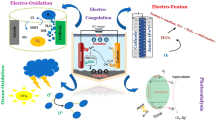
Abstract
Cyanide is the main organic pollutant obtained in the water bodies of steel manufacturing plants. Cyanide generation takes place during the pyrolysis of coal for coke formation. Organic compounds such as cyanide, ammonia, phenol, and others are found in bodies of water after the coke oven gas is quenched. Highly hazardous nature and fatality of cyanide demand its effective remediation, with environmental norms stating (< 0.2 ppm cyanide) must be fulfilled before its release. The objective of the review is to include all the studies till date and the discomfort associated with the techniques. The study emphasizes on both primitive and advanced scientific techniques employed around the globe for cyanide remediation. Advanced techniques are also accompanied with challenges such as in case of strong oxidants; the storage and handling of explosive oxidants can be of concern. Strong oxidants, such as ozone, hydrogen peroxide, or hypochlorite, are extremely effective, but they significantly increase chemical demand. Strong oxidants like H2O2 were found to be very explosive in nature, hence limit their uses. Greener bioremediation alternatives are available, but their longer reaction times are their main detriment. Because of their high effectiveness even in the absence of any chemical requirement, photocatalysts and their actions are intensely studied. Efficiency and economics being the primary parameter, this paper focuses on the sustainability and recovery of catalyst reagents. Alongside, how large-scale industries can utilize designated techniques for higher yield and economic benefits is also retrospected here.














Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- WWTP:
-
Wastewater treatment plant
- PAHs:
-
Polycyclic aromatic hydrocarbons
- WAD:
-
Weak-acid dissociable
- SAD:
-
Strong-acid dissociable
- AC:
-
Activated carbon
- BAF:
-
Biological aerated filters
- AOP:
-
Advanced oxidation process
- GO:
-
Graphene oxide
- GAC:
-
Granular activated carbon
- SBBR:
-
Sequencing batch biofilm reactor
- EPA:
-
Environmental Protection Agency
- FCC:
-
Fluid catalytic cracking
- EMSTM:
-
The engineered membrane separation treatment method
- COD:
-
Chemical oxygen demand
- DLHF:
-
Dual layer hollow fibre
References
Aazam, E. (2014). Environmental remediation of cyanide solutions by photocatalytic oxidation using Au/CdS nanoparticles. Journal of Industrial and Engineering Chemistry, 20, 2870–2875.
Acheampong, M. A., Meulepas, R. J., & Lens, P. N. (2010). Removal of heavy metals and cyanide from gold mine wastewater. Journal of Chemical Technology & Biotechnology, 85, 590–613.
Adams, M., & Lloyd, V. (2008). Cyanide recovery by tailings washing and pond stripping. Minerals Engineering, 21, 501–508.
Adhoum, N., & Monser, L. (2002). Removal of cyanide from aqueous solution using impregnated activated carbon. Chemical Engineering and Processing: Process Intensification, 41, 17–21.
Aguado, J., Van Grieken, R., Lopez-Munoz, M., & Marugán, J. (2002). Removal of cyanides in wastewater by supported TiO2-based photocatalysts. Catalysis Today, 75, 95–102.
Ahmed, M., Mavukkandy, M. O., Giwa, A., Elektorowicz, M., Katsou, E., Khelifi, O., Naddeo, V., & Hasan, S. W. (2022). Recent developments in hazardous pollutants removal from wastewater and water reuse within a circular economy. NPJ Clean Water, 5, 12.
Akcil, A. (2003). Destruction of cyanide in gold mill effluents: Biological versus chemical treatments. Biotechnology Advances, 21, 501–511.
Albarelli, J. Q., Santos, D. T., Murphy, S., & Oelgemöller, M. (2009). Use of Ca-alginate as a novel support for TiO2 immobilization in methylene blue decolorisation. Water Science and Technology, 60, 1081–1087.
Alexander, J. T., Hai, F. I., & Al-aboud, T. M. (2012). Chemical coagulation-based processes for trace organic contaminant removal: Current state and future potential. Journal of Environmental Management, 111, 195–207.
Alguacil, F. J. (2006), 'The Chemistry of Gold Extraction (2nd edn), John, O., Marsden, C., Iain House SME', Gold Bulletin 39, 138–138.
Alonso-González, O., Nava-Alonso, F., & Uribe-Salas, A. (2009). Copper removal from cyanide solutions by acidification. Minerals Engineering, 22, 324–329.
Alonso-González, O., Nava-Alonso, F., Uribe-Salas, A., & Dreisinger, D. (2010). Use of quaternary ammonium salts to remove copper–cyanide complexes by solvent extraction. Minerals Engineering, 23, 765–770.
