Abstract
Previous studies focused on investigating particulate matter with aerodynamic diameter ≤ 2.5 µm (PM2.5) have shown the risk of disease development, and association with increased morbidity and mortality rates. The current review investigate epidemiological and experimental findings from 2016 to 2021, which enabled the systemic overview of PM2.5’s toxic impacts on human health. The Web of Science database search used descriptive terms to investigate the interaction among PM2.5 exposure, systemic effects, and COVID-19 disease. Analyzed studies have indicated that cardiovascular and respiratory systems have been extensively investigated and indicated as the main air pollution targets. Nevertheless, PM2.5 reaches other organic systems and harms the renal, neurological, gastrointestinal, and reproductive systems. Pathologies onset and/or get worse due to toxicological effects associated with the exposure to this particle type, since it can trigger several reactions, such as inflammatory responses, oxidative stress generation and genotoxicity. These cellular dysfunctions lead to organ malfunctions, as shown in the current review. In addition, the correlation between COVID-19/Sars-CoV-2 and PM2.5 exposure was also assessed to help better understand the role of atmospheric pollution in the pathophysiology of this disease. Despite the significant number of studies about PM2.5's effects on organic functions, available in the literature, there are still gaps in knowledge about how this particulate matter can hinder human health. The current review aimed to approach the main findings about the effect of PM2.5 exposure on different systems, and demonstrate the likely interaction of COVID-19/Sars-CoV-2 and PM2.5.
Similar content being viewed by others
1 Introduction
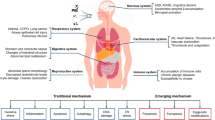
Over the last few years, epidemiological and experimental studies have been reporting association between adverse effects of individuals’ exposure to particulate matter (PM) and the development of several diseases (Jang et al., 2018; Kioumourtzoglou et al., 2016; Nephew et al., 2020; Tanwar et al., 2017; Zhang et al., 2018). PM is classified based on its particles’ size; thus, coarse particles (PM10) range from 10 to 2.5 µm; fine particles (PM2.5), from 2.5 to 0.1 µm; and ultrafine particles (PM0.1) are smaller than 0.1 µm (Al-Thani et al., 2018). PM2.5 toxicity has been assessed in many studies focused on investigating its health-related risks (Gallo et al., 2020; Yang et al., 2020; Younan et al., 2020). PM2.5 can derive from natural sources, such as volcanoes, fire events, dust storms, as well as from anthropogenic activities, mainly from biomass and fuel combustion, and from industrial processes (Al-Thani et al., 2018). The human body is constantly exposed to PM2.5, which can get to individuals’ respiratory tract and reach other systems through the bloodstream; particles’ translocation triggers tissue responses to these airborne pollutants in one’s body (Milani et al., 2020). Mechanisms involved in organic system injuries resulting from individuals’ exposure to PM2.5 are based on biomolecular damage, as well as on signaling pathway alterations and involvement in cell function and survival processes (Fu et al., 2017; Li et al., 2017; Ribeiro et al., 2016; Wei et al., 2016). PM2.5-organic systems’ interaction induces immune system responsiveness, whereas activated pathways include multiple inflammatory cells’ recruitment, cytokine production and increased reactive oxygen species (ROS) production in tissues (Jan et al., 2020; Li et al., 2019a, 2019b; Libalova et al., 2018). Stressful conditions triggered by excessive ROS production lead to an unbalanced normal redox cycle, which, in its turn, triggers pro-oxidant reactions capable of destabilizing the cellular environment and, consequently, of compromising tissues (Jan et al., 2020; Ribeiro et al., 2016). PM2.5 exposure has been associated with systemic disorders in several epidemiological and experimental studies. A broad spectrum of respiratory system-related diseases, such as asthma (Jung et al., 2019; Zhao et al., 2018), lung cancer (Tomczak et al., 2016; Zhang et al., 2020), chronic obstructive pulmonary disease, (COPD) (Liu et al., 2017b) and pneumonia (Lv et al., 2017), can onset and/or worsen due to exposure to PM2.5. Changes in the cardiovascular system, such as arrhythmias, increased blood pressure and variations in heart rate (Folino et al., 2017; Fuks et al., 2016; Gallo et al., 2020; Honda et al., 2018; Kim et al., 2019; Xie et al., 2018; Yang et al., 2020) are also observed in the aforementioned context. Studies focused on investigating the human nervous system evidenced that exposure to PM2.5 increased individuals’ risk of developing Parkinson’s disease, Alzheimer's disease, memory deficit and dementia (Kioumourtzoglou et al., 2016; Liu et al., 2016a, 2016b; Younan et al., 2020). Harmful effects of exposure to PM2.5 and their association with damage in the human renal system were reported in different studies, which evidenced the risk of chronic kidney disease (CKD) (Blum et al., 2020; Bo et al., 2021; Bragg-Gresham et al., 2018; Chan et al., 2018), of progression to end-stage kidney disease—ESKD (Bowe et al., 2018) and of lower glomerular filtration rate (GFR) (Mehta et al., 2016). The aforementioned data indicate the scientific community’s growing interest in investigating the association between exposure to PM2.5 and renal dysfunction (Mehta et al., 2016; de Paula et al., 2019; Xu et al., 2016). Concerning the gastrointestinal system, studies have also reported increased mortality rates associated with liver, colorectal, and gastrointestinal cancer, as well as with dysbiosis resulting from exposure to PM2.5 (Liu et al., 2021; Pan et al., 2016; Weinmayr et al., 2018; Wong et al., 2016). As for the reproductive system, studies have indicated that exposure to PM2.5 has a negative effect on individuals’ fertility (Guo et al., 2020), hinders fetal development (Liu et al., 2016b; Percy et al., 2019; Soto et al., 2017) and leads to plancentary and circulatory impairment (Kingsley et al., 2017; Liu et al., 2016b; Soto et al., 2017; Wylie et al., 2017). In addition, the world was exposed to the COVID-19 pandemic, whose causative virus mainly enters individuals’ bodies through their respiratory system. Therefore, studies were conducted to help better understand the association between exposure to PM2.5, viral cycle and clinical state worsening (Loaiza-Ceballos et al., 2021; Yang et al., 2020). Investigating particulate matter interactions is essential to help develop strategies to reduce global pollution and to help better understand the association between air pollution and health issues. The current review aimed to gather information about organic system alterations and the pathways that contribute to this process, and present epidemiological and experimental findings about exposure to PM2.5 and systemic damages resulting from it (Fig. 1).
2 Cellular Environment and PM2.5 Interactions
2.1 Inflammation and Exposure to PM 2.5
The inflammatory state is the most common response to individuals’ exposure to PM2.5, whose chemical compounds are linked to several changes in body systems, a fact that can onset and/or worsen different pathological conditions (Guan et al., 2019). The interaction of PM2.5 and its compounds with airways induces the formation of local pro-inflammatory and pro-oxidant molecules that use individuals’ bloodstream to reach other organs and change tissue functions (Li et al., 2019a, 2019b). PM2.5 changes cellular biomolecules and activates the immune response; this process starts with immune cells’ recruitment and cytokine production to promote pro-inflammatory signaling (Li et al., 2019a, 2019b; Xu et al., 2020). Cytokines are chemical messengers used as inflammatory markers capable of changing cellular activity (Zheng et al., 2019). Their secretion is susceptible to exposure to PM2.5. Assays conducted in vitro have evidenced increased IL-6, IL-8, and IL-1β cytokine levels in human bronchial epithelial cells, after 24-h exposure to PM2.5 (Zou et al., 2020). Increased cytokine mRNA expression was observed in experimental studies, after individuals’ exposure to PM2.5; this event was associated with neural changes (Liu et al., 2018). Modified mRNA expression of IL4, IL13 and IL17 inflammatory cytokines and decreased number of autophagy markers (ULK1 and LC3A/B) were observed in bronchoalveolar fluid and lungs in asthmatic mice, and it indicated loss of an important inflammation control mechanism, namely: autophagy (Wang et al., 2016). Studies have shown that exposure to PM2.5 for 3, 6, and 12 weeks increased plasma neutrophil and monocyte concentrations, as well as the level of pro-inflammatory cytokines, such as Interferon-gamma (IFN-γ) and Interleukin-10 (IL-10), in mice (Li et al., 2019a, 2019b). These findings corroborate experimental results described by Yang et al. (2019), according to whom, increased levels of immune cells (lymphocytes, neutrophils, eosinophils), Interleukin 4 (IL-4), tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF beta) were observed in the serum and lungs of mice, after three-month exposure to PM2.5. Mast cells are an important target used in studies focused on investigating exposure to PM2.5 since they a play key part in allergic and inflammatory processes, besides being involved in innate and adaptive immune responses (Jin et al., 2019). Wang et al. (2021) have shown that pre-treatment for instillation with PM2.5 in mice at different concentrations (0.187, 0.375, 0.75 mg kg−1), induced mast cells’ activation through immunoglobulin E (IgE) highlighting by β-hexosaminidase (mast degranulation marker) levels. This finding has evidenced that PM2.5 compounds contribute to worsening allergic processes. As previously described, exposure to PM2.5 -at early stages—triggers local immune system activation, and respiratory system inflammatory state, leading to tissue dysfunction (Guan et al., 2019; He et al., 2017; Yang et al., 2018).
2.2 Oxidative Stress and Exposure to PM 2.5
Exposure to PM2.5’s organic and inorganic compounds is linked to increased reactive oxygen species (ROS) formation, and to unbalanced antioxidant defense. The resultant oxidative stress leads to biomolecular injuries (Fang et al., 2019; Lakey et al., 2016). Antioxidant defenses (enzymatic and non-enzymatic) account for protecting the body from damage to macromolecules resulting from ROS production. It does so, by neutralizing ROS action and by maintaining the appropriate cellular environment (Zeng et al., 2018). PM2.5 presents heterogeneous composition, and its compounds can interact in different ways. Among the aforementioned compounds, metals emerge as an important pro-oxidative factor capable of increasing ROS formation (Fang et al., 2019; Ribeiro et al., 2016; de Paula et al., 2019). Although metallic ions are closely related to ROS formation, researchers attribute the oxidative condition to quinones, such as polycyclic aromatic hydrocarbons (PAHs). Both aggravating PM2.5 compounds can interact with biomolecules and lead to oxidative stress condition (Fang et al., 2019; Lakey et al., 2016; Li et al., 2019b; Libalova et al., 2018). Based on findings observed both in vitro and in vivo, PM2.5 inhalation has induced ROS formation, a fact that indicates redox imbalance (Cao et al., 2016; Jin et al., 2019; Park et al., 2018; Ren et al., 2020) and contributes to local and systemic inflammatory state (Crobeddu et al., 2020; Guan et al., 2019). An assay performed in vitro by Xu et al., (2020) has evidenced that macrophage cells exposed to PM2.5 solution (200 µg mL−1) for 6 h showed increased ROS levels and secretion of pro-inflammatory cytokines, such as IL-1β and TNF-α, which are associated with systemic inflammation. Reports have indicated that exposure to PM2.5 decreased antioxidant defense in different organs, such as heart, kidney, liver and lung, by reducing antioxidants’ mechanism, and by affecting glutathione levels and total antioxidant capacity (Ribeiro et al., 2016; de Paula et al., 2019), as well as the activity of enzymes such as superoxide dismutase and glutathione peroxidase (Wu et al., 2016). Exposure to PM2.5 plays a critical part in oxidative stress conditions, since it increases lipoperoxidation (Qiu et al., 2019; de Paula et al., 2019; Wang et al., 2019; Yu et al., 2017) and leads to mitochondrial damage (Guo et al., 2017; Jin et al., 2018; Wang et al., 2019). Accordingly, mitochondrial injury opens the transition pore and hinders the potential of the mitochondrial membrane (Qiu et al., 2019; Wang et al., 2019). Studies have suggested that ROS exacerbation deriving from exposure to PM2.5 accounts for cellular apoptosis and necrosis processes (Santa-Helena et al., 2021; Shan et al., 2021), as well as for increased DNA damage rate and cellular senescence (Gao et al., 2016).
