Abstract
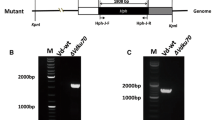
In the phytopathogenic fungus Ustilago maydis the mating-type loci control the transition from yeast-like to filamentous growth required for pathogenic development. In a large REMI (restriction enzyme mediated integration) screen, non-pathogenic mutants were isolated in a haploid strain that had been engineered to be pathogenic. In one of these mutants, which showed a specific morphological phenotype, the tagged gene, glo1 , was found to encode a product that is highly homologous to a glyoxal oxidase gene from the wood-rot fungus Phanerochaete chrysosporium. Glyoxal oxidase homologues are found in human, plant pathogenic fungi and in plants, but not in other mammals or yeasts. To confirm the function of the glo1 gene, null mutations were generated in compatible haploid U. maydis strains. In crosses null mutants were unable to generate filamentous dikaryons, and were completely non-pathogenic. Using a Glo1-overproducing strain we demonstrated that Glo1 is membrane bound, oxidizes a series of small aldehydes (<C4) and produces H2O2. The enzyme needs to be activated, presumably by auto-oxidation, to show full activity. A potential role for Glo1 during filamentous growth and pathogenic development of U. maydis is proposed.





Similar content being viewed by others
References
Banuett F, Herskowitz I (1989) Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc Natl Acad Sci USA 86:5878–5882
Bölker M, Urban M, Kahmann R (1992) The a mating type locus of U. maydis specifies cell signalling components. Cell 68:441–450
Bölker M, Böhnert HU, Braun K-H, Görl J, Kahmann R (1995a) Tagging pathogenicity genes in Ustilago maydis by restriction enzyme-mediated integration (REMI). Mol Gen Genet 248:547–552
Bölker M, Genin S, Lehmler C, Kahmann R (1995b) Genetic regulation of mating and dimorphism in Ustilago maydis. Can J Bot 73:S320-S325
Brachmann A, Weinzierl G, Kämper J, Kahmann R (2001) Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol Microbiol 42:1047–1063
Brachmann A, Koenig J, Julius C, Feldbruegge M (2004) A reverse genetic approach for generating gene replacement mutants in Ustilago maydis. Mol Gen Genomics 272:216–226
Dürrenberger F, Wong K, Kronstad JW (1998) Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc Natl Acad Sci USA 95:5684–5689
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstaed P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Giasson L, Kronstad JW (1995) Mutations in the myp1 gene of Ustilago maydis attenuate mycelial growth and virulence. Genetics 141:491–501
Gillissen B, Bergemann J, Sandmann C, Schroeer M, Bölker M, Kahmann R (1992) A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell 68:647–657
Gold S, Duncan G, Barrett K, Kronstad J (1994) cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev 8:2805–2816
Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10:751–757
Hoffman CS, Winston F (1987) A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267–272
Holliday R (1974) Molecular aspects of genetic exchange and gene conversion. Genetics 78:273–287
Ito N, Phillips SE, Stevens C, Ogel ZB, McPherson MJ, Keen JN, Yadav KD, Knowles PF (1991) Novel thioether bond revealed by a 1.7 Å crystal structure of galactose oxidase. Nature 350:87–90
Janse JHB, Gaskell J, Akhtar M, Cullen D (1998) Expression of Phanerochaete chrysosporium genes encoding lignin peroxidases, manganese peroxidases, and glyoxal oxidase in wood. Appl Environ Microbiol 64:3536–3538
Kahmann R, Steinberg G, Basse C, Feldbrügge, M, Kämper J (2000) Ustilago maydis, the causative agent of corn smut disease. In: Kronstad JW (ed) Fungal pathology. Kluwer Academic Publishers, Dordrecht, pp. 347–371
Kalapos MP (1999) Methylglyoxal in living organisms. Chemistry, biochemistry, toxicology and biological implications. Toxicol Lett 110:145–75
Kämper J (2003, 2004) A PCR-based system for highly efficient generation of gene replacement mutants in Ustilago maydis. DOI 10.1007/s00438-003-0962-8 Mol Gen Genomics 271:103–110
Keon JP, White GA, Hargraves JA (1991) Isolation, characterization and sequence of a gene conferring resistance to the systemic fungicide carboxin from the maize smut pathogen Ustilago maydis. Curr Genet 19:475–481
Kersten JP, Cullen D (1993) Cloning and characterization of a cDNA encoding glyoxal oxidase, a hydrogen peroxide-producing enzyme from the lignin-degrading basidiomycete Phanerochaete chrysosporium. Proc Natl Acad Sci USA 90:7411–7413
Kersten PJ, Kirk TK (1987) Involvement of a new enzyme, glyoxal oxidase, in extracellular hydrogen peroxide production by Phanerochaete chrysosporium. J Bac 169:2195–2201
