Abstract
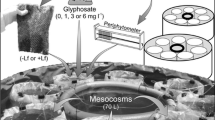
Water ecosystems face enormous, variable anthropogenic stressors that contribute to water degradation. This study explores the effects of three substances frequently found in high concentrations in natural environments linked to agricultural areas on plankton abundance, composition, and trophic interactions. We conducted a microcosm experiment where we exposed a natural plankton assemblage to excess copper (Cu), atrazine (At), and dissolved organic matter (OM), along with a control group encompassing all possible treatment combinations. Each treatment was replicated three times (n = 24), and the experiment lasted 96 h. At the beginning and end of the experiment, relevant limnological variables, including inorganic dissolved nutrients, were measured. We used one-way ANOVA analyses with total abundances and species richness as response variables for data analysis. Results showed that the response of each plankton group to these substances was unequal, with some benefiting at tested concentrations. For example, Cu was beneficial for Chlorophyceae and Euglenophyceae, while CuOM was beneficial for Cyanobacteria and Cryptophyceae. Regarding zooplankton, Copepoda density was depleted in all treatments where stressors were combined and when exposed to OM compared to the control. When richness was considered, phytoplankton showed depletion in CuOMAt compared to OM and AtCu. No treatments affected zooplankton richness. Zooplankton:phytoplankton ratios showed significant effects for total zooplankton and Copepoda in all treatments compared to the control. These results indicate that the effects of contaminants on natural assemblages strongly depend on the plankton group and the combinations of contaminants, making predictions and extrapolations a challenging task that requires further study.






Similar content being viewed by others
Data Availability
Data are available upon reasonable request.
References
Akhtar, N., Syakir Ishak, M. I., Bhawani, S. A., & Umar, K. (2021). Various natural and anthropogenic factors responsible for water quality degradation: A review. Water, 13, 2660.
APHA. (2005). Standard methods for the examination of water and wastewater (21st ed.). American Public Health Association.
Arvola, L., & Tulcnen, T. (1998). Effects of allochthonous dissolved organic matter and inorganic nutrients on the growth of bacteria and algae from a highly humic lake. Environment International, 24, 509–520.
Bai, L., Jub, Q., Wang, C., Tian, L., Wang, C., Zhang, H., & Jiang, H. (2022). Responses of steroid estrogen biodegradation to cyanobacterial organic matter biodegradability in the water column of a eutrophic lake. Science of the Total Environment, 805, 150058.
Bashir, I., Lone, F. A., Bhat, R. A., Mir, S. A., Dar, Z. A., & Dar, S. A. (2020). Concerns and threats of contamination on aquatic ecosystems. Bioremediation and Biotechnology, 27, 1–26.
Bérard, A., & Benninghoff, C. (2001). Pollution-induced community tolerance (PICT) and seasonal variations in the sensitivity of phytoplankton to atrazine in nanocosms. Chemosphere, 45, 427–437.
Bergström, A. K., Jansson, M., Drakare, S., & Blomqvist, P. (2003). Occurrence of mixotrophic flagellates in relation to bacterioplankton production, light regime and availability of inorganic nutrients in unproductive lakes with differing humic contents. Freshwater Biology, 48, 868–877.
Bláha, M., Grabicova, K., Shaliutina, O., Kubec, J., Randák, J., Zlabek, V., Buřič, M., & Veselý, L. (2019). Foraging behavior of top predators mediated by pollution of psychoactive pharmaceuticals and effects on ecosystem stability. Science of the Total Environment, 662, 655–661.
Bottrell, H. H., Duncan, A., Gliwicz, Z. M., Grygierek, E., Herzig, A., Hillbricht-Ilkowska, A., Kurasawa, H., Larsson, P., & Weglenska, T. (1976). A review of some problems in zooplankton production studies. Norwegian Journal of Zoology, 24, 419–456.
Bowszys, M., Dunalska, J. A., & Jaworska, B. (2014). Zooplankton response to organic carbon level in lakes of differing trophic states. Aquatic Ecosystems, 412, 10.