2.3 DNA Changes and Cell Death Pathways Associated with Exposure to PM 2.5
Exposure to PM2.5 has been associated with genotoxicity (DNA strand breaks) and DNA damage signaling ( Kim et al., 2018; Lemos et al., 2016), as well as with chromosomal abnormalities (Miousse et al., 2016) and with changes in DNA methylation (Jiang et al., 2019; Liu et al., 2017a, 2017b; Panni et al., 2016; Shi et al., 2019; Wei et al., 2016, 2017). DNA methylation is a DNA repair mechanism that tends to adjust gene transcription (Harris et al., 2018); however, methylation changes associated with oxidative-stress increase can lead to epigenetic changes (Wei et al., 2017) and induce the development of different diseases (Miousse et al., 2016; Sun et al., 2018;). The study conducted invitro by Wei et al. (2017) exposed SH-SY5Y nervous cell lineage to PM2.5 for 72 h at concentrations of 0, 2.5, 5, 10, 20, 40, 80, 160, and 320 µg mL−1 for the cell viability tests. For the assays, a proportion of 15% of these values was used from organic fraction of PM2.5 (0, 0.375, 0.75, 1.5, 3, 6, 12, 24, and 48 µg mL−1); results have shown changes in DNA methylation associated with redox imbalance, a fact that was attenuated by the use of antioxidants, indicated close correlation between DNA damage and oxidative stress generation. Xu et al. (2018) have shown that PM2.5 compounds found in human bronchial epithelial cells and mouse lungs have activated the early growth-response gene expression (Egr-1) involved in different signaling pathways associated with pathological processes. However, further studies should be performed to help better understand the association between methylation and oxidative stress, as well as the target genes and the mechanisms involved in this process. Besides being associated with DNA damage, exposure to PM2.5 also affects cell survival rates. Studies have indicated that cellular interaction with PM2.5 compounds decreased cell viability by activating different cell death pathways (apoptosis, necrosis, and autophagy) depending on PM composition, concentration, exposure time and on cell type (Cao et al., 2016; Fu et al., 2017; Shan et al., 2021). Studies conducted with human corneal epithelial cells (Fu et al., 2017) have shown decreased cell viability associated with the activation of apoptosis and autophagy pathways, after treatment application with PM2.5 solution (50 µg mL−1). This finding has evidenced time-dependent exposure effects on the investigated cells. According to Santa-Helena et al. (2021), acute exposure of another cell type—H9c2 cardiomyocyte—to PM2.5 for 24 h increases cell apoptosis and necrosis rates associated with ROS formation. The aforementioned authors also reported the activation of different cell death signaling pathways; lower PM2.5 concentrations activated apoptosis, whereas higher concentrations of it led to necrosis and resulted in decreased cell viability (Santa-Helena et al., 2021). In addition, studies in vitro conducted with cortical neurons exposed to different PM2.5 concentrations (12.5, 25, 50, 100, and 200 μg mL−1) have shown that this pollutant triggered cell apoptosis and neurotoxicity (Chen et al., 2017b). PM2.5 also induced pro-apoptotic events due to Bax proteins’ expression; these proteins are associated with apoptotic mechanisms, as well as with anti-apoptotic BCL-2 proteins’ inhibition. As for the seasonal factor, Chen et al. (2017b) reported that winter was the most critical season associated with both exposure to PM2.5 and neurotoxicity events (Chen et al., 2017b). Apoptotic events were also described in mast cells (Jin et al., 2018), as well as in human bronchial epithelial cells (Dornhof et al., 2017) exposed to different PM2.5 concentrations (Table 1). These studies aimed to explain how cellular toxicity occurs after cellular exposure to PM2.5 to help better understand the mechanisms underlying cellular damage (Fig. 2).
3 Methodology
The research question guiding the current Systematic Literature Review was: “What are the PM2.5 effects on different systems?” The present study aimed to assess PM2.5 exposure-related risks to human health, based on experimental and epidemiological findings. Inclusion and exclusion criteria were adjusted to generate sensitive research, as described: 1) articles published in internationa1 journals; 2) studies on exposure to PM2.5 that have excluded PM10 and PM0.1; and 3) articles published in peer-reviewed journals. A search for eligible experimental and epidemiological studies published from January 2016 to December 2021 was carried out in the Web of Science database (Fig. 3).
The following descriptors were used: “fine particulate matter and systems’ diseases” (respiratory, cardiovascular, kidney, gastrointestinal, neurological and reproductive); “fine particulate matter and toxicity”; “fine particulate matter and COVID-19 or Sars-CoV-2”. The herein used descriptors resulted in 4,413 articles, as shown (Fig. 3 and Table 2).
Applied the search in the databases mentioned above, the works found were downloaded and uploaded to the Rayyan semi-automatic platform. Duplicates and triplicates were detected automatically, but the exclusions were made manually. Next, 26 of the eligibility criteria for selecting articles by titles and abstracts were applied. After the inclusion and exclusion criteria application, 109 articles were eligible for the current review. Using the Rayyan software, the most referred keyword found was “particulate.” Two authors in the current study have independently analyzed articles’ titles, abstracts and full texts, based on the herein adopted inclusion and exclusion criteria.
4 Results
4.1 Systems’ Imbalance Versus Exposure to PM 2.5
4.1.1 Respiratory System and Exposure to PM2.5
With respect to exposure to PM2.5 types, individuals’ respiratory system is the major pathway enabling PM penetration in, and damage to lung tissues (Kilian and Kitazawa, 2018; Yang et al., 2019). Scientific evidence has suggested an association between respiratory system exposure to PM2.5 and exacerbation of pre-existing cardiopulmonary diseases, which, in their turn, leads to increased morbidity and mortality rates (Lin et al., 2016a; Morantes-Caballero et al., 2019). Studies observed respiratory disorders, such as chronic obstructive pulmonary disease (COPD), pneumonia, lung cancer, asthma and morphological changes in the epithelium of individuals exposed to this pollutant (Gharibvand et al., 2017; Jung et al., 2019; Liu et al., 2017a, 2017b; Lv et al., 2017; Sun et al., 2017; Yoshizaki et al., 2017; Zhang et al., 2020; Zhao et al., 2018). Data derived from population cohort studies have indicated that PM2.5 induced systemic inflammatory response and increased hospitalization rates due to lung diseases, such as asthma (Tian et al., 2017), pneumonia, and influenza (Croft et al., 2019; Zhang et al., 2017). Asthma is the most common respiratory disease associated with exposure to PM2.5. Jung and collaborators (2019) conducted a cohort study comprising children born in Taichung City from 2004 to 2011. Results have evidenced the incidence of this pathology after individuals were exposed to PM2.5 during pregnancy and childhood, as well as a positive correlation between such exposure and subsequent asthma emergence. Tian et al. (2017) investigated PM2.5 effects on asthma-related morbidity rates in Beijing City, China (2010–2012) and found an association between high PM2.5 levels and the risk of asthma worsening. The ssociation between pediatric pneumonia and environmental PM2.5 levels (10 µg m−3) were reported for Lv and collaborators (2017) , since the interaction between PM2.5 compounds and individuals’ respiratory epithelium that induced an inflammatory response and, in its turn, increased children’s hospitalization rate due to pneumonia. Another study adopted case-crossover methods to analyze adult individuals in New York City, from 2005 to 2016. The authors of the aforementioned study found increased pneumonia and influenza rates associated with increased PM2.5 concentrations the week before disease diagnosis (Croft et al., 2019). A cross-sectional study conducted in four cities of Guangdong province, in Southern China, associated PM2.5 levels with an increased risk of chronic COPD development. COPD prevalence was significantly associated with high PM2.5 concentrations (10 µg m−3), and with the spirometric decrease in individuals forced expiratory volume and vital capacity, indicating decreased lung capacity (Liu et al., 2017b). In meta-analysis study performed by Li et al., (2016a, 2016b) associated the same increase (10 μg m−3) in daily exposure to PM2.5 with increase by 3.1% in the rate of hospitalizations due to COPD, and by 2.5%, in COPD-related mortality rates. In addition, Chinese study conducted with school-age children exposed to acute PM2.5 levels has shown airway inflammation, pulmonary function loss and changes in oral mucosal microbiota (Wu et al., 2021). The authors of the aforementioned study have also suggested that long-term exposure to this pollutant can lead to different respiratory disease-development patterns. In addition to increased respiratory disease development caused by impaired lung function, PM can also increase the risk of lung cancer incidence rates (Raaschou-Nielsen et al., 2016; Zhang et al., 2020). According to recent studies, the genotoxicity caused by PM2.5 is key mechanism in cancer development processes (Gharibvand et al., 2017; Zhang et al., 2020). Accordingly, a study focused on monitoring a Canadian cohort exposed to PM2.5, based on remote sensing data, has shown that lung cancer incidence was also associated with prolonged periods of PM2.5 presence in airborne (Tomczak et al., 2016). Factors such as air pollution enhance the chance of lung cancer development with the elevation of 10 μg m−3 of PM2.5 in airborne (Gharibvand et al., 2017). Likewise, a study conducted in the US has shown that lung cancer rates increased by 31% as PM2.5 concentrations increased by 10 µg m−3 (Gharibvand et al., 2017). An experimental study conducted with rats indicated worsened asthma after exposure to PM2.5 for 4 h per day, for 8 weeks (Zhao et al., 2018). Yoshizaki et al. (2017) compared the PM2.5 effects in male and female mice and, based on the analyzed parameters reported differences in PM2.5 response between sexes; the male sex was more susceptible to exposure to PM. Interactions among estrogen-like molecules, such as polycyclic aromatic hydrocarbons in PM2.5 can explain this differences. Airway epithelial cells are the first line of defense against PM2.5; besides working as barrier, they account for mucociliary clearance, as well as for secreting antimicrobial proteins and peptides. According to He et al. (2017), exposure to PM2.5 can affect mucociliary movement in rats by increasing mucus production. Furthermore, morphological injuries and changes in lung tissue during short-term exposure to PM2.5, such as alveolar collapse, neutrophilic inflammation and increased TNF-α production, were also reported (Wang et al., 2017a). A study conducted in vivo with mice has proved that PM2.5 triggers lung fibrosis, since lung inflammation and fibrosis symptoms were observed after models’ exposure to this pollutant (Xu et al., 2019). Other studies have indicated that individuals’ exposure to PM2.5 is an important epidemiological factor for respiratory disease development and/or worsening, as well as that such exposure could be associated with the population’s increased susceptibility to different lung disorders (Hehua et al., 2017). Yang et al. (2018) have shown that rats exposed to PM2.5 for two weeks presented increased total protein expression levels in bronchoalveolar lavage fluid, as well as increased levels of cytokines, such as IL-6, IL-10, and MCP-1, in blood tissue. Such an exposure has also activated macrophages, induced oxidative stress and generated acute inflammatory response, which led to damage in lung tissues. According to Jeong et al. (2019), tracheal exposure to PM2.5 led to macrophage activation in the lung tissue of mice, as well as significant changes in the IL-17 signaling pathway.