Alvillo-Rivera, A., Garrido-Hoyos, S., Buitrón, G., Thangarasu-Sarasvathi, P., & Rosano-Ortega, G. (2021). Biological treatment for the degradation of cyanide: A review. Journal of Materials Research and Technology, 12, 1418–1433.
Arellano, C. A. P., & Martínez, S. S. (2007). Indirect electrochemical oxidation of cyanide by hydrogen peroxide generated at a carbon cathode. International Journal of Hydrogen Energy, 32, 3163–3169.
Babuponnusami, A., & Muthukumar, K. (2014). A review on Fenton and improvements to the Fenton process for wastewater treatment. Journal of Environmental Chemical Engineering, 2, 557–572.
Bagabas, A., Alshammari, A., Aboud, M. F., & Kosslick, H. (2013). Room-temperature synthesis of zinc oxide nanoparticles in different media and their application in cyanide photodegradation. Nanoscale Research Letters, 8, 1–10.
Barakat, M., Chen, Y., & Huang, C. (2004). Removal of toxic cyanide and Cu (II) Ions from water by illuminated TiO2 catalyst. Applied Catalysis B: Environmental, 53, 13–20.
Betancourt-Buitrago, L. A., Hernandez-Ramirez, A., Colina-Marquez, J. A., Bustillo-Lecompte, C. F., Rehmann, L., & Machuca-Martinez, F. (2019). Recent developments in the photocatalytic treatment of cyanide wastewater: An approach to remediation and recovery of metals. Processes, 7, 225.
Bhattacharya, S., Das, A. A., Dhal, G. C., Sahoo, P. K., Tripathi, A., & Sahoo, N. K. (2022). Evaluation of N doped rGO-ZnO-CoPc (COOH) 8 nanocomposite in cyanide degradation and its bactericidal activities. Journal of Environmental Management, 302, 114022.
Biswas, J. (2013). Evaluation of various method and efficiencies for treatment of effluent from iron and steel industry—A review. International Journal of Mechanical Engineering and Robotics Research, 2, 67–73.
Biswas, P., Bhunia, P., Saha, P., Sarkar, S., Chandel, H., & De, S. (2020). In situ photodecyanation of steel industry wastewater in a pilot scale. Environmental Science and Pollution Research, 27, 33226–33233.
Bodamer, G. W. (1977). Electrodialysis for closed loop control of cyanide rinse waters. U.S. Environmental Protection Agency, Office of Research and Development, Washington, D.C., EPA/600/2-77/161.
Bokare, A. D., & Choi, W. (2014). Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. Journal of Hazardous Materials, 275, 121–135.
Botz, M., Dimitriadis, D., Polglase, T., Phillips, W., & Jenny, R. (2001). Processes for the regeneration of cyanide from thiocyanate. Mining, Metallurgy & Exploration, 18, 126–132.
Botz, M., Mudder, T., & Akcil, A. (2005). Cyanide treatment: Physical, chemical and biological processes. Advances in Gold Ore Processing, 4528, 672–702.
Botz, M. M.: (2001), 'Overview of cyanide treatment methods'. Mining Environmental Management, Mining Journal Ltd., London, UK, 28-30
Breuer, P., Jeffery, C., & Meakin, R. (2011). 'Fundamental investigations of the SO2/air, peroxide and Caro’s acid cyanide destruction processes by'. Proceedings of ALTA 2011 Gold Conference, ALTA Metallurgical Services Perth, 154–168.
Brillas, E., Sirés, I., & Oturan, M. A. (2009). Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chemical Reviews, 109, 6570–6631.
Byrne, J., Eggins, B., Brown, N., McKinney, B., & Rouse, M. (1998). Immobilisation of TiO2 powder for the treatment of polluted water. Applied Catalysis B: Environmental, 17, 25–36.
Cheng, S., Gattrell, M., Guena, T., & MacDougall, B. (2002). The electrochemical oxidation of alkaline copper cyanide solutions. Electrochimica Acta, 47, 3245–3256.
Chi, G., Fuerstenau, M. C., & Marsden, J. O. (1997). Study of Merrill-Crowe processing. Part I: Solubility of zinc in alkaline cyanide solution. International Journal of Mineral Processing, 49, 171–183.
Chiam, S.-L., Pung, S.-Y., & Yeoh, F.-Y. (2020). Recent developments in MnO2-based photocatalysts for organic dye removal: A review. Environmental Science and Pollution Research, 27, 5759–5778.
Chiang, K., Amal, R., & Tran, T. (2002). Photocatalytic degradation of cyanide using titanium dioxide modified with copper oxide. Advances in Environmental Research, 6, 471–485.