Broccoli, A., Anselmi, S., Cavallo, A., Ferrari, V., Prevedelli, D., Pastorino, P., & Renzi, M. (2021). Ecotoxicological effects of new generation pollutants (nanoparticles, amoxicillin and white musk) on freshwater and marine phytoplankton species. Chemosphere, 279, 130623.
Cid, A., Herrero, C., Torres, E., & Abalde, J. (1995). Copper toxicity on the marine microalga Phaeodactylum tricornutum: Effects on photosynthesis and related parameters. Aquatic Toxicology, 31, 165–174.
Clayton, J., Williams, P. C., Frost, A. M., Morales-Williams, J. H., Larson, W. B., Richardson, A. S. C., & Xenopoulos, M. A. (2016). Human activities cause distinct dissolved organic matter composition across freshwater ecosystems. Global Change Biology, 22, 613–626.
Cyrino de Oliveira-Filho, E., Matos Lopes, R., & Roma Paumgartten, J. (2004). Comparative study on the susceptibility of freshwater species to copper-based pesticides. Chemosphere, 56, 369–374.
Dahms, H. U., Hagiwara, A., & Lee, J. S. (2011). Ecotoxicology, ecophysiology, and mechanistic studies with rotifers. Aquatic Toxicology, 101, 1–12.
Dumont, H. J., Van de Velde, I., & Dumont, S. (1975). The dry weight estimates of biomass in a selection of Cladocera, Copepoda and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia, 19, 75–97.
Estlander, S., & Horppila, J. (2023). Effects of dissolved organic carbon gradient on epilimnetic zooplankton communities in lakes. Hydrobiologia. https://doi.org/10.1007/s10750-023-05284-6
Evans, A. E. V., Mateo Sagasta, J., Qadir, M., Boelee, E., & Ippolito, A. (2019). Agricultural water pollution: Key knowledge gaps and research needs. Current Opinion in Environmental Sustainability, 36, 20–27.
Evans, C. D., Monteith, D. T., & Cooper, D. M. (2005). Long-term increases in surface water dissolved organic carbon: Observations, possible causes and environmental impacts. Environmental Pollution, 137, 55–71.
Filimonova, V., De Trochc, M., Gonçalves, F., Marquesa, J. C., Marques, S. M., Gonçalvesa, A. M. M., & De Laender, F. (2018). Effects of an herbicide and copper mixture on the quality of marine plankton. Ecotoxicology and Environmental Safety, 156, 9–17.
Fleeger, J. W., Carman, K. R., & Nisbet, R. M. (2003). Indirect effects of contaminants in aquatic ecosystems. Science of the Total Environment, 317, 207–233.
Frau, D. (2022). Grazing impacts on phytoplankton from South American water ecosystems: A synthesis. Hydrobiologia, 849, 833–860.
Frau, D., Andrade, V. S., Lares, B. A., & Gutierrez, M. F. (2023). Effects of bifenthrin on microcrustaceans grazing behavior on a phytoplankton assemblage dominated by Cyanobacteria. Environmental Science and Pollution Research, 31, 3754–3762.
Frau, D., Battauz, Y., Alvarenga, P., Scarabotti, P. A., & Mayora, G. (2019). Rodrigo Sinistro Assessing the relevance of top-down and bottom-up effects as phytoplankton structure drivers in a subtropical hypereutrophic shallow lake. Aquatic Ecology, 53, 265–280.
Frau, D., Battauz, Y., & Sinistro, R. (2017). Why predation is not a controlling factor of phytoplankton in a Neotropical shallow lake: A morpho-functional perspective. Hydrobiologia, 788, 115–130.
Frau, D., Gutierrez, M. F., Regaldo, L., Saigo, M., & Licursi, M. (2021). Plankton community responses in Pampean lowland streams linked to intensive agricultural pollution. Ecological Indicators, 120, 106934.
Gimeno García, E., Andreu, V., & Boluda, R. (1996). Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environmental Pollution, 92, 19–25.
Gonçalves, A. M. M., Rocha, C., Marques, J. C., & Gonçalves, F. J. M. (2021). Enzymes as useful biomarkers to assess the response of freshwater communities to pesticide exposure – A review. Ecological Indicators, 122, 107303.