4.1.2 Cardiovascular Damages and Exposure to PM2.5
Exposure to PM2.5 can reach distant organs causing numerous dysfunctions in individuals exposed to it. Among them, one finds the cardiovascular system, which is significantly affected by air pollution and presents several changes already reported in experimental studies (Dai et al., 2017; Li et al., 2017; Ribeiro et al., 2016; Wan et al., 2019). Epidemiological studies conducted with humans have indicated an association between increased PM2.5 concentrations in inhaled air and disorders, such as cardiac arrhythmias (Folino et al., 2017; Yang et al., 2020), cardiac fibrillation (Gallo et al., 2020; Kim et al., 2019), hypertension (Fuks et al., 2016; Honda et al., 2018) and variations in heart rate (Xie et al., 2018). Chen et al. (2017c) have analyzed individuals with pre-existing cardiovascular comorbidities and their association with environmental exposure to PM2.5. Their findings have evidenced a decline in indices, such as heart rate, autonomic imbalance, increased thrombosis development risk and inflammation state. Epidemiological cohort study was performed in China with participants aged 18–80, who lived in different geographic regions and were exposed to different pollution rates, by considering mean PM2.5 concentrations in the air, from 2014 to 2016 (Cao et al., 2021). Collected data have indicated that long-term exposure to PM2.5 was significantly associated with first-stage hypertension parameters—i.e., systolic blood pressure (SBP): 130–139 Torr and diastolic blood pressure (DBP): 80–89 Torr (Cao et al., 2021). Lozano-Sabido et al. (2021) conducted an epidemiological study associated with air pollution in individuals hospitalized in Mexico City who presented with pre-existing coronary disease. This work was accomplished from January 2012 to April 2019 and results have shown ST segment elevation in the electrocardiogram, which was associated with myocardial infarction owing raise PM2.5 concentrations. Another epidemiological study investigated the effects of exposure to PM2.5 on variations in the heart rate of 35 individuals living in an urban community in Taiwan. The adopted correlated variables comprised different weather conditions, seasons, and normal-weight and overweight individuals; PM2.5 mass concentrations were monitored in real-time. The major finding in the aforementioned study concerned changes in the heart rate of overweight individuals right after their exposure to PM2.5, compared to that of normal-weight individuals. This report corroborates the understanding that being overweight is a risk factor for the development of cardiovascular disease and that exposure to pollutants can worsen this condition (Tsou et al., 2021). Experimental studies have also provided evidence that exposure to PM2.5 leads to tissue damage. Li et al. (2017) investigated the association between exposure to PM2.5 and myocardial infarction (MI) in mice models exposed to this pollutant through intranasal instillation. Collected data have indicated cardiac remodeling due to increased apoptosis (increased Caspase 3 and Bax expression), reduced left ventricular function and increased size of infarction area. Ribeiro et al. (2016) exposed rat models to PM2.5 samples collected from two different areas (urban and industrial). Their findings comprised metal bioaccumulation, increased oxidative stress, as well as histological changes, such as decreased number of cardiomyocytes and an increased number of fibroblast cells in the heart of rats from both regions. Wan et al. (2019) observed an association between exposure to PM2.5 and atherosclerosis development in the vascular system of ApoE −/− mice. The aforementioned models presented increased IL-6 and TNF-α cytokine levels, which account for accelerating atherosclerosis progress, as well as decreased IL-10 and TGF-β levels that, in turn, inhibit atherosclerotic plaques’ formation. Heart failure induction in mice, which were subsequently exposed to PM2.5, resulted in inflammation and vascular remodeling in their lungs, as well as in cardiac hypertrophy (Yue et al., 2019). Furthermore, exposure to PM2.5 was associated with endothelial vascular dysfunction. The study conducted with Sprague Dawley rats exposed to PM2.5 for 4 weeks reported abnormalities in miRNA (miR-21) which account for regulating the endothelial barrier; these abnormalities resulted in vascular endothelial-cadherin gene regulation suppression and led to endothelial integrity impairment (Dai et al., 2017). A recent study conducted invitro by Santa-Helena et al. (2021) investigated PM2.5’s toxicological effects on cardioblast cells (H9c2). The cells were treated with PM2.5 extracts, at different concentrations, for 24 h. Post-treatment findings comprised cell damage, evidenced by increased lactate dehydrogenase activity, lipid membrane damage, increased ROS formation and decreased antioxidant catalase activity.
4.1.3 Nervous System and Exposure to PM2.5
Several studies associated neurotoxicity with inhaled PM2.5, which reaches the central nervous system through individuals’ bloodstream and olfactory pathways (Wang et al., 2017c). Reports in the literature have indicated strong association between exposure to PM and a wide variety of neurological disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and dementia. Younan et al. (2020) conducted a study in California (USA) and observed episodic memory decline in women in the age group over 65 years, who did not have dementia symptoms. Results suggested a correlation between such a decline and AD caused by grey matter atrophy due to long exposure to PM2.5. Kioumourtzoglou et al. (2016) had previously observed correlations between long-term exposure to PM2.5 and hospitalizations due to neurological causes in epidemiological studies carried out in different cities in the United States. Collected data have indicated an association between exposure to PM2.5 and the incidence of AD, PD and dementia. Mortamais et al. (2019) also linked behavioral disorders to air pollution, in a study carried out in Barcelona (Spain). Maternal exposure to PM2.5 during the third pregnancy trimester has indicated that an increase of 7 μg m−3 in the concentration of this pollutant in the household was associated with decreased volume in children’s corpus callosum. This finding can be linked to behavioral changes. Female pregnant and lactating rats were exposed to traffic tunnel PM2.5 (200 μg m−3) over 6 weeks in order to assess nervous tissue formation in their offspring. Newborn rats exposed to PM2.5 recorded lower scores for social activities, as well as presented anxiety and decreased cognition levels; changes observed in their social behavior suggested an association with autism (Nephew et al., 2020). Kang et al. (2021) developed a cell-based human brain model to investigate neuroinflammation induced by chronic pulmonary exposure to PM2.5. They reported that such an interaction can disrupt the tight junctions between adjacent endothelial cells, increase neuroinflammation levels and compromise astrocytes, as well as that these impairments can lead to neurodegeneration. The association between AD development and exposure to PM2.5 was investigated in a study conducted in vitro by Wang et al.(2018), who treated mouse microglial cells (C57BL/6) with PM2.5 (50 µg mL−1) for 4 h. Findings comprised neuronal lesions, inflammatory state, apoptotic pathway induction and increased ROS formation (Wang et al., 2018). In addition, experimental studies conducted with an AD model exposed to PM2.5 observed glial activation and increased β—amyloid (Aβ) deposits, which are indicators of AD in the hippocampus of individuals exposed to this pollutant (Jang et al., 2018). Furthermore, astrocyte models investigated in study conducted in vitro produced a significant number of pro-inflammatory chemokines and cytokines capable of recruiting and activating microglia. This outcome has indicated that brains exposed to PM2.5 are more susceptible to present synaptic dysfunction and to develop dementia (Kang et al., 2021). In addition, an assay conducted in vitro with SH-SY5Y cells exposed to PM2.5 has indicated changes in DNA methylation and mRNA expression of genes likely linked to autism (Wei et al., 2016). Thus, if one consider PM2.5’s effect on neurodevelopment and nervous functions, it is essential to investigate the mechanism underlying neurodegeneration associated with air pollution to help minimize neurological injuries associated with this process.
4.1.4 Renal System and Exposure to PM2.5
Environmental exposure to PM2.5 has been associated with kidney issues, such as chronic kidney disease (CKD) (Blum et al., 2020; Bo et al., 2021; Bragg-Gresham et al., 2018; Chan et al., 2018; Li et al., 2021), progression to end-stage kidney disease (ESKD) (Bowe et al., 2018) and lower glomerular filtration (GFR) (Mehta et al., 2016). The combination of pre-existing CKD and exposure to PM2.5 can lead to cardiovascular dysfunction development (Ran et al., 2020). PM2.5-CKD associations in Taiwanese individuals were investigated for an extended period, based on GFR analysis. Results have evidenced that an increase of 10 µg m−3 in PM2.5 concentrations was associated with significant risk (6%) of developing CKD (Chan et al., 2018). Data have indicated likely ESKD progression associated with an increase of 10 µg m−3 in PM2.5 concentrations, as well as with GFR decrease by more than 30% (Bowe et al., 2018). Bo et al. (2021) reported association between PM2.5 concentration and CKD incidence; they also observed that a reduction in airborne PM2.5 (5 μg m−3) concentrations was associated with significant CKD decline (Bo et al., 2021). A study reported kidney injuries in Spontaneously Hypertensive Rats (SHR) subjected to subchronic exposure to environmental PM2.5 (29.4 µg m−3, over three months). The aforementioned study has evidenced a slight increase in the number of leukocytes in the renal interstice, fibrosis, decreased number of glomerular lumens and increased urea level in the investigated models (Tavera Busso et al., 2018). Inhalation-based exposure to PM2.5 for 5 h per day, four days per week, for eight weeks in rats also resulted in kidney dysfunction, featured by altered kidney injury markers, such as creatinine β-2-microglobulin and cystatin- C. Exposure to PM2.5 has also contributed to investigated individuals’ fibrotic state (Aztatzi-Aguilar et al., 2016). Renal redox imbalance was observed in rats exposed to PM2.5, as well as a significant decrease in glutamate-cysteine ligase activity and increased glutathione S-transferase activity. Changes observed in total antioxidant capacity (ACAP) in the cortex and renal medulla have shown that the models’ kidneys were sensitive to oxidative stress caused by exposure to PM2.5 (de Paula et al., 2019). Morphological changes (glomerular atrophy, tubular damage), creatinine rise and caspase-3 expression were observed in animals exposed to PM2.5 for six months (Hsu et al., 2019). Although studies focused on assessing the renal system of individuals exposed to PM2.5 have gained room in this research field, it is necessary to conduct further investigations about this topic to help fill the gaps in current knowledge about the effects of this pollutant on individuals’ renal system.
