) Microbial pretreatment of cotton stalks by submerged cultivation ofPhanerochaete chrysosporium. Bioresour Technol 100:4388–4395
Sindhu R, Binod P, Pandey A (2016) Biological pretreatment of lignocellulosic biomass—an overview. Bioresour Technol 199:76–82
Son YL, Kim HY, Thiyagarajan S, Xu JJ, Park SM (2012) Heterologous expression of Phanerochaete chrysoporium glyoxal oxidase and its application for the coupled reaction with manganese peroxidase to decolorize malachite green. Mycobiology 40:258–262.
Song XS, Xing S, Li HP, Zhang JB, Qu B, Jiang JH, Fan C, Yang P, Liu JL, Hu ZQ, Xue S, Liao YC (2016) An antibody that confers plant disease resistance targets a membrane-bound glyoxal oxidase in Fusarium. New Phytol 210:997–1010
Sousa AF, Vilela C, Fonseca AC, Matos M, Freire CSR, Gruter GJM, Coelho JFJ, Silvestre AJD (2015) Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: a tribute to furan excellency. Polym Chem 6:5961–5983.
Suzuki H, Macdonald J, Syed K et al (2012) Comparative genomics of the white-rot fungi, Phanerochaete carnosa and P. chrysosporium, to elucidate the genetic basis of the distinct wood types they colonize. BMC Genom 13:444
Takano M, Nakamura M, Yamaguchi M (2010) Glyoxal oxidase supplies hydrogen peroxide at hyphal tips and on hyphal wall to manganese peroxidase of white-rot fungus Phanerochaete crassa WD1694. J Wood Sci 56:307–313
Tang JD, Perkins AD, Sonstegard TS, Schroeder SG, Burgess SC, Diehl SV (2012) Short-Read Sequencing for Genomic Analysis of the Brown Rot Fungus Fibroporia radiculosa. Appl Environ Microbiol 78:2272–2281
Tien M, Kirk TK (1983) Lignin-degrading enzyme from the hymenomycetePhanerochaete chrysosporiumBurds. Science 221:661–662
Uchida H, Okamura Y, Yamanaka H, Fukuda T, Haneda S, Aisaka K, Fujii Y (2006) Purification and some properties of an aldehyde oxidase fromStreptomyces rimosusATCC10970. World J Microbiol Biotechnol 22:469–474
Vanden Wymelenberg A, Sabat G, Mozuch M, Kersten PJ, Cullen D, Blanchette RA (2006) Structure, organization, and transcriptional regulation of a family of copper radical oxidase genes in the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol 72:4871–4877
Varela E, Martínez AT, Martínez MJ (1999) Molecular cloning of aryl-alcohol oxidase from the fungusPleurotus eryngii, an enzyme involved in lignin degradation. Biochem J 341:113–117
Wada R, Hyon SH, Ikada Y (1996) New biodegradable oligoesters for pharmaceutical application. J Biomat Sci 7:715–725
Wan C, Li Y (2010) Microbial pretreatment of corn stover withCeriporiopsis subvermisporafor enzymatic hydrolysis and ethanol production. BioresTechnol 101:6398–6403.
Wang P, Woodward CA, Kaufman EN (1999) Poly(ethylene glycol)-modified ligninase enhances pentachlorophenol biodegradation in water-solvent mixtures. Biotechnol Bioeng 64:290–297
Wariishi H, Akileswaran L, Gold MH (1988) Manganese peroxidase from the basidiomycete Phanerochaete chrysosporium: spectral characterization of the oxidized states and the catalytic cycle. Biochem 27:5365–5370.
Whittaker JW (1994) The free radical-coupled copper active site of galactose oxidase. In: Sigel H, Sigel A (eds) Metal Ions in biological systems: Volume 30: Metalloenzymes involving amino acid-residue and related radicals. CRC Press, New York, pp 315–360
Whittaker JW (2005) The radical chemistry of galactose oxidase. Arch Biochem Biophys 433:227–239
Whittaker MM, Whittaker JW (1993) Ligand interactions with galactose oxidase: mechanistic insights. Biophys J 64:762–772
Whittaker MM, Chuang YY, Whittaker JW (1993) Models for the redox-active site in galactose-oxidase. J Am Chem Soc 115:10029–10035
Whittaker MM, Kersten PJ, Nakamura N, Sanders-Loehr J, Schweizer ES, Whittaker JW (1996) Glyoxal oxidase from Phanerochaete chrysosporium is a new radical-copper oxidase. J Biol Chem 271:681–687
Whittaker MM, Kersten PJ, Cullen D, Whittaker JW (1999) Identification of catalytic residues in glyoxal oxidase by targeted mutagenesis. J Biol Chem 274:36226–36232
Wyman CE, Decker SR, Himmel ME, Brady JW, Skopec CE, Viikari L (2005) Hydrolysis of cellulose and hemicellulose. Polysaccharides 1:1023–1062.
Yin D, URRESTI S, Lafond M, Johnston EM, Derikvand F, Ciano L, Berrin J-G, Henrissat B, Walton PH, Davies GJ, Brumer H (2015) Structure function characterization reveals new catalytic diversity in the galactose oxidase and glyoxal oxidase family. Nat Commun 6:10197
Zhou BJ, Wang XP, Wang YJ (2007) cDNA cloning, expression, protein purification, and characterization of a novel glyoxal oxidase related gene from Vitis pseudoreticulata. Biol Plant 51:458–466
Acknowledgements
We are grateful for the European Commission for funding this work within the INDOX Project (KBBE-2013-7-613549).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daou, M., Faulds, C.B. Glyoxal oxidases: their nature and properties. World J Microbiol Biotechnol 33, 87 (2017). https://doi.org/10.1007/s11274-017-2254-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-017-2254-1