Goni-Urriza, M., Moussard, H., Lafabrie, C., Carre, C., Bouvy, M., Sakka Hlaili, A., & Pringault, O. (2018). Consequences of contamination on the interactions between phytoplankton and bacterioplankton. Chemosphere, 195, 212–222.
Graymore, M., Stagnitti, F., & Allinson, G. (2001). Impacts of Atrazine in aquatic ecosystems. Environment International, 26, 483–495.
Gutierrez, M. A., Gagneten, A. M., & Paggi, J. C. (2011). Acute and chronic effects of copper, chromium and insecticide-endosulfan on littoral cladocera, Pseudosida Variabilis. Fresenius Environmental Bulletin, 20, 3286–3294.
Gutierrez, M. A., Gagneten, A. M., & Paggi, J. C. (2013). Acute and behavioral sensitivity of Mesocyclops longisetus to atrazine and endosulfan formulations under predation pressure. Water Air and Soil Pollution, 224, 1375.
Gutierrez, M. F., Andrade, V. S., Flores-Mendez, D. N., Frau, D., Licursi, M., Negro, L. (2024). The relative importance of salinization in lowland stream zooplankton: Implications of the ecosystem nutrient status. Science of the Total Environment, 912, 169240.
Gutierrez, M. F., Nadson, R. S., Frau, D., Saigo, M., & Licursi, M. (2020a). Responses of stream zooplankton diversity metrics to eutrophication and temporal environmental variability in agricultural catchments. Environmental Monitoring and Assessment, 192, 792.
Gutierrez, M. A., Paggi, J. C., & Gagneten, A. M. (2012). Microcrustaceans escape behavior as an early bioindicator of copper, chromium and endosulfan toxicity. Ecotoxicology, 21, 428–438.
Gutierrez, M. F., Rojas Molina, F., Frau, D., Mayora, G., & Battauz, B. (2020b). Interactive effects of fish predation and sublethal insecticide concentrations on freshwater zooplankton communities. Ecotoxicology and Environmental Safety, 196, 110497.
Hammer, Ø., Harper, D.A. & Ryan, P.D. (2018). PAST palaeontological statistics, version 3.18. Norway: University of Oslo.
Hanazato, T. (2001). Pesticide effects on freshwater zooplankton: An ecological perspective. Environmental Pollution, 112, 1–10.
Havens, K. E., & Beaver, J. R. (2007). Composition, size, and biomass of zooplankton in large productive Florida lakes. Hydrobiologia, 668, 49–60.
Hillebrand, H., Dürselen, C., Kirschtel, D., Pollingher, U., & Zohary, T. (1999). Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology, 35, 403–424.
Husak, V. (2015). Copper and copper-containing pesticides: Metabolism, toxicity and oxidative stress. Journal of Vasyl Stefanyk Precarpathian National University, 2, 38–50.
Jfittner, I., Peither, A., Lay, J. P., Kettrup, A., & Ormerod, S. J. (1995). An outdoor mesocosm study to assess ecotoxicological effects of atrazine on a Natural Plankton Community. Archives of Environmental Contamination and Toxicology, 29, 435–441.
Jones, R.I. (1998). Phytoplankton, primary production and nutrient cycling. In: Aquatic Humic Substances—Ecology and Biogeochemistry. Hessen, D.O., Tranvik, L.J. (eds). Springer-Verlag, Berlin.
Joniak, T. (2007). Seasonal variations of dominant phytoplankton in humic forest lakes. Oceanological and Hydrobiological Studies, 36, 49–59.
Jonkers, L., Hillebrand, H., & Kucera, M. (2019). Global change drives modern plankton communities away from the pre-industrial state. Nature, 570, 372–375.
José de Paggi, S. B., & Paggi, J. C. (2008). Hydrological connectivity as a shaping force in the zooplankton community of two lakes in the Paraná River Floodplain. International Review of Hydrobiology, 93, 659–678.
Kaplan, D. (2013). Absorption and Adsorption of Heavy Metals by Microalgae. In Richmond, A., Hu, Q. (eds.). Handbook of Microalgal Culture: Applied Phycology and Biotechnology, Second Edition. John Wiley & Sons, Ltd, U.S.