Crini, G., & Lichtfouse, E. (2019). Advantages and disadvantages of techniques used for wastewater treatment. Environmental Chemistry Letters, 17, 145–155.
Cui, J., Wang, X., Yuan, Y., Guo, X., Gu, X., & Jian, L. (2014). Combined ozone oxidation and biological aerated filter processes for treatment of cyanide containing electroplating wastewater. Chemical Engineering Journal, 241, 184–189.
Dai, X., Simons, A., & Breuer, P. (2012). A review of copper cyanide recovery technologies for the cyanidation of copper containing gold ores. Minerals Engineering, 25, 1–13.
Daneshvar, N., Salari, D., Niaei, A., Rasoulifard, M., & Khataee, A. (2005). Immobilization of TiO2 nanopowder on glass beads for the photocatalytic decolorization of an azo dye CI Direct Red 23. Journal of Environmental Science and Health, Part A, 40, 1605–1617.
Das, P. P., Mondal, P., Sinha, A., Biswas, P., Sarkar, S., & Purkait, M. (2021a). Integrated ozonation assisted electrocoagulation process for the removal of cyanide from steel industry wastewater. Chemosphere, 263, 128370.
Das, P. P., Mondal, P., Sinha, A., Biswas, P., Sarkar, S., & Purkait, M. K. (2021b). Treatment of steel plant generated biological oxidation treated (BOT) wastewater by hybrid process. Separation and Purification Technology, 258, 118013.
Dash, R. R., Balomajumder, C., & Kumar, A. (2008). Treatment of metal cyanide bearing wastewater by simultaneous adsorption and biodegradation (SAB). Journal of Hazardous Materials, 152, 387–396.
Dash, R. R., Balomajumder, C., & Kumar, A. (2009a). Removal of cyanide from water and wastewater using granular activated carbon. Chemical Engineering Journal, 146, 408–413.
Dash, R. R., Gaur, A., & Balomajumder, C. (2009b). Cyanide in industrial wastewaters and its removal: A review on biotreatment. Journal of Hazardous Materials, 163, 1–11.
de Andrade, F. V., de Lima, G. M., Augusti, R., da Silva, J. C. C., Coelho, M. G., Paniago, R., & Machado, I. R. (2015). A novel TiO2/autoclaved cellular concrete composite: From a precast building material to a new floating photocatalyst for degradation of organic water contaminants. Journal of Water Process Engineering, 7, 27–35.
Depci, T. (2012). Comparison of activated carbon and iron impregnated activated carbon derived from Gölbaşı lignite to remove cyanide from water. Chemical Engineering Journal, 181, 467–478.
Deveci, H., Yazıcı, E., Alp, I., & Uslu, T. (2006). Removal of cyanide from aqueous solutions by plain and metal-impregnated granular activated carbons. International Journal of Mineral Processing, 79, 198–208.
Dhamo, N. (1996). Electrochemical oxidation of cyanide in the hydrocyclone cell. Waste Management, 16, 257–261.
Dubey, S., & Holmes, D. (1995). Biological cyanide destruction mediated by microorganisms. World Journal of Microbiology and Biotechnology, 11, 257–265.
Dzinun, H., Othman, M. H. D., Ismail, A., Puteh, M. H., Rahman, M. A., & Jaafar, J. (2015). Morphological study of co-extruded dual-layer hollow fiber membranes incorporated with different TiO2 loadings. Journal of Membrane Science, 479, 123–131.
Eskandari, P., Farhadian, M., Solaimany Nazar, A. R., & Jeon, B.-H. (2019). Adsorption and photodegradation efficiency of TiO2/Fe2O3/PAC and TiO2/Fe2O3/zeolite nanophotocatalysts for the removal of cyanide. Industrial & Engineering Chemistry Research, 58, 2099–2112.
Farrokhi, M., Yang, J.-K., Lee, S.-M., & Shirzad-Siboni, M. (2013). Effect of organic matter on cyanide removal by illuminated titanium dioxide or zinc oxide nanoparticles. Journal of Environmental Health Science and Engineering, 11, 1–8.
Felföldi, T., Nagymáté, Z., Székely, A. J., Jurecska, L., & Márialigeti, K. (2020). Biological treatment of coke plant effluents: From a microbiological perspective. Biologia Futura, 71, 359–370.
Fry, W. & Millar, R. (1972). Cyanide degradion by an enzyme from Stemphylium loti. Archives of biochemistry and biophysics, 151, 468–474.
Gar Alalm, M., Tawfik, A., & Ookawara, S. (2016). Enhancement of photocatalytic activity of TiO2 by immobilization on activated carbon for degradation of pharmaceuticals. Journal of Environmental Chemical Engineering, 4, 1929–1937.
