Abstract
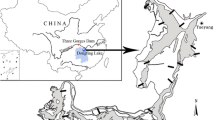
We investigated compositions of plant communities and their abiotic determinants in the Yellow River estuary, China. Along a topographic gradient, we quantified plant compositions and abiotic factors in different vegetation zones, and examined the relationships between plant communities and abiotic factors using canonical correspondence analysis and the effects of vegetation shading using a removal experiment. The relationships between plant communities and abiotic factors differed between high elevations and low elevations. Salinity and flooding oppositely related with distributions of plant communities at low elevations, but they appeared to operate synergically at high elevations. The effects of vegetation shading were found to vary across the topographic gradient, indicating spatial variations in potential positive interactions among plants. These results suggest that spatial variations in determinants of community structure should be addressed in future studies in estuarine and coastal systems, as well as in other natural habitats.




Similar content being viewed by others
References
Adam, P. 1990. Saltmarsh ecology. Cambridge: Cambridge University Press.
Adams, J.B., and G.C. Bate. 1999. Growth and photosynthetic performance of Phragmites australis in estuarine waters: A field and experimental evaluation. Aquatic Botany 64: 359–367.
Alberti, J., M. Escapa, O. Iribarne, B. Silliman, and M. Bertness. 2008. Crab herbivory regulates plant facilitative and competitive processes in Argentinean marshes. Ecology 89: 155–164.
Bertness, M.D. 1991a. Interspecific interactions among high marsh perennials in a New England salt marsh. Ecology 72: 125–137.
Bertness, M.D. 1991b. Zonation of Spartina patens and Spartina alterniflora in a New England salt marsh. Ecology 72: 138–148.
Bertness, M.D., and A.M. Ellison. 1987. Determinants of pattern in a New England salt marsh plant community. Ecological Monographs 57: 129–147.
Bertness, M.D., and S.D. Hacker. 1994. Physical stress and positive associations among marsh plants. The American Naturalist 144: 363–372.
Bertness, M.D., and S.C. Pennings. 2000. Spatial variation in process and pattern in salt marsh plant communities in eastern North America. In Concepts and controversies in tidal marsh ecology, ed. M.P. Weinstein and D.A. Kreeger, 39–57. Dordrecht: Kluwer.
Bertness, M.D., and S.M. Yeh. 1994. Cooperative and competitive interactions in the recruitment of marsh elders. Ecology 75: 2416–2429.
Bertness, M.D., L. Gaugh, and S.W. Shumway. 1992. Salt tolerances and the distribution of plants across a New England salt marsh. Ecology 72: 1842–1851.
Bockelmann, A., and R. Neuhaus. 1999. Competitive exclusion of Elymus athericus from a high-stress habitat in a European salt marsh. Journal of Ecology 87: 503–513.
Brewer, J.S., J.M. Levine, and M.D. Bertness. 1997. Effects of biomass removal and elevation on species richness in a New England salt marsh. Oikos 80: 333–341.
Chapman, V.J. 1974. Salt marshes and salt deserts of the world. New York: Interscience.
Chapman, V.J. 1977. Introduction. In Ecosystems of the world 1: Wet coastal ecosystems, ed. V.J. Chapman. Amsterdam: Elsevier.
Costa, C.S.B., J.C. Marangoni, and A.G. Azevedo. 2003. Plant zonation in irregularly flooded salt marshes: Relative importance of stress tolerance and biological interactions. Journal of Ecology 91: 951–965.
Editorial Committee of Wetland Vegetation in China. 1999. Wetland vegetation in China. Beijing: Science.
Fariña, J.M., B.R. Silliman, and M.D. Bertness. 2009. Can conservation biologists rely on established community structure rules to manage novel systems? ... Not in salt marshes. Ecological Applications 19: 413–422.
Gormally, C.L., and L.A. Donovan. 2010. Responses of Uniola paniculata L. (Poaceae), an essential dune-building grass, to complex changing environmental gradients on the coastal dunes. Estuaries and Coasts, doi:10.1007/s12237-010-9269-2.
Hacker, S.D., and M.D. Bertness. 1999. Experimental evidence for factors maintaining plant species diversity in a New England salt marsh. Ecology 80: 2064–2073.