Kirk, J. T. O. (1994). Light and Photosynthesis in Aquatic Ecosystems. Cambridge University Press.
Klug, J. L. (2002). Positive and negative effects of allochthonous dissolved organic matter and inorganic nutrients on phytoplankton growth. Canadian Journal of Fisheries and Aquatic Sciences, 59, 85–95.
Komárek, J. & Fott, B. (1983). Chlorophyceae, chlorococcales. In: Huber-Pestalozzi, G. (Ed.), Das Phytoplankton des Sdwasswes. Die Binnenggewasser, Vol. 16(5). Schweizerbart’sche Verlagsbuchhandlung, Germany.
Komárek, J. & Anagnostidis, K. (1998). Cyanoprokaryota. Teil 1: Chroococcales. In: Ettl, H, Gärtner G, Heynig H, Mollenhauer D (Eds.), Süsswasserflora von Mitteleuropa 19/1. Jena: Gustav Fisher Verlag, Germany.
Komárek, J., Anagnostidis, K. (2005). Cyanoprokaryota. Teil 2: Oscillatoriales. In: Büdel B, Gärtner G, Krienitz L, Schagerl M (Eds.), Süsswasserflora von Mitteleuropa 19/ 2. Elsevier, Germany.
Komárek, J. (2013). Cyanoprokaryota.Teil/3rd part: heterocytous genera. In: Büdel, Gärtner L, Krienitz M, Chagerl M (Eds.). Süswasserflora von Mitteleuropa (Freshwater flora of Central Europe). Springer Spektrum, Germany.
Korinek, V. (2002). Cladocera. A Guide to Tropical Freshwater Zooplankton. Identification, ecology, and impact on fisheries, 69–122. C.H. Fernando. Leiden Backhuys Publishers, The Netherlands.
Koste, W. (1978). Rotatoria. Die Radertiere Mitteleuropas, Gebruder Borntraeger, Germany.
Krammer, K. & Lange-Bertalot, H. (1991). Bacillariophyceae. 3. Teil Centrales, Fragilariaceae, Eunotiaceae. In: Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D. (Eds.), Süsswasserflora von Mitteleuropa. Gustav Fischer Verlag, Germany.
Lair, N. (2006). A review of regulation mechanisms of metazoan plankton in riverine ecosystems: Aquatic habitat versus biota. River Research Applications, 22, 567–593.
Lee, J. L., Kang, H., Park, J. C., & Lee, J. S. (2020). Protective role of the freshwater rotifer Brachionus calyciflorus glutathione S-transferase zeta 3 recombinant protein in response to Hg and Cd. Comparative Biochemistry and Physiology Part b: Biochemistry and Molecular Biology, 110435, 243–244.
Leenheer, J. A., & Croué, J. P. (2003). Characterizing aquatic dissolved organic matter. Environmental Science and Technology, 37, 18A-26A.
Liu, Q., Tian, Y., Liu, Y., Yu, M., Hou, Z., He, K., Xu, H., Cui, B., & Jiang, Y. (2021). Relationship between dissolved organic matter and phytoplankton community dynamics in a human-impacted subtropical river. Journal of Cleaner Production, 289, 125144.
Lomartire, S., Marques, J. C., & Gonçalves, A. M. M. (2021). The key role of zooplankton in ecosystem services: A perspective of interaction between zooplankton and fish recruitment. Ecological Indicators, 129, 107867.
Lopez, J. S., Lee, L., & Mackey, K. R. M. (2019). The toxicity of copper to crocosphaera watsonii and other Marine Phytoplankton: A systematic review. Frontiers in Marine Science, 5, 511.
Lushchak, V. I. (2011). Environmentally induced oxidative stress in aquatic animals. Aquatic Toxicology, 101, 13–30.
Malmqvist, B., & Rundle, S. (2002). Threats to the running water ecosystems of the world. Environmental Conservation, 29, 134–153.
Mateo Sagasta, J., Zadeh, S.M., Turral, H. & Burke, J. (2017). Water pollution from agriculture: a global review. Executive summary. Food and Agriculture Organization of the United Nations, Rome, Italy.