García, V., Häyrynen, P., Landaburu-Aguirre, J., Pirilä, M., Keiski, R. L., & Urtiaga, A. (2014). Purification techniques for the recovery of valuable compounds from acid mine drainage and cyanide tailings: Application of green engineering principles. Journal of Chemical Technology & Biotechnology, 89, 803–813.
Golbaz, S., Jafari, A. J., Rafiee, M., & Kalantary, R. R. (2014). Separate and simultaneous removal of phenol, chromium, and cyanide from aqueous solution by coagulation/precipitation: Mechanisms and theory. Chemical Engineering Journal, 253, 251–257.
Gönen, N., Kabasakal, O., & Özdil, G. (2004). Recovery of cyanide in gold leach waste solution by volatilization and absorption. Journal of Hazardous Materials, 113, 231–236.
Grao, M., Ratova, M., Amorim, C. C., Marcelino, R. B., & Kelly, P. (2020). Crystalline TiO2 supported on stainless steel mesh deposited in a one step process via pulsed DC magnetron sputtering for wastewater treatment applications. Journal of Materials Research and Technology, 9, 5761–5773.
Han, B., Shen, Z., & Wickramasinghe, S. R. (2005). Cyanide removal from industrial wastewaters using gas membranes. Journal of Membrane Science, 257, 171–181.
Harraz, F. A., Abdel-Salam, O. E., Mostafa, A. A., Mohamed, R. M., & Hanafy, M. (2013). Rapid synthesis of titania–silica nanoparticles photocatalyst by a modified sol–gel method for cyanide degradation and heavy metals removal. Journal of Alloys and Compounds, 551, 1–7.
Harris, R. E., & Knowles, C. J. (1983). The conversion of cyanide to ammonia by extracts of a strain of Pseudomonas fluorescens that utilizes cyanide as a source of nitrogen for growth. FEMS Microbiology Letters, 20, 337–341.
Hassanzadeh Fard, Z., Malliakas, C. D., Mertz, J. L., & Kanatzidis, M. G. (2015). Direct extraction of Ag+ and Hg2+ from cyanide complexes and mode of binding by the layered K2MgSn2S6 (KMS-2). Chemistry of Materials, 27, 1925–1928.
Hidaka, H., Nakamura, T., Ishizaka, A., Tsuchiya, M., & Zhao, J. (1992). Heterogeneous photocatalytic degradation of cyanide on TiO2 surfaces. Journal of Photochemistry and Photobiology a: Chemistry, 66, 367–374.
Hosseini, S. N., Borghei, S. M., Vossoughi, M., & Taghavinia, N. (2007). Immobilization of TiO2 on perlite granules for photocatalytic degradation of phenol. Applied Catalysis B: Environmental, 74, 53–62.
Huertas, M., Sáez, L., Roldán, M., Luque-Almagro, V., Martínez-Luque, M., Blasco, R., Castillo, F., Moreno-Vivián, C., & García-García, I. (2010). Alkaline cyanide degradation by Pseudomonas pseudoalcaligenes CECT5344 in a batch reactor. Influence of pH. Journal of Hazardous Materials, 179, 72–78.
Ismail, A., Ibrahim, I., Ahmed, M., Mohamed, R., & El-Shall, H. (2004). Sol–gel synthesis of titania–silica photocatalyst for cyanide photodegradation. Journal of Photochemistry and Photobiology a: Chemistry, 163, 445–451.
Jaszczak, E., Polkowska, Ż, Narkowicz, S., & Namieśnik, J. (2017). Cyanides in the environment—analysis—problems and challenges. Environmental Science and Pollution Research, 24, 15929–15948.
Jawale, R. H., Gogate, P. R., & Pandit, A. B. (2014). Treatment of cyanide containing wastewater using cavitation based approach. Ultrasonics Sonochemistry, 21, 1392–1399.
Jawale, R. H., Tandale, A., & Gogate, P. R. (2017). Novel approaches based on ultrasound for treatment of wastewater containing potassium ferrocyanide. Ultrasonics Sonochemistry, 38, 402–409.
Jiménez-Prieto, Y., Esperanza-Pérez, G., Ramírez-González, S., & Alomas-Vicente, I. D. L. C. (2020). Assessment of technological alternatives for cyanide waste waters management in gold ores processing plant. Revista Cubana de Química, 32, 218–231.
Johnson, C. A. (2015). The fate of cyanide in leach wastes at gold mines: An environmental perspective. Applied Geochemistry, 57, 194–205.
