Abstract
The high elevation salt marsh plant Spartina patens can potentially cope with accelerated sea level rise by migrating inland, but the ability to do so may differ among plant ecotypes. We compared performance among ecotypes collected from three different sites within mesocosms in which we manipulated soil type, plant litter and salinity. Half of our treatment levels simulated conditions plants would encounter when expanding into terrestrial environments (i.e., upland soil, litter present and low salinity); the other half expansion into tidal creeks (i.e., marsh soil, litter absent, and high salinity). Plant litter and salinity did not significantly affect aboveground biomass or rhizome growth and only affected flowering in a three-way interaction with site. However, all three parameters were significantly affected by soil conditions and the site × soil interaction. Upland soil conditions reduced aboveground biomass, rhizome growth and flowering, as compared to marsh soil conditions, for ecotypes from some sites but not others. When just comparing plant performance in the upland soil treatment, ecotypes from some collection sites did better than others. One plausible explanation for this ecotypic variation is pre-adaptation to differences we found in organic matter content among our collection sites, with the ecotype collected from the site with the lowest organic matter content generally being least impacted by upland soil conditions. Our results indicate that S. patens ecotypes can vary in their capacity to successfully expand into uplands, and thus we suggest prioritizing conservation of such ecotypes, as well as their use in restoration efforts. Consideration of ecotypic variation might also prove useful in deciding where to focus conservation efforts for marsh migration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accelerated sea level rise has led to increased rates of salt marsh loss world-wide due to the inability of salt marshes to maintain surface elevation (Warren and Niering 1993; Stammermann and Piasecki 2012; Fagherazzi 2013). Loss of salt marshes can have negative environmental effects due to a reduction of wildlife habitat (Clausen and Clausen 2014) and can cause negative effects on coastal human infrastructure through the increased impact of storm surges and the decrease in shoreline stability (Shepard et al. 2011). Typical New England salt marsh communities are divided into two zones; a high elevation zone dominated by the perennial grass salt hay (Spartina patens) that only floods intermittently, and a low marsh zone dominated by smooth cordgrass (Spartina alterniflora) that is subjected to twice daily inundation by tides (Pennings and Bertness 2001; Lonard et al. 2017). Although interspecies competition generally prevents S. alterniflora from spreading into areas dominated by S. patens (Bertness and Ellison 1987; Ungar 1998) increased tidal inundation associated with sea level rise can facilitate the movement of S. alterniflora populations into the high marsh zone (Donnelly and Bertness 2001; Watson et al. 2017). With increasing rates of sea level rise, S. patens is squeezed between the encroaching S. alterniflora and the adjacent upland unless it can migrate inland.
Upland conditions, including soil composition, the presence of plant litter, and changes in salinity, all have the potential to impede or promote marsh expansion. The upland soil that expanding S. patens encounters will not match its native marsh environment, in part because mineral upland soil is unlikely to convert into more organic marsh soil as quickly as vegetation can move inland (Anisfeld et al. 2017). Soil organic matter is particularly important to marsh plant growth because it pools nutrients and promotes nutrient fixation (Langis et al. 1991; Callaway 2000), and thus upland soil that is low in organic matter content could deter S. patens growth. The forest plant litter that S. patens might encounter when expanding into the upland can also inhibit plant growth by acting as a physical barrier to stem and seedling emergence or by blocking sunlight (Eriksson 1995; Xiong and Nilsson 1999; Sayer 2006). One upland condition that might actually promote marsh expansion is lower salinity (Cui et al. 2011; Anisfeld et al. 2017; Lee et al. 2019), which could release S. patens from the salt stress that can limit aboveground and belowground growth above 7ppt (Pezeshki and DeLaune 1993; Ewing et al. 1995; Snedden et al. 2015). Given that plant allocation to reproduction is closely tied to plant growth (Crosby et al. 2015), a change in S. patens biomass resulting from any of these factors might also impact flowering.