Kersten JP, Witek C, Van den Wymelenberg A, Cullen D (1995) Phanerochaete chrysosporium glyoxal oxidase is encoded by two allelic variants: structure, genomic organization, and heterologous expression of glx1 and glx2. J Bacteriol 177:6106–6110
Klein RD, Gu Q, Goddard A, Rosenthal A (1996) Selection for genes encoding secreted proteins and receptors. Proc Natl Acad Sci USA 93:7108–7113
Krüger J, Loubradou G, Regenfelder E, Hartmann A, Kahmann R (1998) Crosstalk between cAMP and pheromone signalling pathways in Ustilago maydis. Mol Gen Genet 260:193–198
Lara-Ortiz T, Riveros-Rosas H, Aguirre J (2003) Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol Microbiol 50:1241–1255
Lau GW, Hamer JE (1998) Acropetal: a genetic locus required for conidiophore architecture and pathogenicity in the rice blast fungus. Fungal Genet Biol 24:228–239
Lehmler C, Steinberg G, Snetselaar KM, Schliwa M, Kahmann R, Bölker M (1997) Identification of a motor protein required for filamentous growth in Ustilago maydis. EMBO J 16:3464–3473
Martinez D, Larrondo LF, Putnam N, Gelpke MDS, Huang K, Chapman J, Helfenbein KG, Ramaiya P, Detter JC, Larimer F, Coutinho PM, Henrissat B, Berka R, Cullen D, Rokhsar D (2004) Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat Biotechnol 22:695–700
Martins AM, Cordeiro CA, Ponces Freire AM (2001) In situ analysis of glyoxalase I and glyoxalase II in Saccharomyces cerevisiae. FEBS Lett 499:41–44
Müller P, Aichinger C, Feldbrügge M, Kahmann R (1999) The MAP kinase Kpp2 regulates mating and pathogenic development in Ustilago maydis. Mol Microbiol 34:1007–1017
Nakai K, Horton P (1999) PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci 24:34–36
Navarro RE, Stringer MA, Hansberg W, Timberlake WE, Aguirre J (1996) catA, a new Aspergillus nidulans gene encoding a developmentally regulated catalase. Curr Genet 29:352–359
Paiva S, Kruckeberg AL, Casal M (2002) Utilization of green fluorescent protein as a marker for studying the expression and turnover of the monocarboxylate permease Jen1p of Saccharomyces cerevisiae. Biochem J 363:737–744
Petersson J, Pattison J, Kruckeberg AL, Berden JA, Persson BL (1999) Intracellular localization of an active green fluorescent protein-tagged Pho84 phosphate permease in Saccharomyces cerevisiae. FEBS Lett 462:37–42
Pick E, Mizel D (1981) Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods 46:211–226
Romeis T, Brachmann A, Kahmann R, Kämper J (2000) Identification of a target gene for the bE-bW homeodomain protein complex in Ustilago maydis. Mol Microbiol 37:54–66
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Schiestl RH, Petes TD (1991) Integration of DNA fragments by illegitimate recombination in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 88:7585-7589
Schulz B, Banuett F, Dahl M, Schlesinger R, Schäfer W, Martin T, Herskowitz I, Kahmann R (1990) The b alleles of Ustilago maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell 60:295–306
Spellig T, Bölker M, Lottspeich F, Frank RW, Kahmann R (1994) Pheromones trigger filamentous growth in Ustilago maydis. EMBO J 13:1620–1627
Spellig T, Bottin A, Kahmann R (1996) Green fluorescent protein (GFP) as a new vital marker in the phytopathogenic fungus Ustilago maydis . Mol Gen Genet 252:503–509
Toledo I, Rangel P, Hansberg W (1995) Redox imbalance at the start of each morphogenetic step of Neurospora crassa conidiation. Arch Biochem Biophys 319:519–524
Tsukada T, Carleton S, Fotherinham S, Holloman WK (1988) Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol Cell Biol 8:3703–3709
Wedlich-Soldner R, Schulz I, Straube A, Steinberg G (2002) Dynein supports motility of endoplasmic reticulum in the fungus Ustilago maydis. Mol Biol Cell 13:965–977
Whittaker MM, Kersten JP, Nakamura N, Sanders-Loehr J (1996) Glyoxal oxidase from Phanerochaete chrysosporium is a new radical-copper oxidase. J Biol Chem 271:681–687
Whittaker MM, Kersten PJ, Cullen D, Whittaker JW (1999) Identification of catalytic residues in glyoxal oxidase by targeted mutagenesis. J Biol Chem 274:36226–36232
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. J. Punt
The first two authors contributed equally to this work
We dedicate this work to the memory of Jeff Schell, a charismatic and outstanding person who loved science and respected people
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Leuthner, B., Aichinger, C., Oehmen, E. et al. A H2O2-producing glyoxal oxidase is required for filamentous growth and pathogenicity in Ustilago maydis. Mol Genet Genomics 272, 639–650 (2005). https://doi.org/10.1007/s00438-004-1085-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-004-1085-6