4.1.5 Gastrointestinal System and Exposure to PM2.5
Studies have shown that the gastrointestinal tract and its annex glands are also susceptible to the components present in PM2.5. The exposure of this system owing to air pollution occurs through pulmonary ventilation (way circulation) but also through mucociliary movement of the respiratory system and contaminated water and food (Feng et al., 2020). A study has investigated deaths from liver, colorectal and gastrointestinal cancer and confirmed the positive correlation between them and long-term exposure to PM2.5 (Guo et al., 2020). Similar findings were observed in an epidemiological cohort study conducted in six European countries; results have indicated the development of gastric cancer and risk of death associated with cancer in the upper digestive tract (Weinmayr et al., 2018), as well as in other digestive tract organs, breast and lung (Wong et al., 2016). The epidemiological study in Beijing (China) reported increased peptic ulcer blood loss in elderly individuals who sought emergency hospital services after short-term exposure to increasing PM2.5 concentrations (Duan et al., 2019). In experimental assays, mice exposed to PM2.5 inhalation for 3 weeks have shown changes in intestinal absorptive epithelium, as well as in microbiota composition and quantity, in different parts of the gastrointestinal tract. These changes were associated with the risk of inflammatory processes taking place in the intestine (Mutlu et al., 2018). Inflammation, immune reaction, and metabolic changes were pointed out as likely consequences of changes observed in murine microbiota and gut environment deterioration attributed to exposure to PM2.5 in the study conducted by Liu et al. (2021). Intestinal microbiota coordinates lipid metabolism and triggers inflammatory and intestinal diseases (Feng et al., 2020). Accordingly, metabolic pathways in the liver tissue of mice subjected to PM2.5 instillation were analyzed in the study conducted by Shi et al. (2019); pollutant concentrations of 7.5, 20 and 37.5 mg mL−1 were classified by them as low, moderate and high pollution levels, respectively. Based on results in the aforementioned study, the authors inferred that PM2.5 instillation had changed amino acids’ metabolism, which led to decreased threonine, alanine and serine levels, affected tricarboxylic acid cycle (TCA), changed the urea cycle and increased purine levels, as well as that such an increase has contributed to ROS formation. However, similar to what was reported for the renal system, only few studies focused on investigating bowel diseases resulting from exposure to PM2.5 are available in the literature. Further studies should be conducted to help better understand the origin of the herein mentioned damages and pathologies, as well as to fill the gap in knowledge about the consequences of PM2.5-gastrointestinal system interaction.
4.1.6 Reproductive System and Exposure to PM2.5
Like the others, the reproductive system also seems susceptible to increased levels of atmospheric pollution. A study conducted by Percy et al. (2019), focused on investigating the likely association between increased PM2.5 indices and pregnancy issues observed in the third gestational trimester, from 2007 to 2010. Analyzed data have indicated inadequate fetal size in pregnant women exposed to high PM2.5 indices during the investigated period. Kingsley et al. (2017) observed links between air pollution and changes in the expression of seven genes found in the human placenta that, in turn, can negatively affect fetal growth. Women’s daily exposure to PM2.5, associated with different levels of traumatic events during pregnancy, have indicated mitochondrial changes in cord blood samples. In contrast, placental samples have shown reduced mitochondrial indicators and multiple mitochondrial DNA copies (mtDNAcn), which are used to check the fetal health status (Brunst et al., 2018). Studies have pointed toward a possible association between increased exposure to PM2.5 and worsened placental pathologies, such as fetal thrombotic vasculopathy, as reported in a cohort study conducted with Tanzanian pregnant women (Wylie et al., 2017). An experimental study was recently conducted with mice exposed to concentrated PM2.5 (115.60 ± 7.77 μg m−3) or filtered air (14.07 ± 0.38 μg m−3) for 12 weeks before pregnancy, in order to investigate PM2.5-exposure effects on mice reproductive system (Guo et al., 2021). The investigated experimental model presented decreased anti-Müllerian hormone and oocyte levels. This outcome has evidenced that PM2.5 triggered ROS production increase and degeneration, altered mitochondrial expression genes, increased degeneration rate observed for these cells and interfered in embryos’ development (Guo et al., 2021). According to Liu et al. (2016b), pregnant rats exposed to PM2.5 through intratracheal instillation (15 mg kg−1, in two days) have shown reduced maternal and fetal weight, as well as placental thrombus, whereas blood analysis has evidenced an increased number of platelets, IL-6 and mononuclear cells. Based on an experimental study conducted by Soto et al. (2017), Wistar rats exposed to PM2.5 (15 days, 600 μg m−3 per day) during pregnancy presented changes in placental mass, size and surface area. According to the aforementioned study, renin-angiotensin system destabilization in the placenta was observed, as well as decreased levels of both angiotensin II and its receptors (AT1R and AT2R) (Soto et al., 2017). Besides, pregnant rats exposed to PM2.5 (1.0 mg kg−1) through the oropharyngeal route have shown increased blood pressure in adult offspring, as well as reduced sodium and water excretion rates (Ye et al., 2018). Ye et al. (2018) have shown decreased expression of the dopamine receptor, which accounts for blood pressure regulation, sodium excretion, and its regulatory kinase (kinase 4). Table 3 shows a broad overview of damage caused to different tissues, as well as epidemiological and experimental findings associated with PM2.5’s toxic effects.
5 COVID-19 and Exposure to PM2.5
Severe restrictions on people’s movement and economic activities were imposed on global society in 2020, due to the COVID-19 pandemic, and it decreased PM2.5 concentrations in the air from 9 to 60%, if one compares air-quality data from early 2020 to data recorded in the same period in previous years (IQAir 2020). Scholars from different countries have assessed PM2.5 levels in different cities, on all five continents, between December 2019 and March 2020; they compared collected data with those recorded in the same period, in previous years. Studies pointed towards significant reductions, of up to 60%, in PM2.5 concentrations, and it has clearly shown improved air quality (Berman & Ebisu, 2020; Chauhan & Singh, 2020; Collivignarelli et al., 2020; Nakada & Urban, 2020; Sharma et al., 2020; Shrestha et al., 2020). A valid explanation for this phenomenon lies in the fact that anthropogenic PM2.5 levels observed in big cities during regular working days are higher than those observed in smaller and less urbanized cities (Zhao et al., 2009). PM2.5 reduction can also be attributed to a simultaneous reduction in the concentration of other pollutants, such as volatile organic compounds, which act as precursors for PM2.5 formation (Chen et al., 2017a, 2017b, 2017c; Han et al., 2018). An epidemiological study published in 2003 suggested positive correlation between PM concentration and SARS lethality: mortality rate increased by 100 and 84% in patients living in regions recording high and moderate air pollution rates, respectively, in comparison to rates recorded in areas presenting low air pollution index (Cui et al., 2003). This finding was corroborated by another epidemiological study, according to which, each increment by 10 µg m−3 of PM in 5 days resulted in a mean increase of 1.06 in the risk of daily mortality rate linked to SARS (Kan et al., 2005). PM can act as a virus carrier or intensifier, and it has a sublayer capable of keeping the virus alive in air flows, for hours or even days (Setti et al., 2020a). Chen et al. (2017a, 2017b, 2017c) observed an association between short-term exposure to PM and the incidence of measles in several Chinese cities; this finding has confirmed the association between PM and viral diseases. Based on these data, air pollution is expected to play an essential part in the pandemic caused by the new coronavirus, given the positive correlation between the number of COVID-19 cases and PM2.5 concentrations in the air. A recent epidemiological study in Wuhan City investigated the association between PM levels and the COVID-19 lethality rate. A positive correlation was observed between PM10 and PM2.5 concentrations and COVID-19 lethality rate (Yao et al., 2020b). Another national study conducted in China included more than 60 cities in and outside the province most affected by the virus; results have shown that short-term exposure to PM10 and PM2.5 was strongly associated with COVID-19 mortality rates (Yao et al., 2020a). The European Public Health Alliance recently reported that individuals living in polluted cities are the ones mostly threatened by COVID-19 disease (European Public Health Alliance 2020), a fact that was confirmed in further research conducted in Italy. Recent studies have suggested that the expansion of COVID-19 cases in Northern Italy, mainly in Lombardy, was promoted by high PM concentrations in the air, based on the idea that atmospheric particles act as virus carriers (Sterpetti, 2020). Furthermore, only 0.003% of dwellers in the least polluted provinces were infected with this virus, whereas highly polluted regions recorded approximately nine times more COVID-19 cases than the least polluted ones (Setti et al., 2020b, 2020c). Two independent studies have evidenced that continuous exposure to particulate matter in Northern Italy was the leading cause of COVID-19 cases (Fattorini & Regoli, 2020; Coker et al., 2020). A United States national comprehensive cross-sectional study has shown that each increase of 1 µg cm−3 in long-term exposure to PM2.5 significantly increased COVID-19-related mortality rates (Wu et al., 2020). A similar observation was made in the Netherlands, where exposure to PM2.5 was closely associated with confirmed COVID-19 cases since an increase of approximately 1/5 in pollution concentrations has doubled the number of COVID-19 cases (Andree, 2020). Studies conducted so far aimed at investigating individuals’ sensitivity to the SARS-CoV-2 virus after exposure to PM2.5. However, only epidemiological studies have suggested biomolecular mechanisms acting behind this sensitivity; however, it is yet to be experimentally confirmed through studies conducted both in vitro and in vivo. In addition to reduced PM levels during the COVID-19 pandemic, studies have suggested that long-term exposure to PM2.5 was associated with higher likelihood of hospitalization among patients with COVID-19. PM2.5 can impair both the mucociliary clearance of different pathogens and natural killer cell response, which can increase individuals’ susceptibility to and severity of COVID-19 (Mendy et al., 2021). Changes in host defense mechanisms caused by exposure to PM2.5 may be the key to individuals’ susceptibility to respiratory system infections. The study review conducted by Yang et al. (2020) has shown that exposure to PM2.5 enables the adhesion, colonization and growth of different microorganisms, such as viruses, and makes removing them from individuals’ respiratory system hard. Likely mechanisms pointed out by the aforementioned authors may be associated with mitigation of the host defense function played by the airway epithelium, changes in the natural microbiota of the respiratory tract and insufficient number of, or dysfunction in, immune cells. According to Loaiza-Ceballos et al. (2021), exposure to air pollutants, such as PM, induces ROS production, which, in its turn, leads to an oxidative state, to the activation of transcription factors such as NF-kB and AP-1 and, subsequently, to cytokine production. These mechanisms worsen inflammatory processes in the respiratory tract by changing the respiratory tract homeostasis and making it more susceptible to viral infections (Loaiza-Ceballos et al., 2021). Likewise, pre-existing baseline inflammatory conditions worsened by pollution can easily contribute to better explain why individuals exposed to pollution appear to be at greater risk of developing severe COVID-19 cases (Gao et al., 2020, 2021).