Although upland conditions will likely impact S. patens in the ways described above, little is known about the degree to which marsh plant ecotypes might vary in their response to upland conditions, and thus in their ability to expand inland. However, it seems very likely that ecotypes would differ, given the high degree of intraspecific trait variation in S. patens, or related species, that has been observed in other studies and in some cases even demonstrated to influence the response to environmental factors (Brewer and Bertness 1996, Lessmann et al. 1997, Liu et al. 2022). For example, allocation to aboveground biomass has been shown to vary among populations of S. patens and is likely to influence tolerance to extreme flooding (Lessmann et al. 1997). The proportion of dry mass allocated to roots varies among populations of S. patens and can impact colonization of bare areas created by floating debris that is deposited on the marsh surface (Brewer and Bertness 1996). Although we are not aware of any studies that have examined ecotypic variation in S. patens flowering in response to stress, populations of S. alterniflora have been shown to vary in the onset of flowering and this might also occur in S. patens (Liu et al. 2022).
In order to determine how S. patens ecotypes might differ in their potential to expand into uplands we compared plant performance among S. patens, collected from three different sites in southern Maine USA, within experimental mesocosms that varied in soil type (upland vs. marsh), plant litter (present vs. absent), and salinity levels (low vs. high). Half of the treatment levels were intended to simulate the conditions that a salt marsh would encounter when expanding into an adjacent upland (i.e., upland soil, litter present and low salinity) and the others were chosen for reference, akin to if a marsh were expanding into an adjacent tidal creek (i.e., marsh soil, litter absent, and high salinity). As our primary response measure we quantified aboveground biomass, but also included two additional parameters that could influence the capacity of the marsh to move inland: rhizome growth into adjacent soil, which is a primary mechanism of belowground expansion by this clonal species; and flowering, which is an indicator of reproductive potential and thus dispersal by seed. We predicted that although plant performance would be lower in the upland soil and when plant litter was present, and be higher at lower salinity levels, S. patens ecotypes would vary in the magnitude and/or direction of their response to the experimental treatments. This information could prove useful to the development of successful management tools to assist marsh expansion inland and help salt marshes persist into the future despite sea level rise.
Methods
Ecotype collections
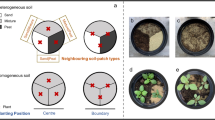
Spartina patens was collected in June 2017 from three geographically distinct populations in southern Maine, hereafter referred to by the name of the city in which the marsh is located (Fig. 1): Scarborough marsh (43°33′52.6"N 70°22′25.9"W), Biddeford marsh (43°27′23.1"N 70°22′52.0"W), Wells marsh (43°19′58.5"N 70°32′45.0"W). These sites were chosen because all three have relatively large, so-called meadow marshes dominated by S. patens and are a minimum of 10 km from one another (Scarborough marsh was 11.8 km north of the Biddeford marsh, which was 19.2 km northeast of the Wells marsh). Adjacent to the Scarborough marsh site is a small open forest containing aspen (Populus sp.) and an understory of honeysuckle (Lonicera sp.), bayberry (Myrica sp.) and mixed herbaceous species. The forest adjacent to the Biddeford marsh is dominated by red oaks (Quercus rubra) and an understory of cinnamon ferns (Osmundastrum cinnamomeum). The Wells marsh is directly connected to a red maple (Acer rubrum) swamp which transitions to an upland forest containing red maple and black cherry (Prunus serotina) trees with an understory dominated by Japanese barberry (Berberis thunbergii). Spartina patens was extracted with spades in approximately 8.5 cm diameter by 21 cm deep plugs, containing approximately 30 stems, and including the intact root mass and soil. Eighty S. patens plugs were collected 2 m apart from each of the three marsh sites (240 total), placed in plastic bins with water from tidal creeks, and then transported back to the University of New England which is adjacent to the Biddeford marsh collection site.