Kadi, M. W., & Mohamed, R. (2013). Enhanced photocatalytic activity of ZrO2-SiO2 nanoparticles by platinum doping. International Journal of Photoenergy, 2013, 812097. https://doi.org/10.1155/2013/812097.
Karunakaran, C., & Senthilvelan, S. (2005). Photocatalysis with ZrO2: Oxidation of aniline. Journal of Molecular Catalysis A: Chemical, 233, 1–8.
Karunakaran, C., Gomathisankar, P., & Manikandan, G. (2010). Preparation and characterization of antimicrobial Ce-doped ZnO nanoparticles for photocatalytic detoxification of cyanide. Materials Chemistry and Physics, 123, 585–594.
Karunakaran, C., Rajeswari, V., & Gomathisankar, P. (2011). Enhanced photocatalytic and antibacterial activities of sol–gel synthesized ZnO and Ag-ZnO. Materials Science in Semiconductor Processing, 14, 133–138.
Kepa, U., Stanczyk-Mazanek, E., & Stepniak, L. (2008). The use of the advanced oxidation process in the ozone+ hydrogen peroxide system for the removal of cyanide from water. Desalination, 223, 187–193.
Kobayakawa, K., Sato, C., Sato, Y., & Fujishima, A. (1998). Continuous-flow photoreactor packed with titanium dioxide immobilized on large silica gel beads to decompose oxalic acid in excess water. Journal of Photochemistry and Photobiology A: Chemistry, 118, 65–69.
Koohestani, H., & Sadrnezhaad, S. K. (2016). Photocatalytic degradation of methyl orange and cyanide by using TiO2/CuO composite. Desalination and Water Treatment, 57, 22029–22038.
Kumar, M. S., Gopinath, A., Ranjith, N., Ummar, S., & Nidheesh, P. V. (2023). Treatment of integrated steel plant wastewater using conventional and advanced techniques. CLEAN–Soil, Air, Water, 51, 2100169.
Kunz, D. A., Nagappan, O., Silva-Avalos, J., & Delong, G. (1992). Utilization of cyanide as nitrogenous substrate by Pseudomonas fluorescens NCIMB 11764: Evidence for multiple pathways of metabolic conversion. Applied and Environmental Microbiology, 58, 2022–2029.
Kurama, H., & Çatalsarik, T. (2000). Removal of zinc cyanide from a leach solution by an anionic ion-exchange resin. Desalination, 129, 1–6.
Kuyucak, N., & Akcil, A. (2013). Cyanide and removal options from effluents in gold mining and metallurgical processes. Minerals Engineering, 50, 13–29.
Li, J., Zhang, S., Nie, Y., Ma, X., Xu, L., & Wu, L. (2020). A holistic life cycle evaluation of coking production covering coke oven gas purification process based on the subdivision method. Journal of Cleaner Production, 248, 119183.
Lintzos, L., Koumaki, E., Mendrinou, P., Chatzikonstantinou, K., Tzamtzis, N., & Malamis, S. (2020). Biological cyanide removal from industrial wastewater by applying membrane bioreactors. Journal of Chemical Technology & Biotechnology, 95, 3041–3050.
Lu, J., Dreisinger, D., & Cooper, W. (2002). Copper electrowinning from dilute cyanide solution in a membrane cell using graphite felt. Hydrometallurgy, 64, 1–11.
Luo, M., Liu, Y., Hu, J., Liu, H., & Li, J. (2012). One-pot synthesis of CdS and Ni-doped CdS hollow spheres with enhanced photocatalytic activity and durability. ACS Applied Materials & Interfaces, 4, 1813–1821.
Mamelkina, M. A., Herraiz-Carboné, M., Cotillas, S., Lacasa, E., Sáez, C., Tuunila, R., Sillanpää, M., Häkkinen, A., & Rodrigo, M. A. (2020). Treatment of mining wastewater polluted with cyanide by coagulation processes: A mechanistic study. Separation and Purification Technology, 237, 116345.
Maulana, I., & Takahashi, F. (2018). Cyanide removal study by raw and iron-modified synthetic zeolites in batch adsorption experiments. Journal of Water Process Engineering, 22, 80–86.
McNulty, K. J., & Hoover, P. R. (1980). Evaluation of reverse osmosis membranes for treatment of electroplating rinsewater. Environmental Protection Agency, Office of Research and Development, Industrial Environmental Research Laboratory, Springfield, Va.
Mishra, L., Paul, K. K., & Jena, S. (2018). Characterization of coke oven wastewater. IOP Conference Series: Earth and Environmental Science, 167, 012011.
Mo, S.-D., & Ching, W. (1995). Electronic and optical properties of three phases of titanium dioxide: Rutile, anatase, and brookite. Physical Review B, 51, 13023.