He, Q., B.S. Cui, X.S. Zhao, and H.L. Fu. 2008. Niches of plant species in wetlands of the Yellow River Delta under gradients of water table depth and soil salinity. Chinese Journal of Applied Ecology 19: 969–975.
He, Q., B. Cui, Y. Cai, J. Deng, T. Sun, and Z. Yang. 2009a. What confines an annual plant to two separate zones along coastal topographic gradients? Hydrobiologia 630: 327–340.
He, Q., B. Cui, X. Zhao, H. Fu, and X. Liao. 2009b. Relationships between salt marsh vegetation distribution/diversity and soil chemical factors in the Yellow River Estuary, China. Acta Ecologica Sinica 29: 676–687.
Huckle, J.M., R.H. Marrs, and J.A. Potter. 2002. Interspecific and intraspecific interactions between salt marsh plants: Integrating the effects of environmental factors and density on plant performance. Oikos 96: 307–319.
Ji, Y., G. Zhou, and T. New. 2009. Abiotic factors influencing the distribution of vegetation in coastal estuary of the Liaohe Delta, Northeast China. Estuaries and Coasts 32: 937–942.
Jobbágy, E.G., and R.B. Jackson. 2004. Groundwater use and salinization with grassland afforestation. Global Chang Biol 10: 1299–1312.
Kunza, A.E., and S.C. Pennings. 2008. Patterns of plant diversity in Georgia and Texas salt marshes. Estuaries and Coasts 31: 673–681.
Lenssen, J.P.M., F.B.J. Menting, W.H. van der Putten, and C.W.P.M. Blom. 1999. Control of plant species richness and zonation of functional groups along a freshwater flooding gradient. Oikos 86: 523–534.
Li, S., G. Wang, W. Deng, Y. Hu, and W.W. Hu. 2009a. Influence of hydrology process on wetland landscape pattern: A case study in the Yellow River Delta. Ecological Engineering 35: 1719–1726.
Li, B., C. Liao, X. Zhang, H. Chen, Q. Wang, Z. Chen, X. Gan, J. Wu, B. Zhao, Z. Ma, X. Cheng, L. Jiang, and J. Chen. 2009b. Spartina alterniflora invasions in the Yangtze River estuary, China: An overview of current status and ecosystem effects. Ecological Engineering 35: 511–520.
Matthews, J.W., A.L. Peralta, D.N. Flanagan, P.M. Baldwin, A. Soni, A.D. Kent, and A.G. Endress. 2009. Relative influence of landscape vs. local factors on plant community assembly in restored wetlands. Ecological Applications 19: 2108–2123.
Montemayor, M.B., J.S. Price, L. Rochefort, and S. Boudreau. 2008. Temporal variations and spatial patterns in saline and waterlogged peat fields. 1. Survival and growth of salt marsh graminoids. Environmental and Experimental Botany 62: 333–342.
Nikolaevskii, V.G., and A.N. Smirnova. 1968. The effect of salinization of soil on the growth and yield of the common reed under conditions of a vegetation experiment. Rastit Resur 44: 552–554.
Noe, G.B., and J.B. Zedler. 2000. Differential effects of four abiotic factors on the germination of salt marsh annuals. American Journal of Botany 87: 1679–1692.
Palmer, M.W. 1993. Putting things in even better order: The advantages of canonical correspondence analysis. Ecology 74: 2215–2230.
Pennings, S.C., and M.D. Bertness. 2000. Salt marsh communities. In Marine community ecology, ed. M.D. Bertness, S.D. Gaines, and M.E. Hay, 289–316. Sunderland: Sinauer Associates.
Pennings, S.C., and R.M. Callaway. 1992. Salt marsh plant zonation: the relative importance of competition and physical factors. Ecology 73: 681–690.
Pennings, S.C., and B.R. Silliman. 2005. Linking biogeography and community ecology: Latitudinal variation in plant–herbivore interaction strength. Ecology 86: 2310–2319.
Pennings, S.C., E.R. Selig, L.T. Houser, and M.D. Bertness. 2003. Geographic variation in positive and negative interactions among salt marsh plants. Ecology 84: 1527–1538.
Pennings, S.C., M.B. Grant, and M.D. Bertness. 2005. Plant zonation in low-latitude salt marshes: disentangling the roles of flooding, salinity and competition. Journal of Ecology 93: 159–167.