Study design
We used the 240 S. patens plug in an experimental design consisting of ecotypes from 3 sites (Scarborough, Biddeford, Wells) × 2 soil types (marsh, upland) × 2 litter conditions (present, absent) × 2 salinities (high, low) × 10 replicates. The two soil treatments were created by first placing a S. patens plug in the center of a circular fabric 19 L pot (30 cm diameter × 25 cm depth) and then filling the remainder of the pot surrounding the plug with either marsh or upland soil. The marsh soil consisted of sediment collected from tidal creeks at each of the three marsh sites and the upland soil was from a forest located ca. 7.7 km north of the Scarborough marsh (Fig. 1), dominated by common hardwood species for the region, red oak and red maple (43°37′51.0"N 70°24′02.2"W). For the plugs assigned to the litter present treatment, plant litter (ca. 3 cm depth) was placed over the surface of the soil around the plug and the composition of the litter corresponded to the soil treatment, such that the litter placed over marsh soil was clipped dead S. patens from the three marsh sites, and the litter placed over the upland soil was mostly red oak and red maple leaves from the upland forest site. For the plugs assigned to the litter absent treatment the soil was left exposed. To create the salinity treatments, we randomly assigned 24 pots containing S. patens plugs to one of ten 1.8 m diameter by 0.4 m deep plastic wading pools, such that each pool contained two replicates of each ecotype × soil × litter treatment combination. Half the pools received a low salinity treatment, created by adding 500 g of aquarium salt to approximately 70 L of freshwater, and half of the wading pools received a high salinity treatment, created by adding 3120 g of aquarium salt to a similar volume of freshwater. The low salinity treatment averaged 4.5 ppt ± 0.2 (standard error) over the course of the growing season and the high salinity averaged 14.5ppt ± 0.5. Water levels within the pools were maintained at a depth of 5 cm (i.e., only the bottom 5 cm of the ca. 21 cm tall plugs and surrounding soil were inundated), with occasional fluctuations due to rainfall.
We quantified three measures of plant response to the treatment factors over the course of one growing season: aboveground biomass, rhizome growth, and flowering. In September, we clipped the stems where they exited the soil surface, dried them to a constant mass in a 60 °C oven, and then recorded aboveground biomass as dried grams per pot (g/pot). In September and October we also collected rhizomes that grew into surrounding soil during the experiment by clipping them where they exited from the S. patens plug (which maintained its structural integrity throughout the study), dried them to a constant mass in a 60 °C oven and recorded rhizome growth as dried g/pot. Pairs of low salinity and high salinity pools containing 48 pots (3 sites × 2 soil types × 2 litter conditions × 2 salinities × 2 replicates) were processed in turn, so as to ensure that any temporal effects due to processing times were distributed among all treatment combinations (see “Data analysis” section for details on how this was also addressed in statistical tests). Flowering occurred in August, at which time we recorded the presence or absence of flowering stems in each pot, and report the percentage of plots in each treatment combination that had flowers.
To understand whether variation in soil properties among sites might explain differential performance in upland soil among ecotypes, we collected 12 soil samples (approximately 8.5 cm in diameter × 21 cm deep) from each of the three S. patens collection sites. The samples were collected in April 2018 and were sent to the Analytical lab and Maine Soil Testing Service (University of Maine-Orono), where organic matter content was determined by loss on ignition at 375 °C for 2 h (Schulte and Hoskins 2011).
Data analysis
For aboveground biomass and rhizome growth, we used a two-step modeling approach, equivalent to a stepwise analysis of covariance (ANCOVA). Both aboveground biomass and rhizome growth were logarithmically transformed to meet model assumptions of normality prior to analysis. The first step in our stepwise process involved multiple linear regression modeling to identify potential covariates that might impact our results. Given that differences among pots in plant biomass at the start of the experiment could potentially influence aboveground biomass or rhizome growth during our experiment, we used the initial number of stems per pot as a surrogate for initial plant biomass). Because it took several weeks to process all the pots for both aboveground biomass and rhizome growth, which potentially could have led to differences among pots depending on when they were processed, we also quantified the number of grow days (i.e., the days that plants grew prior to taking measurements). For aboveground biomass, we included the presence or absence of flowering, and for belowground rhizome growth we measured the depth of soil subsidence (i.e., the amount that soil around the plug settled during the experiment which exposed some of the rhizomes around the plug). Model results indicated that all potential covariates were significant, as well as several interaction terms. In the second step we used the residuals from these multiple regression models to run a multi-factor analysis of variance (ANOVA). Given that each pool by necessity could only contain one salinity treatment, we considered site × soil × litter combinations to be nested within pools, which, in turn, were nested within salinity treatments. Any significant main or interactive effects were further explored via pairwise F-tests using sequential Bonferroni-corrected alpha levels. Although statistical tests were performed on adjusted values, graphical representations below use unadjusted values so as to facilitate comparisons with other published literature.