Mondal, M., Mukherjee, R., Sinha, A., Sarkar, S., & De, S. (2019). Removal of cyanide from steel plant effluent using coke breeze, a waste product of steel industry. Journal of Water Process Engineering, 28, 135–143.
Mondal, K., & Sharma, A. (2014) 'Photocatalytic oxidation of pollutant dyes in wastewater by TiO2 and ZnO nano-materials—A mini-review'. Nanoscience & Technology for Mankind, 36–72.
Mondal, A., Saha, P., Sarkar, S. & Nair, U. G. (2021). Mitigation of cyanide from coke plant wastewater using chemical oxidation process. PREPRINT (Version 1) available at Research Square. https://doi.org/10.21203/rs.3.rs-727204/v1.
Monser, L., & Adhoum, N. (2002). Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Separation and Purification Technology, 26, 137–146.
Morillo Esparza, J., Cevallos Cueva, N., Sandoval Pauker, C., Vargas Jentzsch, P., & Muñoz Bisesti, F. (2019). Combined treatment using ozone for cyanide removal from wastewater: A comparison. Revista Internacional De Contaminación Ambiental, 35, 459–467.
Moussavi, G., Majidi, F., & Farzadkia, M. (2011). The influence of operational parameters on elimination of cyanide from wastewater using the electrocoagulation process. Desalination, 280, 127–133.
Ning, P., Qiu, J., Wang, X., Liu, W., & Chen, W. (2013). Metal loaded zeolite adsorbents for hydrogen cyanide removal. Journal of Environmental Sciences, 25, 808–814.
Núñez-Salas, R. E., Hernández-Ramírez, A., Hinojosa-Reyes, L., Guzmán-Mar, J. L., Villanueva-Rodriguez, M., & de Lourdes Maya-Trevino, M. (2019). Cyanide degradation in aqueous solution by heterogeneous photocatalysis using boron-doped zinc oxide. Catalysis Today, 328, 202–209.
Osathaphan, K., Boonpitak, T., Laopirojana, T., & Sharma, V. (2008). Removal of cyanide and zinc–cyanide complex by an ion-exchange process. Water, Air, and Soil Pollution, 194, 179–183.
Oudjehani, K., Zagury, G., & Deschenes, L. (2002). Natural attenuation potential of cyanide via microbial activity in mine tailings. Applied Microbiology and Biotechnology, 58, 409–415.
Pan, Y., Zhang, Y., Huang, Y., Jia, Y., & Chen, L. (2022). Enhanced photocatalytic oxidation degradability for real cyanide wastewater by designing photocatalyst GO/TiO2/ZSM-5: Performance and mechanism research. Chemical Engineering Journal, 428, 131257.
Paredes, L., Murgolo, S., Dzinun, H., Dzarfan Othman, M. H., Ismail, A. F., Carballa, M., & Mascolo, G. (2019). Application of immobilized TiO2 on PVDF dual layer hollow fibre membrane to improve the photocatalytic removal of pharmaceuticals in different water matrices. Applied Catalysis B: Environmental, 240, 9–18.
Parga, J., Shukla, S., & Carrillo-Pedroza, F. (2003). Destruction of cyanide waste solutions using chlorine dioxide, ozone and titania sol. Waste Management, 23, 183–191.
Pathak, U., Kumari, S., Das, P., Kumar, T., & Mandal, T. (2019). Role of advanced oxidation process in treatment of coke oven wastewater—A review. Waste Water Recycling and Management: 7th IconSWM -- ISWMAW 2017, 3, 37–51.
Pauna, H., Willms, T., Aula, M., Echterhof, T., Huttula, M., & Fabritius, T. (2021). Cyanide recombination in electric arc furnace plasma. Plasma Research Express, 3, 025008.
Pawar, V., & Gawande, S. (2015). An overview of the Fenton process for industrial wastewater. IOSR Journal of Mechanical and Civil Engineering, 2, 127–136.
Petelin, A., Yusfin, Y. S., & Travyanov, A. Y. (2008). Possibility of cyanide formation in blast furnaces. Steel in Translation, 38, 5.
Pueyo, N., Rodríguez-Chueca, J., Ovelleiro, J., & Ormad, M. (2016). Limitations of the removal of cyanide from coking wastewater by treatment with hydrogen peroxide. Water, Air, & Soil Pollution, 227, 1–10.
Ravuru, S. S., Jana, A., & De, S. (2019). Synthesis of NiAl-layered double hydroxide with nitrate intercalation: Application in cyanide removal from steel industry effluent. Journal of Hazardous Materials, 373, 791–800.