6 Conclusion
The present study addressed the effects of PM2.5 in several potential health-damage contexts, by comparing epidemiological studies to experimental findings. Based on the analyzed results, PM2.5 is strongly associated with the pathological genesis of the most diverse systems. Its toxicological potential is linked to inflammation generation pathways, which comprise genes involved in inflammation processes and signaling molecules. Based on the analysis of the herein selected studies, PM2.5 is remarkably capable of generating ROS, which is well-known for its involvement in inflammatory processes, cell death, genotoxicity, and epigenetic changes. It was also proved that the antioxidant defenses of individuals exposed to PM2.5 are affected and contribute to the emergence of cellular oxidative stress. PM2.5 an damage several organs, likely due to its ability to travel through individuals’ bloodstream and cause systemic damage. Organs are affected at the cellular level, and injury accumulation associated with exposure time can generate different pathologies, as seen in the epidemiological studies mentioned above. Cardiopulmonary, nervous, renal, gastrointestinal, and even the reproductive system, suffer from exposure to PM2.5, which harms individuals’ health and hinders their quality of life. Studies linking PM2.5 emissions to the current COVID-19 pandemic were performed in 2020. They reported a significant decrease in PM2.5 emissions when different cities and countries adopted partial or total lockdown measures. Interestingly, several researchers associated high environmental PM2.5 concentrations with a higher likelihood of being contaminated with and, consequently, of dying of COVID-19. This phenomenon can be related to virus permanence in PM2.5 particles; therefore, places presenting a larger number of particles would also enable longer virus permanence. Thus, although many studies focus on explaining mechanisms linked to the toxicity caused by PM2.5, several points need to be further investigated to help better understand PM2.5 interactions in the body. Nevertheless, these data, altogether, make us reason about how important it is to establish measures to help mitigate PM2.5 emissions and avoid its associated damages (Fig. 4).
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Al-Thani, H., Koç, M., & Isaifan, R. J. (2018). A review on the direct effect of particulate atmospheric pollution on materials and its mitigation for sustainable cities and societies. Environmental Science and Pollution Research, 25, 27839–27857. https://doi.org/10.1007/s11356-018-2952-8
Andree, B. P. J. (2020). Incidence of COVID-19 and Connections with Air Pollution Exposure: Evidence from the Netherlands. World Bank Policy Res Work Pap. https://doi.org/10.1101/2020.04.27.20081562
Aztatzi-Aguilar, O. G., Uribe-Ramírez, M., Narváez-Morales, J., et al. (2016). Early kidney damage induced by subchronic exposure to PM2.5 in rats. Particle and Fibre Toxicology, 13, 1–20. https://doi.org/10.1186/s12989-016-0179-8
Berman, J. D., & Ebisu, K. (2020). Changes in U.S. air pollution during the COVID-19 pandemic. Science of the Total Environment, 739, 139864. https://doi.org/10.1016/j.scitotenv.2020.139864
Blum MF, Surapaneni A, Stewart JD, Liao D, Yanosky JD, Whitsel EA, ... & Grams ME (2020). Particulate matter and albuminuria, glomerular filtration rate, and incident CKD. Clinical Journal of the American Society of Nephrology, 15: 311–319. https://doi.org/10.2215/CJN.08350719
Bo, Y., Brook, J. R., Lin, C., et al. (2021). Reduced Ambient PM2.5 Was Associated with a Decreased Risk of Chronic Kidney Disease: A Longitudinal Cohort Study. Environmental Science and Technology, 55, 6876–6883. https://doi.org/10.1021/ACS.EST.1C00552
Bowe, B., Xie, Y., Li, T., et al. (2018). Particulate matter air pollution and the risk of incident CKD and progression to ESRD. Journal of the American Society of Nephrology, 29, 218–230. https://doi.org/10.1681/ASN.2017030253
Bragg-Gresham, J., Morgenstern, H., McClellan, W., et al. (2018). County-level air quality and the prevalence of diagnosed chronic kidney disease in the US Medicare population. PLoS ONE, 13, 1–13. https://doi.org/10.1371/journal.pone.0200612
Brunst, K. J., Sanchez-Guerra, M., Chiu, Y. H. M., et al. (2018). Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: Effect modification by maternal lifetime trauma and child sex. Environment International, 112, 49–58. https://doi.org/10.1016/j.envint.2017.12.020
Cao, J., Qin, G., Shi, R., et al. (2016). Overproduction of reactive oxygen species and activation of MAPKs are involved in apoptosis induced by PM2.5 in rat cardiac H9c2 cells. Journal of Applied Toxicology, 36, 609–617. https://doi.org/10.1002/jat.3249
Cao, Y., Zhu, M., Guo, Y., et al. (2019). Fine particulate matter (PM 2.5) enhances FcεRI-mediated signaling and mast cell function. Cellular Signalling, 57, 102–109. https://doi.org/10.1016/j.cellsig.2019.01.010
Cao H, Li B, Liu K, et al (2021) Association of long-term exposure to ambient particulate pollution with stage 1 hypertension defined by the 2017 ACC/AHA Hypertension Guideline and cardiovascular disease: The CHCN-BTH cohort study. Environ Res 199:. https://doi.org/10.1016/J.ENVRES.2021.111356
Chan TC, Zhang Z, Lin BC, et al (2018) Long-term exposure to ambient fine particulate matter and chronic kidney disease: A cohort study. Environ Health Perspect 126:. https://doi.org/10.1289/EHP3304
Chauhan, A., & Singh, R. P. (2020). Decline in PM25 concentrations over major cities around the world associated with COVID-19. Environmental Research, 187, 109634. https://doi.org/10.1016/j.envres.2020.109634
Chen, G., Zhang, W., Li, S., et al. (2017a). Is short-term exposure to ambient fine particles associated with measles incidence in China? A multi-city study. Environmental Research, 156, 306–311. https://doi.org/10.1016/j.envres.2017.03.046
Chen, M., Li, B., & Sang, N. (2017b). Particulate matter (PM2.5) exposure season-dependently induces neuronal apoptosis and synaptic injuries. Journal of Environmental Sciences (china), 54, 336–345. https://doi.org/10.1016/j.jes.2016.10.013
Chen, S. Y., Chan, C. C., & Su, T. C. (2017c). Particulate and gaseous pollutants on inflammation, thrombosis, and autonomic imbalance in subjects at risk for cardiovascular disease. Environmental Pollution, 223, 403–408. https://doi.org/10.1016/j.envpol.2017.01.037
Coker, E. S., Cavalli, L., Fabrizi, E., Guastella, G., Lippo, E., Parisi, M. L., Pontarollo, N., Rizzati, M., Varacca, A., & Vergalli, S. (2020). The Effects of Air Pollution on COVID-19 Related Mortality in Northern Italy. Environmental and Resource Economics, 76, 611–634. https://doi.org/10.1007/s10640-020-00486-1
Collivignarelli, M. C., Abbà, A., Bertanza, G., et al. (2020). Lockdown for CoViD-2019 in Milan: What are the effects on air quality? Science of the Total Environment, 732, 139280. https://doi.org/10.1016/j.scitotenv.2020.139280
Crobeddu, B., Baudrimont, I., Deweirdt, J., et al. (2020). Lung Antioxidant Depletion: A Predictive Indicator of Cellular Stress Induced by Ambient Fine Particles. Environmental Science and Technology, 54, 2360–2369. https://doi.org/10.1021/acs.est.9b05990
Croft DP, Zhang W, Lin S, Thurston SW, Hopke PK, Masiol M, ... & Rich DQ, (2019). The association between respiratory infection and air pollution in the setting of air quality policy and economic change. Annals of the American Thoracic Society, 16:321–330. https://doi.org/10.1513/AnnalsATS.201810-691OC
Cui, Y., Zhang, Z. F., Froines, J., et al. (2003). Air pollution and case fatality of SARS in the People’s Republic of China: An ecologic study. Environ Heal A Glob Access Sci Source, 2, 1–5. https://doi.org/10.1186/1476-069X-2-1
Dai, J., Chen, W., Lin, Y., et al. (2017). Exposure to concentrated ambient fine particulate matter induces vascular endothelial dysfunction via miR-21. International Journal of Biological Sciences, 13, 868–877. https://doi.org/10.7150/ijbs.19868
de Paula, R. J., Kalb, A. C., de Bastos, M. S., et al. (2019). The impact of polar fraction of the fine particulate matter on redox responses in different rat tissues. Environmental Science and Pollution Research, 26, 32476–32487. https://doi.org/10.1007/s11356-019-06452-9
de Ribeiro, J., P, Kalb AC, Campos PP, et al. (2016). Toxicological effects of particulate matter (PM2.5) on rats: Bioaccumulation, antioxidant alterations, lipid damage, and ABC transporter activity. Chemosphere, 163, 569–577. https://doi.org/10.1016/j.chemosphere.2016.07.094
Dornhof, R., Maschowski, C., Osipova, A., et al. (2017). Stress fibers, autophagy and necrosis by persistent exposure to PM2.5 from biomass combustion. PLoS ONE, 12, 1–20. https://doi.org/10.1371/journal.pone.0180291
Duan, R., Tian, Y., Hu, Y., & Duan, L. (2019). Exploring the association between short-term exposure to ambient fine particulate matter pollution and emergency admissions for peptic ulcer bleeding in Beijing, China. Atmospheric Environment, 213, 485–490. https://doi.org/10.1016/j.atmosenv.2019.06.037
European Public Health Alliance (2020) Coronavirus threat greater for polluted cities.