Pennings, S.C., C.-K. Ho, C.S. Salgado, K. Więski, N. Davé, A.E. Kunza, and E.L. Wason. 2009. Latitudinal variation in herbivore pressure in Atlantic Coast salt marshes. Ecology 90: 183–195.
R Development Core Team. 2008. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing
Saintilan, N. 2009. Distribution of Australian saltmarsh plants. In Australian saltmarsh ecology, ed. N. Saintilan, 23–39. Collingwood: Csiro Publishing.
Salama, R.B., C.J. Otto, and R.W. Fitzpatrick. 1999. Contributions of groundwater conditions to soil and water salinization. Hydrogeology Journal 7: 46–64.
Song, J., H. Fan, Y. Zhao, Y. Jia, X. Du, and B. Wang. 2008. Effect of salinity on germination, seedling emergence, seedling growth and ion accumulation of a euhalophyte Suaeda salsa in an intertidal zone and on saline inland. Aquatic Botany 88: 331–337.
ter Braak, C.J.F., and P. Šmilauer. 2002. Canoco for Windows 4.5. Wageningen: Biometris-Plant Research International.
Traut, B.H. 2005. The role of coastal ecotones: a case study of the salt marsh/upland transition zone in California. Journal of Ecology 93: 279–290.
van Wijnen, H.J., and J.P. Bakker. 1999. Nitrogen and phosphorus limitation in a coastal barrier salt marsh: the implications for vegetation succession. Journal of Ecology 87: 265–272.
Vince, S.W., and A.A. Snow. 1984. Plant zonation in an Alaskan salt marsh: I. Distribution, abundance and environmental factors. Journal of Ecology 72: 651–667.
Wang, Q., C.H. Wang, B. Zhao, Z.J. Ma, Y.Q. Luo, J.K. Chen, and B. Li. 2006. Effects of growing conditions on the growth of and interactions between salt marsh plants: Implications for invasibility of habitats. Biological Invasions 8: 1547–1560.
Wang, H., Y.P. Hsieh, M.A. Harwell, and W. Huang. 2007. Modeling soil salinity distribution along topographic gradients in tidal salt marshes in Atlantic and Gulf coastal regions. Ecological Modelling 201: 429–439.
Wang, C.H., M. Lu, B. Yang, Q. Yang, X.D. Zhang, T. Hara, and B. Li. 2010. Effects of environmental gradients on the performances of four dominant plants in a Chinese saltmarsh: Implications for plant zonation. Ecological Research 25: 347–358.
Werner, A.D., and D.A. Lockington. 2006. Tidal impacts on riparian salinities near estuaries. Journal of Hydrology 328: 511–522.
Wu, Y.T., C.H. Wang, X.D. Zhang, B. Zhao, L.F. Jiang, J.K. Chen, and B. Li. 2008. Effects of saltmarsh invasion by Spartina alterniflora on arthropod community structure and diets. Biological Invasions 11: 635–649.
Yang, S., and J. Chen. 1995. Coastal salt marshes and mangrove swamps in China. Chinese Journal of Oceanology and Limnology 13: 318–324.
Acknowledgments
We thank Xianxian Liu, Yanyan Hua, Yanzi Cai, and Jiafeng Deng for invaluable field and/or laboratory assistance, Chenghuan Wang, Xiao Sun, two anonymous reviewers for critical comments, and Yueliang Liu and Juanzhang Lv for access to the study site. This study was funded by National Natural Science Foundation of China (U0833002), National Key Basic Research Program of China (2006CB403303), and the Fundamental Research Funds for the Central Universities (2009SD-24).
Conflicts of Interest Notification
We state that this manuscript have been read and approved by all authors listed on the title page, and that all authors have made substantial contributions to the design, execution, and reporting of this study. All financial interests held by the authors in any reported findings and all sources of financial support for the work have been described in the acknowledgments of the paper. We do not have a financial relationship with the organization that sponsored the research and no potential conflicts of interest exist to our knowledge and understanding. We maintain full control of all primary data, and we agree to allow the journal to review our data if requested.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
CUI, BS., HE, Q. & AN, Y. Community Structure and Abiotic Determinants of Salt Marsh Plant Zonation Vary Across Topographic Gradients. Estuaries and Coasts 34, 459–469 (2011). https://doi.org/10.1007/s12237-010-9364-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-010-9364-4