Ravuru, S. S., Jana, A., & De, S. (2022). Cyanide removal from blast furnace effluent using layered double hydroxide based mixed matrix beads: Batch and fixed bed study. Journal of Cleaner Production, 371, 133634.
Ritcey, G. M. (2005). Tailings management in gold plants. Hydrometallurgy, 78, 3–20.
Roques, H. (1996). Chemical water treatment: Principles and practice (p. 620). VCH Publishers. Inc.
Rubio, J., Souza, M., & Smith, R. (2002). Overview of flotation as a wastewater treatment technique. Minerals Engineering, 15, 139–155.
Saleh, T. A., Gondal, M., & Drmosh, Q. (2010). Preparation of a MWCNT/ZnO nanocomposite and its photocatalytic activity for the removal of cyanide from water using a laser. Nanotechnology, 21, 495705.
Sathya, K., Nagarajan, K., Carlin Geor Malar, G., Rajalakshmi, S., & Raja Lakshmi, P. (2022). A comprehensive review on comparison among effluent treatment methods and modern methods of treatment of industrial wastewater effluent from different sources. Applied Water Science, 12, 70.
Sboui, M., Nsib, M. F., Rayes, A., Swaminathan, M., & Houas, A. (2017). TiO2–PANI/Cork composite: A new floating photocatalyst for the treatment of organic pollutants under sunlight irradiation. Journal of Environmental Sciences, 60, 3–13.
Sharma, V. K., Yngard, R. A., Cabelli, D. E., & Baum, J. C. (2008). Ferrate (VI) and ferrate (V) oxidation of cyanide, thiocyanate, and copper (I) cyanide. Radiation Physics and Chemistry, 77, 761–767.
Sharma, M., Akhter, Y., & Chatterjee, S. (2019). A review on remediation of cyanide containing industrial wastes using biological systems with special reference to enzymatic degradation. World Journal of Microbiology and Biotechnology, 35, 70.
Shen, Z., Han, B., & Wickramasinghe, R. (2004). Cyanide removal from wastewater using gas membranes: Pilot-scale study. Water Environment Research, 76, 15–22.
Shokoohi, R., Poureshgh, Y., Parastar, S., Ahmadi, S., Shabanloo, A., Rahmani, Z., Asl, F. B., & Tabar, M. V. (2018). Comparing the efficiency of UV/ZrO2 and UV/H2O2/ZrO2 photocatalytic processes in furfural removal from aqueous solution. Applied Water Science, 8, 1–8.
Siboni, M. S., Samarghandi, M., Yang, J.-K., & Lee, S.-M. (2011). Photocatalytic removal of cyanide with illuminated TiO2. Water Science and Technology, 64, 1383–1387.
Simovic, L., Snodgrass, W. J., Murphy, K. L. S. (1985). Development of a model to describe the natural degradation of cyanide in gold milling effluents. In Van Zyl, D. (Ed.). Cyanide and the environment., (vol. II ,pp. 413–432). Colorado State University, USA.
Sinbuathong, N., Kongseri, B., Plungklang, P., & Khun-anake, R. (2000). Cyanide removal from laboratory wastewater using sodium hypochlorite and calcium hypochlorite. Agriculture and Natural Resources, 34, 74–78.
Singh, N., & Balomajumder, C. (2016). Batch growth kinetic studies for elimination of phenol and cyanide using mixed microbial culture. Journal of Water Process Engineering, 11, 130–137.
Sonune, A., & Ghate, R. (2004). Developments in wastewater treatment methods. Desalination, 167, 55–63.
Sousa, R. R. M. D., Araújo, F. O. D., Costa, J. A. P. D., Nishimoto, A., Viana, B. C., & Alves, C., Jr. (2016). Deposition of TiO 2 film on duplex stainless steel substrate using the cathodic cage plasma technique. Materials Research, 19, 1207–1212.
Teixeira, L. A. C., Andia, J. P. M., Yokoyama, L., da Fonseca Araújo, F. V., & Sarmiento, C. M. (2013). Oxidation of cyanide in effluents by Caro’s Acid. Minerals Engineering, 45, 81–87.
Tu, Y., Han, P., Wei, L., Zhang, X., Yu, B., Qian, P., & Ye, S. (2019). Removal of cyanide adsorbed on pyrite by H2O2 oxidation under alkaline conditions. Journal of Environmental Sciences, 78, 287–292.
Walling, S. A., Um, W., Corkhill, C. L., & Hyatt, N. C. (2021). Fenton and Fenton-like wet oxidation for degradation and destruction of organic radioactive wastes. npj Materials Degradation, 5, 50. https://doi.org/10.1038/s41529-021-00192-3.