Fang, T., Lakey, P. S. J., Weber, R. J., & Shiraiwa, M. (2019). Oxidative Potential of Particulate Matter and Generation of Reactive Oxygen Species in Epithelial Lining Fluid. Environmental Science and Technology, 53, 12784–12792. https://doi.org/10.1021/acs.est.9b03823
Fattorini, D., & Regoli, F. (2020). Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environmental Pollution, 264, 114732. https://doi.org/10.1016/j.envpol.2020.114732
Feng, J., Cavallero, S., Hsiai, T., & Li, R. (2020). Impact of air pollution on intestinal redox lipidome and microbiome. Free Radical Biology & Medicine, 151, 99–110. https://doi.org/10.1016/j.freeradbiomed.2019.12.044
Ferraro, S. A., Astort, F., Yakisich, J. S., & Tasat, D. R. (2016). Particulate matter cytotoxicity in cultured SH-SY5Y cells is modulated by simvastatin: Toxicological assessment for oxidative damage. Neurotoxicology, 53, 108–114. https://doi.org/10.1016/j.neuro.2016.01.003
Folino, F., Buja, G., Zanotto, G., et al. (2017). Association between air pollution and ventricular arrhythmias in high-risk patients (ARIA study): A multicentre longitudinal study. Lancet Planet Heal, 1, e58–e64. https://doi.org/10.1016/S2542-5196(17)30020-7
Fu, Q., Lyu, D., Zhang, L., et al. (2017). Airborne particulate matter (PM2.5) triggers autophagy in human corneal epithelial cell line. Environmental Pollution, 227, 314–322. https://doi.org/10.1016/j.envpol.2017.04.078
Fuks, K. B., Weinmayr, G., Hennig, F., et al. (2016). Association of long-term exposure to local industry- and traffic-specific particulate matter with arterial blood pressure and incident hypertension. International Journal of Hygiene and Environmental Health, 219, 527–535. https://doi.org/10.1016/j.ijheh.2016.05.008
Gallo, E., Folino, F., Buja, G., et al. (2020). Daily exposure to air pollution particulate matter is associated with atrial fibrillation in high-risk patients. International Journal of Environmental Research and Public Health, 17, 1–10. https://doi.org/10.3390/ijerph17176017
Gao, Z. X., Song, X. L., Li, S. S., et al. (2016). Assessment of DNA damage and cell senescence in corneal epithelial cells exposed to airborne particulate matter (PM2.5) collected in Guangzhou. China. Investig Ophthalmol vis Sci, 57, 3093–3102. https://doi.org/10.1167/iovs.15-18839
Gao, F., Zheng, K. I., Wang, X.-B., et al. (2020). Obesity Is a Risk Factor for Greater COVID-19 Severity. Diabetes Care, 43, e72–e74. https://doi.org/10.2337/dc20-0682
Gao, F., Zheng, K. I., Wang, X. B., et al. (2021). Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. Journal of Gastroenterology and Hepatology, 36, 204–207. https://doi.org/10.1111/jgh.15112
Gharibvand, L., Lawrence Beeson, W., Shavlik, D., et al. (2017). The association between ambient fine particulate matter and incident adenocarcinoma subtype of lung cancer. Environ Heal A Glob Access Sci Source, 16, 1–9. https://doi.org/10.1186/s12940-017-0268-7
Guan, Y., Kang, L., Wang, Y., Zhang, N. N., & Ju, M. T. (2019). Health loss attributed to PM2. 5 pollution in China’s cities: Economic impact, annual change and reduction potential. Journal of Cleaner Production, 217, 284–294. https://doi.org/10.1016/j.jclepro.2019.01.284
Guo, C., Chan, T. C., Teng, Y. C., et al. (2020). Long-term exposure to ambient fine particles and gastrointestinal cancer mortality in Taiwan: A cohort study. Environment International, 138, 105640. https://doi.org/10.1016/j.envint.2020.105640
Guo Z, Hong Z, Dong W, Deng C, Zhao R, Xu J, ... & Zhang R (2017). PM2. 5-induced oxidative stress and mitochondrial damage in the nasal mucosa of rats. International journal of environmental research and public health, 14:134. https://doi.org/10.3390/ijerph14020134
Guo Y, Cao Z, Jiao X, et al (2021) Pre-pregnancy exposure to fine particulate matter (PM2.5) increases reactive oxygen species production in oocytes and decrease litter size and weight in mice. Environ Pollut 268:. https://doi.org/10.1016/J.ENVPOL.2020.115858
Han, D., Gao, S., Fu, Q., et al. (2018). Do volatile organic compounds (VOCs) emitted from petrochemical industries affect regional PM2.5? Atmospheric Research, 209, 123–130. https://doi.org/10.1016/j.atmosres.2018.04.002
Harris CJ, Scheibe M, Wongpalee SP, et al (2018) A DNA methylation reader complex that enhances gene transcription. Science (80- ) 362:1182–1186. https://doi.org/10.1126/science.aar7854
Hassani, M., Brown, J. M., Morandi, M. T., & Holian, A. (2004). Particulate matter immunomodulatory effects on autoantibody development in New Zealand mixed mice. Journal of Immunotoxicology, 1, 95–102. https://doi.org/10.1080/15476910490505644
He F, Liao B, Pu J, Li C, Zheng, M., Huang, L., ... & Ran, P. (2017). Exposure to ambient particulate matter induced COPD in a rat model and a description of the underlying mechanism. Scientific reports, 7(1), 1–15. https://doi.org/10.1038/srep45666
Hehua, Z., Qing, C., Gaoyan, G., et al. (2017). The impact of prenatal exposure to air pollution on childhood wheezing and asthma: A systematic review. Environmental Research, 159, 519–530. https://doi.org/10.1016/J.ENVRES.2017.08.038
Herman, J., Zhang, Y., Castranova, V., & Neal, S. L. (2018). Emerging technologies for optical spectral detection of reactive oxygen species. Analytical and Bioanalytical Chemistry, 410, 6079–6095. https://doi.org/10.1007/s00216-018-1233-1
Honda, T., Pun, V. C., Manjourides, J., & Suh, H. (2018). Associations of long-term fine particulate matter exposure with prevalent hypertension and increased blood pressure in older Americans. Environmental Research, 164, 1–8. https://doi.org/10.1016/j.envres.2018.02.008
Hsu, Y. H., Chuang, H. C., Lee, Y. H., et al. (2019). Traffic-related particulate matter exposure induces nephrotoxicity in vitro and in vivo. Free Radical Biology & Medicine, 135, 235–244. https://doi.org/10.1016/j.freeradbiomed.2019.03.008
IQAir (2020) Covid-19 Air Quality report. 1–14
Jan, R., Roy, R., Bhor, R., et al. (2020). Toxicological screening of airborne particulate matter in atmosphere of Pune: Reactive oxygen species and cellular toxicity. Environmental Pollution, 261, 113724. https://doi.org/10.1016/j.envpol.2019.113724
Jang, S., Kim, E. W., Zhang, Y., et al. (2018). Particulate matter increases beta-amyloid and activated glial cells in hippocampal tissues of transgenic Alzheimer’s mouse: Involvement of PARP-1. Biochemical and Biophysical Research Communications, 500, 333–338. https://doi.org/10.1016/j.bbrc.2018.04.068
Jeong S, Park SA, Park I, Kim P, Cho NH, Hyun JW, & Hyun YM, (2019). PM2. 5 Exposure in the respiratory system induces distinct inflammatory signaling in the lung and the liver of mice. Journal of immunology research, 2019. https://doi.org/10.1155/2019/3486841
Jiang, Y., Li, J., Ren, F., Ji, C., Aniagu, S., & Chen, T. (2019). PM2. 5-induced extensive DNA methylation changes in the heart of zebrafish embryos and the protective effect of folic acid. Environmental Pollution, 255, 113331. https://doi.org/10.1016/j.envpol.2019.113331
Jin, X., Xue, B., Zhou, Q., Su, R., & Li, Z. (2018). Mitochondrial damage mediated by ROS incurs bronchial epithelial cell apoptosis upon ambient PM2. 5 exposure. The Journal of Toxicological Sciences, 43, 101–111. https://doi.org/10.2131/jts.43.101
Jin L, Xie J, Wong CK, Chan SK, Abbaszade G, Schnelle-Kreis J, ... & Li X (2019). Contribuições do material particulado fino específico da cidade (PM2. 5) para diferenças de estresse oxidativo in vitro e implicações de toxicidade entre Pequim e Guangzhou da China. Ciência e tecnologia ambiental , 53: 2881–2891. https://doi.org/10.1021/acs.est.9b00449
Jung, C. R., Chen, W. T., Tang, Y. H., & Hwang, B. F. (2019). Fine particulate matter exposure during pregnancy and infancy and incident asthma. The Journal of Allergy and Clinical Immunology, 143, 2254-2262.e5. https://doi.org/10.1016/j.jaci.2019.03.024
Kan, H. D., Chen, B. H., Fu, C. W., et al. (2005). Relationship between ambient air pollution and daily mortality of SARS in Beijing. Biomedical and Environmental Sciences, 18, 1–4.
Kang YJ, Tan H-Y, Lee CY, Cho H (2021) An Air Particulate Pollutant Induces Neuroinflammation and Neurodegeneration in Human Brain Models. Adv Sci 2101251. https://doi.org/10.1002/ADVS.202101251
Kessel, D., & Oleinick, N. L. (2018). Cell Death Pathways Associated with Photodynamic Therapy: An Update. Photochemistry and Photobiology, 94, 213–218. https://doi.org/10.1111/php.12857
Kilian, J., & Kitasawa, M. (2018). The emerging risk of exposure to air pollution on cognitive decline and Alzheimer’s disease e Evidence from epidemiological and animal studies. Biomedical Journal, 141, 141–162. https://doi.org/10.1016/j.bj.2018.06.001
Kim, I. S., Yang, P. S., Lee, J., et al. (2019). Long-term exposure of fine particulate matter air pollution and incident atrial fibrillation in the general population: A nationwide cohort study. International Journal of Cardiology, 283, 178–183. https://doi.org/10.1016/j.ijcard.2018.12.048
Kim W, Jeong SC, Shin CY, Song MK, Cho Y, Lim JH, ... & Ryu JC (2018). A study of cytotoxicity and genotoxicity of particulate matter (PM2. 5) in human lung epithelial cells (A549). Molecular & Cellular Toxicology, 14:163–172. https://doi.org/10.1007/s13273-018-0018-0
Kingsley, S. L., Deyssenroth, M. A., Kelsey, K. T., et al. (2017). Maternal residential air pollution and placental imprinted gene expression. Environment International, 108, 204–211. https://doi.org/10.1016/j.envint.2017.08.022
Kioumourtzoglou, M. A., Schwartz, J. D., Weisskopf, M. G., et al. (2016). Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environmental Health Perspectives, 124, 23–29. https://doi.org/10.1289/ehp.1408973
Lakey, P. S. J., Berkemeier, T., Tong, H., et al. (2016). Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Science and Reports, 6, 1–6. https://doi.org/10.1038/srep32916
Lelieveld, J., Klingmüller, K., Pozzer, A., et al. (2019). Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. European Heart Journal, 40, 1590–1596. https://doi.org/10.1093/eurheartj/ehz135
Lemos, A. T., de Lemos, C. T., Flores, A. N., Pantoja, E. O., Rocha, J. A. V., & Vargas, V. M. F. (2016). Genotoxicity biomarkers for airborne particulate matter (PM2. 5) in an area under petrochemical influence. Chemosphere, 159, 610–618. https://doi.org/10.1016/j.chemosphere.2016.05.087
Li, Y., Rittenhouse-Olson, K., Scheider, W. L., & Mu, L. (2012). Effect of particulate matter air pollution on C-reactive protein: A review of epidemiologic studies. Reviews on Environmental Health, 27, 133–149. https://doi.org/10.1515/reveh-2012-0012