We used a log-linear modeling approach to test the dependency of flowering (number of pots with flowers present) on site, soil, and litter, as well as their interactions—and significant interdependencies were assessed by multiple comparisons using odds-ratio tests. We compared soil organic matter among sites with a one-way ANOVA and tested for significance between pairs of sites using pairwise F-tests with sequential Bonferroni corrected alpha levels. We conducted all analyses in R statistical software (Version 3.3.1 2016), using an alpha-level of 0.05, or an adjusted alpha level when appropriate, as described above.
Results
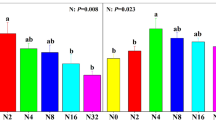
Soil type and the site × soil interaction significantly impacted both aboveground biomass (Table 1) and rhizome growth (Table 2), but neither salinity nor litter treatments (alone or in interactions with other factors) impacted either plant response. Aboveground biomass was lower overall in upland soil as compared to marsh soil, but the pattern varied among sites (Fig. 2A): there were significant biomass reductions in upland soil compared to marsh soil for ecotypes from the Biddeford site (\({F}_{1, 78}\) = 9.71, p < 0.01) and the Wells site (\({F}_{1, 78}\) = 13.17, p < 0.01), but not from the Scarborough site (\({F}_{1, 78}\) = 3.25, p = 0.08).
Ecotype and soil effects on S. patens vegetative response: A aboveground biomass and B rhizome growth. An asterisk indicates a significant difference between marsh and upland soil treatments within a collection site, and letters indicate significant differences among sites within the upland soil treatment
When comparing aboveground biomass just within the upland soil treatment (Fig. 2A), the ecotype from Scarborough had greater biomass than that from Wells (\({F}_{1, 78}\) = 8.42, p < 0.01), but there were no significant differences between Biddeford and the other two sites (Scarborough vs. Biddeford: \({F}_{1, 78}\) = 1.18, p = 0.28 and Wells vs. Biddeford: \({F}_{1, 78}\) = 3.04, p = 0.09). Treatment effects were identical for rhizome growth (Fig. 2b), with rhizome expansion into upland soil being significantly less than into marsh soil for ecotypes from Biddeford (\({F}_{1, 78}\) = 13.09, p < 0.01) and Wells (\({F}_{1, 78}\) = 15.17, p < 0.01), but not from Scarborough (\({F}_{1, 78}\) = 0.10, p = 0.76). The ecotype from Scarborough also had more rhizome growth into upland soil (Fig. 2B) than the ecotype from Wells (\({F}_{1, 78}\) = 9.78, p < 0.01), but rhizome growth by the ecotype from Biddeford was not significantly different from the others (Scarborough vs. Biddeford: \({F}_{1, 78}\) = 0.95, p = 0.33 and Wells vs. Biddeford: \({F}_{1, 78}\) = 3.86, p = 0.05)
Flowering was significantly influenced by site, soil, the site × soil interaction, and the three-way interaction of site, litter, and salinity (Table 3). Overall, flowers occurred in approximately 25% of pots. The number of stems with flowers per pot ranged from 0 to 7, and the total number of flowers per pot ranged from 0 to 15 (because some stems produced more than one flower). The interaction between site and soil (Fig. 3) was slightly different than the pattern found for the other plant performance measures (Fig. 2), with only the ecotype from the Biddeford site having a significant decrease in number of pots with flowers in upland as compared to marsh soil (a 95% confidence interval, CI, which does not include 1.00 indicates a significant difference: Scarborough odds ratio = 1.11, 95% CI = 0.46–2.70; Biddeford odds ratio = 14.80, 95% CI = 1.81- 121.15; Wells odds ratio = 2.15, 95% CI = 0.71–6.53). When flowering was compared just within the upland soil treatment, more pots from Scarborough contained flowers than either Biddeford (odds ratio = 26.00, 95% CI = 3.24-208.81) or Wells (odds ratio = 3.78, 95% CI = 1.29–11.06), but Biddeford and Wells did not significantly differ (odds ratio = 0.15, 95% CI = 0.02–1.27). There was no consistent pattern in the interaction between litter and salinity among sites. For example, when litter was present, a higher number of pots with ecotypes from Scarborough and Wells contained flowers in the high salinity treatment compared to the low salinity treatment, but Biddeford pots had the exact opposite flowering trend with more pots containing flowers in the low salinity treatment.