Mateo Sagasta, J., Marjani Zadeh, S. & Turral, H. (2018). More people, more food, worse water? a global review of water pollution from agriculture. FAO Colombo, Rome, Italy.
Mazanti, L., Rice, C., Bialek, C., Sparling, D., Stevenson, C., Johnson, W. E., Kangas, P., & Rheinstein, J. (2003). Aqueous-phase disappearance of atrazine, metolachlor, and chlorpyrifos in laboratory aquaria and outdoor macrocosms. Archives of Environmental Contamination and Toxicology, 44, 67–76.
Mitrovic, S. M., Westhorpe, D. P., Kobayashi, T., Baldwin, D. S., Ryan, D., Hitchcock, J. N. (2014). Short-term changes in zooplankton density and community structure in response to different sources of dissolved organic carbon in an unconstrained lowland river: evidence for food web support. Journal of Plankton Research, 36, 1488–1500.
Mostofa, K.M.G., Liu, C., Mottale, M.A., Wan, G., Ogawa, H., Vione, D., Yoshioka, Y. & Wu, F. (2013). Dissolved Organic Matter in Natural Waters. Mostofa, K.M.G., et al. (eds.), Photobiogeochemistry of Organic Matter. Environmental Science and Engineering. Springer-Verlag, Berlin.
Pace, M. L., & Cole, J. J. (2002). Comparative and experimental approaches to top-down and bottom-up regulation of bacteria. Microbial Ecology, 43, 107–117.
Paggi, J.C. (1995). Crustacea cladocera. Ecosistemas de Aguas Continentales, Metodologías para su estudio.
Paggi, J. C. (1979). Revisión de las especies argentinas del género Bosmina Baird agrupadas en el sugénero Neobosmina Lieder (Crustacea, Cladocera). Acta Zoologica Lilloana, 35, 137–162.
Paraskevopoulou, S., Dennis, A. B., Weithoff, G., Hartmann, S., & Tiedemann, R. (2019). Within species expressed genetic variability and gene expression response to different temperatures in the rotifer Brachionus calyciflorus sensu stricto. PLoS ONE, 14, e0223134.
Patel, N., Khan, M. D., Shahane, S., Rai, D., Chauhan, D., Kant, C., & Chaudhary, V. K. (2020). Emerging pollutants in aquatic environment: Source, effect, and challenges in biomonitoring and bioremediation- a review. Pollution, 6, 99–113.
Pereira de Albuquerque, F., de Oliveira, J. L., Moschini-Carlos, V., & Fernandes Fraceto, L. (2020). An overview of the potential impacts of atrazine in aquatic environments: Perspectives for tailored solutions based on nanotechnology. Science of the Total Environment, 700, 134868.
Pete, R., Davidson, K., Hart, M. C., Gutierrez, T., & Miller, A. E. J. (2010). Diatom derived dissolved organic matter as a driver of bacterial productivity: The role of nutrient limitation. Journal of Experimental Marine Biology and Ecology, 391, 20–26.
Popa, C. L., Dontu, S. I., Carstea, E. M., Loja, I. C., Florescu, L. I., Dumitrache, A. C., Vanau, G., Ana-Popa, A. M., & Moldoveanu, M. (2023). Land use impact on the levels of fluorescent dissolved organic matter, phytoplankton, and zooplankton in urban lakes. Limnologica, 99, 126062.
Prakash, A., Rashid, M. A., Jensen, A., & Subba Rao, D. V. (1973). Influence of humic substances on the growth of marine phytoplankton: Diatoms. Limnology and Oceanography, 18, 516–524.
Prosnier, L., Loreau, L., & Hulot, F. D. (2015). Modeling the direct and indirect effects of copper on phytoplankton–zooplankton interactions. Aquatic Toxicology, 162, 73–81.
Qian, H., Sheng, D., Liu, W., Lu, Y., Liu, Z., & Fu, Z. (2008). Inhibitory effects of atrazine on chlorella vulgaris as assessed by real-time polymerase chain reaction. Environmental Toxicology and Chemistry, 27, 82–187.