Li, M. H., Fan, L. C., Mao, B., Yang, J. W., Choi, A. M., Cao, W. J., & Xu, J. F. (2016a). Short-term exposure to ambient fine particulate matter increases hospitalizations and mortality in COPD: A systematic review and meta-analysis. Chest, 149(2), 447–458. https://doi.org/10.1378/chest.15-0513
Li, X., Lv, Y., Hao, J., et al. (2016). Role of microRNA-4516 involved autophagy associated with exposure to fine particulate matter. Oncotarget, 7, 45385–45397. https://doi.org/10.18632/oncotarget.9978
Li, X., Geng, J., Chen, Y., et al. (2017). Exposure to particulate matter induces cardiomyocytes apoptosis after myocardial infarction through NFκB activation. Biochemical and Biophysical Research Communications, 488, 224–231. https://doi.org/10.1016/j.bbrc.2017.05.047
Li, D., Zhang, R., Cui, L., et al. (2019a). Multiple organ injury in male C57BL/6J mice exposed to ambient particulate matter in a real-ambient PM exposure system in Shijiazhuang, China. Environmental Pollution, 248, 874–887. https://doi.org/10.1016/j.envpol.2019.02.097
Li, Y., Xu, H., He, K., et al. (2019b). Reactive oxygen species induced by personal exposure to fine particulate matter emitted from solid fuel combustion in rural Guanzhong Basin, northwestern China. Air Qual Atmos Heal, 12, 1323–1333. https://doi.org/10.1007/s11869-019-00747-z
Li Q, Wang YY, Guo Y, Zhou H, Wang QM, Shen HP, ... & Ma X (2021). Association between airborne particulate matter and renal function: an analysis of 2.5 million young adults. Environment International, 147:106348. https://doi.org/10.1016/j.envint.2020.106348
Libalova, H., Milcova, A., Cervena, T., et al. (2018). Kinetics of ROS generation induced by polycyclic aromatic hydrocarbons and organic extracts from ambient air particulate matter in model human lung cell lines. Mutat Res - Genet Toxicol Environ Mutagen, 827, 50–58. https://doi.org/10.1016/j.mrgentox.2018.01.006
Lin H, Liu T, Xiao J, Zeng W, Li X, Guo L, et al. (2016a). Mortality burden of ambient fine particulate air pollution in six Chinese cities: results from the Pearl River Delta study. Environment international, 96, 91–97. https://doi.org/10.1016/j.envint.2016.09.007
Lin, N., & Simon, M. C. (2016). Hypoxia-inducible factors: Key regulators of myeloid cells during inflammation. The Journal of Clinical Investigation, 126, 3661–3671. https://doi.org/10.1172/JCI84426
Liu, R., Young, M. T., Chen, J. C., et al. (2016a). Ambient air pollution exposures and risk of parkinson disease. Environmental Health Perspectives, 124, 1759–1765. https://doi.org/10.1289/EHP135
Liu, A., Qian, N., Yu, H., et al. (2017a). Estimation of disease burdens on preterm births and low birth weights attributable to maternal fine particulate matter exposure in Shanghai, China. Science of the Total Environment, 609, 815–821. https://doi.org/10.1016/j.scitotenv.2017.07.174
Liu, S., Zhou, Y., Liu, S., et al. (2017b). Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: Results from a cross-sectional study in China. Thorax, 72, 788–795. https://doi.org/10.1136/thoraxjnl-2016-208910
Liu, X., Qian, X., Xing, J., et al. (2018). Particulate matter triggers depressive-like response associated with modulation of inflammatory cytokine homeostasis and brain-derived neurotrophic factor signaling pathway in mice. Toxicological Sciences, 164, 278–288. https://doi.org/10.1093/toxsci/kfy086
Liu, Y., Wang, T., Si, B., et al. (2021). Intratracheally instillated diesel PM25 significantly altered the structure and composition of indigenous murine gut microbiota. Ecotoxicology Environment Saf, 210, 111903. https://doi.org/10.1016/J.ECOENV.2021.111903
Liu Y, Wang L, Wang F, Li C (2016b) Effect of fine particulate matter (PM25) on rat placenta pathology and perinatal outcomes. Med Sci Monit 22:3274–3280. https://doi.org/10.12659/MSM.897808
Loaiza-Ceballos, M. C., Marin-Palma, D., Zapata, W., & Hernandez, J. C. (2021). Viral respiratory infections and air pollutants. Air Qual Atmos Heal, 15, 105–114. https://doi.org/10.1007/s11869-021-01088-6
Lozano-Sabido E, Berrios-Barcenas E, Cazares-Diazleal A, et al (2021) “ST-elevation myocardial infarction associated with air pollution levels in Mexico City.” Int J Cardiol Hear Vasc 35:. https://doi.org/10.1016/J.IJCHA.2021.100846
Lv, C., Wang, X., Pang, N., et al. (2017). The impact of airborne particulate matter on pediatric hospital admissions for pneumonia among children in Jinan, China: A case-crossover study. Journal of the Air and Waste Management Association, 67, 669–676. https://doi.org/10.1080/10962247.2016.1265026
Mai AS, Dos Santos AB, Beber LCC, et al (2017) Exercise Training under Exposure to Low Levels of Fine Particulate Matter: Effects on Heart Oxidative Stress and Extra-to-Intracellular HSP70 Ratio. Oxid Med Cell Longev 2017:. https://doi.org/10.1155/2017/9067875
Marchini, T., Wolf, D., Michel, N. A., et al. (2016). Acute exposure to air pollution particulate matter aggravates experimental myocardial infarction in mice by potentiating cytokine secretion from lung macrophages. Basic Research in Cardiology, 111, 1–14. https://doi.org/10.1007/s00395-016-0562-5
Mehta, A. J., Zanobetti, A., Bind, M. A. C., et al. (2016). Long-term exposure to ambient fine particulate matter and renal function in older men: The veterans administration normative aging study. Environmental Health Perspectives, 124, 1353–1360. https://doi.org/10.1289/ehp.1510269
Mendy A, Wu X, Keller J, et al (2021) Long-term exposure to fine particulate matter and hospitalization in COVID-19 patients. Respir Med 178:. https://doi.org/10.1016/J.RMED.2021.106313
Milani, C., Farina, F., Botto, L., Massimino, L., Lonati, E., Donzelli, E., Ballarini, E., Crippa, L., Marmiroli, P., Bulbarelli, A., & Palestini, P. (2020). Systemic Exposure to Air Pollution Induces Oxidative Stress and Inflammation in Mouse Brain, Contributing to Neurodegeneration Onset. International Journal of Molecular Science., 21, 3699. https://doi.org/10.3390/ijms21103699
Miousse, I. R., Koturbash, I., Chalbot, M. C., et al. (2016). Analysis of the ambient particulate matter-induced chromosomal aberrations using an in vitro system. Journal of Visualized Experiments, 2016, 1–7. https://doi.org/10.3791/54969
Morantes-Caballero JA., & Fajardo Rodriguez HA (2019). Effects of air pollution on acute exacerbation of chronic obstructive pulmonary disease: a descriptive retrospective study (pol-AECOPD). International Journal of Chronic Obstructive Pulmonary Disease, 1549–1557. https://doi.org/10.2147/COPD.S192047
Mortamais M, Pujol J, Martínez-Vilavella G, et al (2019) Effects of prenatal exposure to particulate matter air pollution on corpus callosum and behavioral problems in children. Environ Res 178:. https://doi.org/10.1016/J.ENVRES.2019.108734
Mutlu, E. A., Comba, I. Y., Cho, T., et al. (2018). Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environmental Pollution, 240, 817–830. https://doi.org/10.1016/j.envpol.2018.04.130
Nakada, L. Y. K., & Urban, R. C. (2020). COVID-19 pandemic: Impacts on the air quality during the partial lockdown in São Paulo state. Brazil Science Total Environment, 730, 139087. https://doi.org/10.1016/j.scitotenv.2020.139087
Nephew BC, Nemeth A, Hudda N, et al (2020) Traffic-related particulate matter affects behavior, inflammation, and neural integrity in a developmental rodent model. Environ Res 183:. https://doi.org/10.1016/j.envres.2020.109242
Neri, T., Pergoli, L., Petrini, S., et al. (2016). Particulate matter induces prothrombotic microparticle shedding by human mononuclear and endothelial cells. Toxicol Vitr, 32, 333–338. https://doi.org/10.1016/j.tiv.2016.02.001
Odutayo, A., Wong, C. X., Hsiao, A. J., et al. (2016). Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ, 354, 4482. https://doi.org/10.1136/bmj.i4482
Ogino, K., Zhang, R., Takahashi, H., et al. (2014). Allergic airway inflammation by nasal inoculation of particulate matter (PM2.5) in NC/Nga mice. PLoS ONE, 9, 1–9. https://doi.org/10.1371/journal.pone.0092710
Olawoyin, R., Schweitzer, L., Zhang, K., et al. (2018). Index analysis and human health risk model application for evaluating ambient air-heavy metal contamination in Chemical Valley Sarnia. Ecotoxicology and Environmental Safety, 148, 72–81. https://doi.org/10.1016/j.ecoenv.2017.09.069
Pan, M. H., Lai, C. S., & Ho, C. T. (2010). Anti-inflammatory activity of natural dietary flavonoids. Food & Function, 1, 15–31. https://doi.org/10.1039/c0fo00103a
Pan, W. C., Da, Wu. C., Chen, M. J., et al. (2016). Fine Particle Pollution, Alanine Transaminase, and Liver Cancer: A Taiwanese Prospective Cohort Study (REVEAL-HBV). Journal of the National Cancer Institute, 108, 1–7. https://doi.org/10.1093/jnci/djv341
Panni, T., Mehta, A., .J, Schwartz, J. D., et al. (2016) Genome-Wide Analysis of DNA Methylation and Fine Particulate Matter Air Pollution in Three Study Populations: KORA F3, KORA F4, and the Normative Aging Study. Environmental Health Perspectives, 124, 983–990. https://doi.org/10.1289/ehp.1509966
Park, J., Park, E. H., Schauer, J. J., et al. (2018). Reactive oxygen species (ROS) activity of ambient fine particles (PM2.5) measured in Seoul. Korea Environment International, 117, 276–283. https://doi.org/10.1016/j.envint.2018.05.018
Peixoto, M. S., de Oliveira Galvão, M. F., & Batistuzzo de Medeiros, S. R. (2017). Cell death pathways of particulate matter toxicity. Chemosphere, 188, 32–48. https://doi.org/10.1016/j.chemosphere.2017.08.076
Percy, Z., DeFranco, E., Xu, F., et al. (2019). Trimester specific PM 2.5 exposure and fetal growth in Ohio, 2007–2010. Environmental Research, 171, 111–118. https://doi.org/10.1016/J.ENVRES.2019.01.031
Pereira, G., Belanger, K., Ebisu, K., & Bell, M. L. (2014). Fine particulate matter and risk of preterm birth in Connecticut in 2000–2006: A longitudinal study. American Journal of Epidemiology, 179, 67–74. https://doi.org/10.1093/aje/kwt216
Pope, C. A., Dockery, D. W., Kanner, R. E., et al. (1999). Oxygen saturation, pulse rate, and particulate air pollution: A daily time-series panel study. American Journal of Respiratory and Critical Care Medicine, 159, 365–372. https://doi.org/10.1164/ajrccm.159.2.9702103
Pun, V. C., Yu, I. T. S., Ho, K. F., et al. (2014). Differential effects of source-specific particulate matter on emergency hospitalizations for ischemic heart disease in Hong Kong. Environmental Health Perspectives, 122, 391–396. https://doi.org/10.1289/ehp.1307213
Pun, V. C., Kazemiparkouhi, F., Manjourides, J., & Suh, H. H. (2017). Long-Term PM2.5 Exposure and Respiratory, Cancer, and Cardiovascular Mortality in Older US Adults. American Journal of Epidemiology, 186, 961–969. https://doi.org/10.1093/aje/kwx166