Lastly, we found a significant difference in percent soil organic matter content among sites (data not shown; ANOVA F1,2 = 50.80, p < 0.01). The average percent organic matter from the Scarborough site (16.7 ± 2.0%, standard error) was less than half of the average from Biddeford (41.2 ± 2.6%) and Wells (42.2 ± 1.3%). The observed difference in percent organic matter between Scarborough and the other two sites was significant (Scarborough versus Biddeford: \({F}_{1, 22}\) = 57.14, p < 0.01, Scarborough versus Wells: \({F}_{1, 22}\) = 111.70, p < 0.01), but there was no significant difference in percent organic matter between the Biddeford and Wells sites (\({F}_{1, 22}\) = 0.12, p = 0.73).
Discussion
Results from our experiment indicate that ecotypes of S. patens differ in their capacity to grow in conditions found in upland sites, notably soil, suggesting that they would have differential success expanding into adjacent uplands. Inland migration enables salt marsh persistence even in the face of marsh submergence due to accelerated sea level rise (Raabe and Stumpf 2016; Schieder et al. 2018; Kirwan and Gedan 2019; Taillie et al. 2019). Documented obstacles to the successful migration of salt marsh plants include anthropogenic barriers, the resistance of forests to retreat ahead of the salt marsh, and the steepness of adjacent upland slopes (Doyle et al. 2010; Feagin et al. 2010; Smith 2013). Furthermore, upland soil typically differs from marsh soil in a number of characteristics, including bulk density, nutrient retention, and organic matter content (Brinson et al. 1995; Callaway 2000; Truog 2016), that could deter marsh plant growth in uplands (Callaway 2000). Insights into whether ecotypes differ in their capacity to contend with upland conditions, including more mineral soil, but also the presence of plant litter and a reduction in salinity, can inform conservation and management strategies intended to protect S. patens dominated marshes.
One plausible explanation for the ecotypic variation in plant response to soil treatment that we observed may be pre-adaptations to differences in marsh soil organic matter content. Upland soils rarely have organic matter content levels above 10% (Truog 2016), which is dramatically lower than most marsh soils (Brinson et al. 1995; Anisfeld et al. 2017). Our finding that S. patens aboveground biomass and rhizome growth of Scarborough ecotypes did not differ between marsh and upland soil may be due to the relatively low organic matter content in that marsh (16.7%) compared to Biddeford (41.2%) and Wells (42.2%), where plant growth was significantly reduced in upland soil (Fig, 2). Flowering of the Scarborough ecotype also did not differ between marsh and upland soil (Fig. 3). Given how long it can take for organic matter to build-up in soils changing from an upland into a marsh environment (e.g., it can take 15–30 years for organic matter content in constructed marsh soils to reach levels found in natural marshes; Morgan et al. 2002), the Scarborough ecotype would potentially be able to expand more successfully into adjacent upland than the Biddeford or Wells ecotypes. However, when comparing patterns within just the upland soil treatment, aboveground biomass and rhizome growth of the Biddeford ecotype was midway between that of the Scarborough and Wells ecotypes and not significantly different from either (Fig. 2), even though the organic matter content at the Biddeford site differed significantly from that at Scarborough. Only flowering paralleled the differences in organic matter among collection site organic matter content, with the Scarborough ecotype having higher values than either of the other two sites (Fig. 3). Thus, it is likely that additional site characteristics besides soil organic matter content could be important. Future investigation into site characteristics such as hydrology and nutrient availability would be needed to further understand what additional factors influence aboveground biomass, rhizome growth, and flowering of S. patens ecotypes in upland soil.