Wan, S., Zhao, W., Xiong, D., Li, S., Ye, Y., & Du, L. (2022). Novel alginate immobilized TiO2 reusable functional hydrogel beads with high photocatalytic removal of dye pollutions. Journal of Polymer Engineering, 42, 978–985.
Wang, M., Han, J., Xiong, H., Guo, R., & Yin, Y. (2015). Nanostructured hybrid shells of r-GO/AuNP/m-TiO2 as highly active photocatalysts. ACS Applied Materials & Interfaces, 7, 6909–6918.
Wang, L. K., Wang, M.-H.S., Shammas, N. K., & Hahn, H. H. (2021). Physicochemical treatment consisting of chemical coagulation, precipitation, sedimentation, and flotation. In L. K. Wang, M.-H.S. Wang, & Y.-T. Hung (Eds.), Integrated Natural Resources Research (pp. 265–397). Springer International Publishing.
Wedl, D. J., & Fulk, R. J. (1991). Cyanide destruction in plating sludges by hot alkaline chlorination. Metal Finishing, 89, 33–37.
White, D. M., Pilon, T. A., & Woolard, C. (2000). Biological treatment of cyanide containing wastewater. Water Research, 34, 2105–2109.
Xie, F., & Dreisinger, D. (2009). Studies on solvent extraction of copper and cyanide from waste cyanide solution. Journal of Hazardous Materials, 169, 333–338.
Xie, F., Dreisinger, D., & Doyle, F. (2013). A review on recovery of copper and cyanide from waste cyanide solutions. Mineral Processing and Extractive Metallurgy Review, 34, 387–411.
Xing, Z., Zhang, J., Cui, J., Yin, J., Zhao, T., Kuang, J., Xiu, Z., Wan, N., & Zhou, W. (2018). Recent advances in floating TiO2-based photocatalysts for environmental application. Applied Catalysis B: Environmental, 225, 452–467.
Xu, H., Li, A., Feng, L., Cheng, X., & Ding, S. (2012). Destruction of cyanide in aqueous solution by electrochemical oxidation method. International Journal of Electrochemical Science, 7, 7516–7525.
Xu, M., Wu, C., & Zhou, Y. (2020). Advancements in the Fenton process for wastewater treatment. Advanced Oxidation Process, 61, 61–77.
Yan, T. Y. (1992). Removal of cyanide from water. U.S. Patent No. 5,112,494. Washington, DC: U.S. Patent and Trademark Office.
Yang, J. L., An, S. J., Park, W. I., Yi, G. C., & Choi, W. (2004). Photocatalysis using ZnO thin films and nanoneedles grown by metal–organic chemical vapor deposition. Advanced Materials, 16, 1661–1664.
Yazıcı, E. Y., Deveci, H., Alp, I., & Uslu, T. (2007). Generation of hydrogen peroxide and removal of cyanide from solutions using ultrasonic waves. Desalination, 216, 209–221.
Yeddou, A. R., Nadjemi, B., Halet, F., Ould-Dris, A., & Capart, R. (2010). Removal of cyanide in aqueous solution by oxidation with hydrogen peroxide in presence of activated carbon prepared from olive stones. Minerals Engineering, 23, 32–39.
Young, C. A., & Jordan, T. S. (1995). Cyanide remediation: current and past technologies. In Proceedings of the 10th annual conference on hazardous waste research (pp. 104-129). Kansas State University: Manhattan, KS.
Zeng, J., Liu, S., Cai, J., & Zhang, L. (2010). TiO2 immobilized in cellulose matrix for photocatalytic degradation of phenol under weak UV light irradiation. The Journal of Physical Chemistry C, 114, 7806–7811.
Zhang, M.-H., Dong, H., Zhao, L., Wang, D.-X., & Meng, D. (2019). A review on Fenton process for organic wastewater treatment based on optimization perspective. Science of the Total Environment, 670, 110–121.
Zhang, M., Cao, Y., Peng, B., Tian, Y., & Barvor, J. B. (2020). Removal of copper cyanide by precipitate flotation with ammonium salts. Process Safety and Environmental Protection, 133, 82–87.
Author information
Authors and Affiliations
Contributions
NP: conceptualization, methodology, software, writing—original draft preparation, visualisation, and supervision. SD: methodology, software, writing—original draft preparation. PB: reviewing, and supervision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Neha Pandeya and Sunanda Duttab have equal contribution.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandey, N., Dutta, S. & Biswas, P. Cyanide Mitigation at Steel Metallurgical Process’s Effluent. Water Air Soil Pollut 234, 682 (2023). https://doi.org/10.1007/s11270-023-06679-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06679-w