Rader, K. J., Carbonaro, R. F., van Hullebusch, E. D., Baken, S., & Delbeke, K. (2019). The fate of copper added to surface water: field, laboratory, and modeling studies. Environmental Toxicology and Chemestry, 38, 1386–1399.
Reynolds, C. (2006). Ecology of Phytoplankton. University Press.
Rocha, O., & Duncan, A. (1985). The relationship between cell carbon and cell volume in freshwater algal species used in zooplanktonic studies. Journal of Plankton Research, 7, 279–294.
Rodríguez, P., Pizarro, H. (2007). Phytoplankton productivity in a highly colored shallow lake of a south american floodplain. Wetlands, 27, 1153–1160.
Salmaso, N., & Tolotti, M. (2021). Phytoplankton and anthropogenic changes in pelagic environments. Hydrobiologia, 848, 251–284.
Sass, J. B., & Colangelo, A. (2006). European Union bans atrazine, while the United States negotiates continued use. International Journal of Occupational and Environmental Health, 12, 260–267.
Seguin, F., Leboulanger, C., Rimet, F., Druart, J. C., & Bérard, A. (2001). Effects of atrazine and nicosulfuron on Phytoplankton in systems of increasing complexity. Archives of Environmental Contamination and Toxicology, 40, 198–208.
Shim, K. Y., Sukumaran, V., Yeo, I., Shin, H., & Jeong, C. B. (2022). Effects of Atrazine and diuron on life parameters, antioxidant response, and multixenobiotic resistance in non-targeted marine zooplankton. Comparative Biochemistry and Physiology Part c: Toxicology & Pharmacology, 258, 109378.
Siemering, G. S., Hayworth, J. D., & Greenfield, B. K. (2008). Assessment of potential aquatic herbicide impacts to California aquatic ecosystems. Archives of Environmental Contamination and Toxicology, 55, 415–431.
Sommer, U., & Lengfellner, K. (2008). Climate change and the timing, magnitude, and composition of the phytoplankton spring bloom. Global Change Biology, 14, 1199–1208.
Suárez-Serrano, A., Alcaraz, C., Ibáñez, C., Trobajo, R., & Barata, C. (2010). Procambarus clarkii as a bioindicator of heavy metal pollution sources in the lower Ebro River and Delta. Ecotoxicology and Environmental Safety, 73, 280–286.
Tamm, L., Thuerig, B., Apostolov, S., Blogg, H., Borgo, E., Corneo, P. E., Fittje, S., de Palma, M., Donko, A., Experton, C., Alcazar Marin, E., Morell Perez, A., Pertot, I., Rasmussen, A., Steinshamn, H., Vetemaa, A., Willer, H., & Herforth-Rahmé, J. (2022). Use of copper-based fungicides in organic agriculture in twelve European countries. Agronomy, 12, 673.
Utermöhl, H. (1958). Zur Vervollkommnung der quantitative Phytoplankton: Methodik. International Association of Theoretical & Applied Limnology, 9, 1–38.
Venrick, E.L. (1978). How many cells to count? In A. Sournia (Ed.), Phytoplankton manual. Paris: UNESCO.
Zhang, Y., Cheng, D., Ren, Y., Song, J., Xu, D., Chen, R., Pang, R., & Xia, J. (2022). Influence of land cover types and phytoplankton community on the distribution and fate of dissolved organic matter in a typical river located in the semi-arid regions of China. Journal of Hydrology, 610, 127818.
Acknowledgements
The authors thank C. Mora for the nutrient concentration estimations and her help during the experimental setup. We also thank M. Licursi for her comments on the experiment design. The present manuscript was highly improved with the comments of anonymous reviewers.
Funding
This research was made possible by financial support from the National Council for Scientific and Technical Research (CONICET) through the project grant PICT n° 1586–2019 awarded by D. Frau.
Author information
Authors and Affiliations
Contributions
DF and MFG contributed to designing, executing, and samples processing. DF analyzed data and wrote the manuscript, DF and MFG revised the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Financial Disclosure
The authors have no relevant financial to disclose.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frau, D., Gutierrez, M.F. Plankton Response to a Mix of Environmental Stressors. Water Air Soil Pollut 235, 316 (2024). https://doi.org/10.1007/s11270-024-07117-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07117-1