Although we had predicted that litter and salinity would also impact plant performance, both factors were relatively unimportant in our study, only influencing flowering via a three-way interaction with site and litter (Table 3), but with no discernible pattern. Our litter treatment involved placing dead plant material on top of the upland or marsh soil surrounding the S. patens plug (i.e., mimicking the environment that plants would expand into), whereas in other studies the litter was also laid directly over existing stems (Tolley and Christian 1999; Xiong et al. 2001), so the discrepancy in plant response between our study and others may have been due to methodological differences. It is also likely that a single growing season was not enough time to detect discernible litter effects. Although we observed some stems emerging from beneath the litter, it is possible that a second growing season would have allowed more time for the rhizomes that expanded into soil during the first season to send up stems. Our results for salinity are consistent with those of Broome et al. (1995) who found no significant differences in S. patens aboveground biomass at five salinity levels ranging from 0 to 20ppt, but conflict with the results of Ewing et al. (1995) who observed an increase in S. patens aboveground biomass at 7ppt compared to 14ppt. We suggest that variation in results might be due to pre-adaptation among ecotypes used in the studies. For example, the source of the plants in Ewing et al.’s (1995) study was a brackish marsh with an approximate salinity of 2ppt, whereas the source for plants in Broom et al. (1995) study had a salinity of 12.4ppt. While we did not record salinity levels at the marsh surface at our study sites, measurements of salinity in the creeks within the sites ranged from 8 to 30ppt, suggesting that our plants in their native environment, like those in Broom et al. (1995) study, had a higher exposure to salt than the plants in Ewing et al.’s (1995) study, and thus may have had an overall higher salt tolerance.
Summary
Our research indicates that S. patens ecotypes vary in their performance in upland soil, which might translate into an advantage for inland migration. This could have important implications for prioritizing areas for salt marsh conservation, choosing restoration plant sources, and prioritizing adjacent uplands for conservation. In particular, we suggest that salt marsh conservation efforts should focus on preserving S. patens populations growing in soil with a low organic matter content. By identifying S. patens populations that could make the transition into upland soil without a significant decrease in growth, our study joins a growing body of research aimed at determining sites where inland migration is likely to be successful (Feagin et al. 2010; Smith 2013). It is important to focus on plant populations with the highest potential to migrate into the upland so that conservation organizations, often limited by finances, may spend their resources on salt marshes and adjacent uplands that would provide the best opportunity for marsh migrations over the long term. Plants from S. patens populations that grow well in upland conditions could also be used in salt marsh restoration sites to improve the migration potential of the restored marsh. However, future research should seek to further elucidate what site characteristics, in addition to marsh soil organic matter content, could influence the ability of S. patens ecotypes to grow well under upland conditions, and compare more ecotypes from a broader geographic distribution. Although we attempted to simulate the conditions that marsh plants might encounter when expanding into uplands, and by conducting a manipulative experiment we were able to control treatment factors and limit the number of variables potentially confounding our results, research expanding on our experiment should include field studies to confirm that our results are reproducible in a natural setting.
Data availability
The data are available from the corresponding author on request.
References
Anisfeld SC, Cooper KR, Kemp AC (2017) Upslope development of a tidal marsh as a function of upland land use. Glob Chang Biol 23:755–766. https://doi.org/10.1111/gcb.13398
Bertness MD, Ellison AM (1987) Determinants of pattern in a New England salt marsh plant community. Ecol Mongr 57:129–147. https://doi.org/10.2307/1942621
Brewer JS, Bertness MD (1996) Disturbance and intraspecific variation in the clonal morphology of salt marsh perennials. Oikos 77:107–116. https://doi.org/10.2307/3545590
Brinson MM, Christian RR, Blum LK (1995) Multiple states in the sea-level induced transition from terrestrial forest to estuary. Estuaries 18:648–659. https://doi.org/10.2307/1352383
Broome SW, Mendelssohn IA, McKee KL (1995) Relative growth of Spartina patens (Ait.) Muhl. and Scirpus olneyi gray occurring in a mixed stand as affected by salinity and flooding depth. Wetlands 15:20–30. https://doi.org/10.1007/BF03160676
Callaway JC (2000) Hydrology and substrate. In: Zedler JB (ed) Handbook for restoring tidal wetlands, 1st edn. CRC Press, Boca Raton, pp 89–112
Clausen KK, Clausen P (2014) Forecasting future drowning of coastal waterbird habitats reveals a major conservation concern. Biol Conserv 171:177–185. https://doi.org/10.1016/j.biocon.2014.01.033
Crosby SC, Ivens-Duran M, Bertness MD et al (2015) Flowering and biomass allocation in U.S. Atlantic coast Spartina alterniflora. Am J Bot 102:669–676. https://doi.org/10.3732/ajb.1400534
Cui B-S, He Q, An Y (2011) Community structure and abiotic determinants of salt marsh plant zonation vary across topographic gradients. Estuaries Coasts 34:459–469. https://doi.org/10.1007/s12237-010-9364-4
Donnelly JP, Bertness MD (2001) Rapid shoreward encroachment of salt marsh cordgrass in response to accelerated sea-level rise. Proc Natl Acad Sci 98:14218–14223. https://doi.org/10.1073/pnas.251209298
Doyle TW, Krauss KW, Conner WH, From AS (2010) Predicting the retreat and migration of tidal forests along the northern Gulf of Mexico under sea-level rise. For Ecol Manag 259:8. https://doi.org/10.1016/j.foreco.2009.10.023
Eriksson O (1995) Seedling recruitment in deciduous forest herbs: the effects of litter, soil chemistry and seed bank. Flora 190:65–70. https://doi.org/10.1016/S0367-2530(17)30626-6
Ewing K, McKee K, Mendelssohn I, Hester M (1995) A comparison of indicators of sublethal salinity stress in the salt marsh grass, Spartina patens (Ait.) Muhl. Aquat Bot 52:59–74. https://doi.org/10.1016/0304-3770(95)00487-K
Fagherazzi S (2013) The ephemeral life of a salt marsh. Geol 41:943–944. https://doi.org/10.1130/focus082013.1
Feagin R, Martinez M, Mendoza-Gonzalez G, Costanza R (2010) Salt marsh zonal migration and ecosystem service change in response to global sea level rise: a case study from an urban region. Ecol Soc. https://doi.org/10.5751/ES-03724-150414
Kirwan ML, Gedan KB (2019) Sea-level driven land conversion and the formation of ghost forests. Nat Clim Change 9:450–457. https://doi.org/10.1038/s41558-019-0488-7
Langis R, Zalejko M, Zedler JB (1991) Nitrogen assessments in a constructed and a natural salt marsh of San Diego Bay. Ecol Appl 1:40–51. https://doi.org/10.2307/1941846
Lee PO, Shoemaker C, Olson JB (2019) Wetland soil properties and resident bacterial communities are influenced by changes in elevation. Wetlands 39:99–112. https://doi.org/10.1007/s13157-018-1077-7
Lessmann JM, Mendelssohn IA, Hester MW, McKee KL (1997) Population variation in growth response to flooding of three marsh grasses. Ecol Eng 8:31–47. https://doi.org/10.1016/S0925-8574(96)00251-0
Liu W, Chen X, Wang J, Zhang Y (2022) Does the effect of flowering time on biomass allocation across latitudes differ between invasive and native salt marsh grass Spartina alterniflora? Ecol Evol 12:e8681. https://doi.org/10.1002/ece3.8681
Lonard RI, Judd FW, Summy KR et al (2017) The biological flora of coastal dunes and wetlands: Avicennia germinans (L.) L. J of Coastal Res 33:191–207. https://doi.org/10.2112/JCOASTRES-D-16-00013.1
Morgan PA, Short FT (2002) Using functional trajectories to track constructed salt marsh development in the Great Bay Estuary, Maine/New Hampshire. USA Restor Ecol 10:461–473. https://doi.org/10.1046/j.1526-100X.2002.01037.x
Pennings SC, Bertness MD (2001) Salt marsh communities. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology, 1st edn. Sinauer Associates, Sunderland, pp 289–316
Pezeshki S, DeLaune R (1993) Effects of soil hypoxia and salinity on gas exchange and growth of Spartina patens. Mar Ecol Prog Ser 96:75–81. https://doi.org/10.3354/meps096075
Raabe EA, Stumpf RP (2016) Expansion of tidal marsh in response to sea-level rise: Gulf Coast of Florida, USA. Estuaries Coasts 39:145–157. https://doi.org/10.1007/s12237-015-9974-y
Sayer EJ (2006) Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol Rev Camb Philos Soc 81:1–31. https://doi.org/10.1017/S1464793105006846
Schieder NW, Walters DC, Kirwan ML (2018) Massive upland to wetland conversion compensated for historical marsh loss in Chesapeake Bay, USA. Estuaries Coasts 41:940–951. https://doi.org/10.1007/s12237-017-0336-9
Schulte EE, Hoskins B (2011) Recommended soil organic matter tests. Northeastern Regional Publication. http://extension.udel.edu/lawngarden/soil-health-composting/recommended-soil-testing-procedures-for-the-northeastern-united-states. Accessed 2 Aug 2018
Shepard CC, Crain CM, Beck MW (2011) The protective role of coastal marshes: a systematic review and meta-analysis. PLoS ONE 6:e27374. https://doi.org/10.1371/journal.pone.0027374
Smith JAM (2013) The role of Phragmites australis in mediating inland salt marsh migration in a mid-atlantic estuary. PLoS ONE 8:e65091. https://doi.org/10.1371/journal.pone.0065091
Snedden GA, Cretini K, Patton B (2015) Inundation and salinity impacts to above- and belowground productivity in Spartina patens and Spartina alterniflora in the Mississippi River deltaic plain: implications for using river diversions as restoration tools. Ecol Eng 81:133–139. https://doi.org/10.1016/j.ecoleng.2015.04.035
Stammermann R, Piasecki M (2012) Influence of sediment availability, vegetation, and sea level rise on the development of tidal marshes in the Delaware Bay: a review. J Coastal Res 28:1536–1549. https://doi.org/10.2112/JCOASTRES-D-11-00143.1
Taillie PJ, Moorman CE, Poulter B et al (2019) Decadal-scale vegetation change driven by salinity at leading edge of rising sea level. Ecosysts 22:1918–1930. https://doi.org/10.1007/s10021-019-00382-w
Tolley PM, Christian RR (1999) Effects of increased inundation and wrack deposition on a high salt marsh plant community. Estuaries 22:944–954. https://doi.org/10.2307/1353074
Truog E (2016) Soil as a medium for plant growth. In: Snood BS (ed) Mineral nutrition of plants, 1st edn. Medtech Publishing Company, New Gloucester, pp 23–55
Ungar IA (1998) Are biotic factors significant in influencing the distribution of halophytes in saline habitats? Bot Rev 64:176–199. https://doi.org/10.1007/BF02856582
Warren RS, Niering WA (1993) Vegetation change on a northeast tidal marsh: interaction of sea-level rise and marsh accretion. Ecol 74:96–103. https://doi.org/10.2307/1939504
Watson EB, Raposa KB, Carey JC et al (2017) Anthropocene survival of southern New England’s salt marshes. Estuaries Coasts 40:617–625. https://doi.org/10.1007/s12237-016-0166-1
Xiong S, Nilsson C (1999) The effects of plant litter on vegetation: a meta-analysis. J Ecol 87:984–994. https://doi.org/10.1046/j.1365-2745.1999.00414.x
Xiong S, Nilsson C, Johansson ME (2001) Effects of litter accumulation on riparian vegetation: importance of particle size. Ecosyst Veg Sci 12:231–236. https://doi.org/10.2307/3236607
Acknowledgements
We would like to thank Maine Department of Inland Fisheries and Wildlife, Maine Audubon Society, and the staff at the Wells National Estuarine Research Reserve for granting us permission to conduct research in their salt marshes. Thanks also to Grondin Construction Company, Bruce Hoskins, Matt Dorman, Hannah Buckley, Carmen Dancy, and Emma White for assistance in collecting and analyzing the soil used in this study. We are grateful for feedback from an anonymous reviewer which improved the quality of the manuscript.
Funding
Dowling received funding from the University of New England, the Society of Wetland Scientists, and the Maine Association of Wetland Scientists.
Author information
Authors and Affiliations
Contributions
TMD conducted the experiment, did the statistical analyses, and wrote the first draft. GPZ coordinated reviews and made final edits to the manuscript. All authors contributed to the study design and reviewed drafts.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dowling, T.M., Travis, S.E., Morgan, P.A. et al. Can the marsh migrate? Factors influencing the growth of Spartina patens under upland conditions. Wetlands Ecol Manage 31, 887–897 (2023). https://doi.org/10.1007/s11273-023-09958-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-023-09958-9