Qiu YN, Wang GH, Zhou F, Hao JJ, Tian L, Guan LF., ... & Zhang KZ (2019). PM2. 5 induces liver fibrosis via triggering ROS-mediated mitophagy. Ecotoxicology and Environmental Safety, 167: 178–187. https://doi.org/10.1016/j.ecoenv.2018.08.050
Raaschou-Nielsen O, Beelen R, Wang M, Hoek G, Andersen ZJ, Hoffmann B, ... & Vineis P, (2016). Particulate matter air pollution components and risk for lung cancer. Environment international, 87, 66–73. https://doi.org/10.1016/j.envint.2015.11.007
Ran, J., Sun, S., Han, L., et al. (2020). Fine particulate matter and cause-specific mortality in the Hong Kong elder patients with chronic kidney disease. Chemosphere, 247, 125913. https://doi.org/10.1016/j.chemosphere.2020.125913
Ren, F., Ji, C., Huang, Y., Aniagu, S., Jiang, Y., & Chen, T. (2020). AHR-mediated ROS production contributes to the cardiac developmental toxicity of PM2 5 in zebrafish embryos. Science of the Total Environment, 719, 135097. https://doi.org/10.1016/j.scitotenv.2019.135097
Riva, D. R., Magalhães, C. B., Lopes, A. A., et al. (2011). Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhalation Toxicology, 23, 257–267. https://doi.org/10.3109/08958378.2011.566290
Santa-Helena, E., Calderon, E. R. D., Gioda, A., et al. (2021). From air to heart: Particle pollution (PM2.5) and induced injury on cardioblast cells. Atmospheric Pollution Research, 12, 152–159. https://doi.org/10.1016/J.APR.2021.03.001
Setti, L., Passarini, F., De Gennaro, G., et al. (2020). SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: First evidence. Environ Res, 188, 109754. https://doi.org/10.1016/j.envres.2020.109754
Setti L, Passarini F, De Gennaro G, et al (2020a) Potential role of particulate matter in the spreading of COVID-19 in Northern Italy: First observational study based on initial epidemic diffusion. BMJ Open 10:. https://doi.org/10.1136/bmjopen-2020-039338
Setti L, Passarini F, De Gennaro G, et al (2020c) Searching for SARS-COV-2 on particulate matter: A possible early indicator of COVID-19 epidemic recurrence. Int J Environ Res Public Health 17:. https://doi.org/10.3390/ijerph17092986
Shan, X., Liu, L., Li, G., Xu, K., Liu, B., & Jiang, W. (2021). PM2 5 and the typical components cause organelle damage, apoptosis and necrosis: Role of reactive oxygen species. Science of The Total Environment, 782, 146785. https://doi.org/10.1016/j.scitotenv.2021.146785
Sharma, S., Zhang, M., Anshika, et al. (2020). Effect of restricted emissions during COVID-19 on air quality in India. Science Total Environment, 728, 138878. https://doi.org/10.1016/j.scitotenv.2020.138878
Shi, C., Han, X., Mao, X., et al. (2019). Metabolic profiling of liver tissues in mice after instillation of fine particulate matter. Science Total Environment, 696, 133974. https://doi.org/10.1016/j.scitotenv.2019.133974
Shrestha AM, Shrestha UB, Sharma R, et al (2020) Lockdown caused by COVID-19 pandemic reduces air pollution in cities worldwide. https://doi.org/10.31223/OSF.IO/EDT4J
Soto, S. D. F., De Melo, J. O., Marchesi, G. D., et al. (2017). Exposure to fine particulate matter in the air alters placental structure and the renin-angiotensin system. PLoS ONE, 12, 1–15. https://doi.org/10.1371/journal.pone.0183314
Sterpetti, A. V. (2020). Lessons Learned During the COVID-19 Virus Pandemic. Journal of the American College of Surgeons, 230, 1092–1093. https://doi.org/10.1016/j.jamcollsurg.2020.03.018
Sun, L., Wu, Q., Liao, K., et al. (2016). Contribution of heavy metals to toxicity of coal combustion related fine particulate matter (PM2.5) in Caenorhabditis elegans with wild-type or susceptible genetic background. Chemosphere, 144, 2392–2400. https://doi.org/10.1016/j.chemosphere.2015.11.028
Sun, X., Wei, H., Young, D. E., et al. (2017). Differential pulmonary effects of wintertime California and China particulate matter in healthy young mice. Toxicology Letters, 278, 1–8. https://doi.org/10.1016/j.toxlet.2017.07.853
Sun, B., Shi, Y., Yang, X., et al. (2018). DNA methylation: A critical epigenetic mechanism underlying the detrimental effects of airborne particulate matter. Ecotoxicology and Environmental Safety, 161, 173–183. https://doi.org/10.1016/j.ecoenv.2018.05.083
Tanwar, V., Adelstein, J. M., Grimmer, J. A., et al. (2017). PM2.5 exposure in utero contributes to neonatal cardiac dysfunction in mice. Environmental Pollution, 230, 116–124. https://doi.org/10.1016/j.envpol.2017.06.035
Tavera Busso, I., Mateos, A. C., Juncos, L. I., et al. (2018). Kidney damage induced by sub-chronic fine particulate matter exposure. Environment International, 121, 635–642. https://doi.org/10.1016/j.envint.2018.10.007
Tian, Y., Xiang, X., Juan, J., Sun, K., Song, J., Cao, Y., & Hu, Y. (2017). Fine particulate air pollution and hospital visits for asthma in Beijing, China. Environmental Pollution, 230, 227–233. https://doi.org/10.1016/j.envpol.2017.06.029
Tomczak, A., Miller, A. B., Weichenthal, S. A., et al. (2016). Long-term exposure to fine particulate matter air pollution and the risk of lung cancer among participants of the Canadian National Breast Screening Study. International Journal of Cancer, 139, 1958–1966. https://doi.org/10.1002/ijc.30255
Tsou, M. C. M., Lung, S. C. C., Shen, Y. S., et al. (2021). A community-based study on associations between PM25 and PM1 exposure and heart rate variabiyty using wearable low-cost sensing devices. Environment Pollution, 277, 116761. https://doi.org/10.1016/J.ENVPOL.2021.116761
Wagner, H., Cheng, J. W., & Ko, E. Y. (2018). Role of reactive oxygen species in male infertility: An updated review of literature. Arab Journal of Urology, 16, 35–43. https://doi.org/10.1016/j.aju.2017.11.001
Wan, Q., Liu, Z., Yang, M., & Wu, J. (2019). Acceleratory effects of ambient fine particulate matter on the development and progression of atherosclerosis in apolipoprotein E knockout mice by down-regulating CD4+CD25+Foxp3+ regulatory T cells. Toxicology Letters, 316, 27–34. https://doi.org/10.1016/j.toxlet.2019.09.005
Wang, C., Cai, J., Chen, R., et al. (2017a). Personal exposure to fine particulate matter, lung function and serum club cell secretory protein (Clara). Environmental Pollution, 225, 450–455. https://doi.org/10.1016/j.envpol.2017.02.068
Wang, J., Yin, Q., Tong, S., et al. (2017b). Prolonged continuous exposure to high fine particulate matter associated with cardiovascular and respiratory disease mortality in Beijing, China. Atmospheric Environment, 168, 1–7. https://doi.org/10.1016/j.atmosenv.2017.08.060
Wang, Y., Xiong, L., & Tang, M. (2017c). Toxicity of inhaled particulate matter on the central nervous system: Neuroinflammation, neuropsychological effects and neurodegenerative disease. Journal of Applied Toxicology, 37, 644–667. https://doi.org/10.1002/jat.3451
Wang, B. R., Shi, J. Q., Ge, N. N., et al. (2018). PM2.5 exposure aggravates oligomeric amyloid beta-induced neuronal injury and promotes NLRP3 inflammasome activation in an in vitro model of Alzheimer’s disease. Journal of Neuroinflammation, 15, 1–12. https://doi.org/10.1186/s12974-018-1178-5
Wang, Y., Zhang, M., Li, Z., et al. (2019). Fine particulate matter induces mitochondrial dysfunction and oxidative stress in human SH-SY5Y cells. Chemosphere, 218, 577–588. https://doi.org/10.1016/j.chemosphere.2018.11.149
Wang Y., H., Lin, Z., Y., Yang, L., W., He, H., J., Chen, T., Xu, W., Y., ... & Liu G, (2016). PM2. 5 exacerbate allergic asthma involved in autophagy signaling pathway in mice. International Journal of Clinical and Experimental Pathology, 9, 12247–12261.
Wang Y, Tang N, Mao M, Zhou Y, Wu Y, Li J, ... & Li J, (2021). Fine particulate matter (PM2. 5) promotes IgE-mediated mast cell activation through ROS/Gadd45b/JNK axis. Journal of Dermatological Science, 102(1), 47–57. https://doi.org/10.1016/j.jdermsci.2021.02.004
Wei, T., & Tang, M. (2018). Biological effects of airborne fine particulate matter (PM2.5) exposure on pulmonary immune system. Environmental Toxicology and Pharmacology, 60, 195–201. https://doi.org/10.1016/j.etap.2018.04.004
Wei, H., Liang, F., Meng, G., et al. (2016). Redox/methylation mediated abnormal DNA methylation as regulators of ambient fine particulate matter-induced neurodevelopment related impairment in human neuronal cells. Science and Reports, 6, 1–13. https://doi.org/10.1038/srep33402
Wei, H., Feng, Y., Liang, F., et al. (2017). Role of oxidative stress and DNA hydroxymethylation in the neurotoxicity of fine particulate matter. Toxicology, 380, 94–103. https://doi.org/10.1016/j.tox.2017.01.017
Weinmayr, G., Pedersen, M., Stafoggia, M., et al. (2018). Particulate matter air pollution components and incidence of cancers of the stomach and the upper aerodigestive tract in the European Study of Cohorts of Air Pollution Effects (ESCAPE). Environment International, 120, 163–171. https://doi.org/10.1016/j.envint.2018.07.030
Wong, C. M., Tsang, H., Lai, H. K., et al. (2016). Cancer mortality risks from long-term exposure to ambient fine particle. Cancer Epidemiology, Biomarkers & Prevention, 25, 839–845. https://doi.org/10.1158/1055-9965.EPI-15-0626
Wu, S., Wang, B., Yang, D., et al. (2016). Ambient particulate air pollution and circulating antioxidant enzymes: A repeated-measure study in healthy adults in Beijing, China. Environmental Pollution, 208, 16–24. https://doi.org/10.1016/j.envpol.2015.06.002
Wu, W., Jin, Y., & Carlsten, C. (2018). Inflammatory health effects of indoor and outdoor particulate matter. The Journal of Allergy and Clinical Immunology, 141, 833–844. https://doi.org/10.1016/j.jaci.2017.12.981
Wu, X., Nethery, R. C., Sabath, M. B., et al. (2020). Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Science Advances, 6, 1–7. https://doi.org/10.1126/SCIADV.ABD4049
Wu, Y., Li, H., Xu, D., et al. (2021). Associations of fine particulate matter and its constituents with airway inflammation, lung function, and buccal mucosa microbiota in children. Science Total Environment, 773, 145619. https://doi.org/10.1016/J.SCITOTENV.2021.145619
Wylie, B. J., Matechi, E., Kishashu, Y., et al. (2017). Placental pathology associated with household air pollution in a cohort of pregnant women from Dar es Salaam, Tanzania. Environmental Health Perspectives, 125, 134–140. https://doi.org/10.1289/EHP256
Xie, X., Wang, Y., Yang, Y., et al. (2018). Long-term exposure to fine particulate matter and tachycardia and heart rate: Results from 10 million reproductive-age adults in China. Environmental Pollution, 242, 1371–1378. https://doi.org/10.1016/j.envpol.2018.08.022
Xu, X., Wang, G., Chen, N., et al. (2016). Long-term exposure to air pollution and increased risk of membranous nephropathy in China. Journal of the American Society of Nephrology, 27, 3739–3746. https://doi.org/10.1681/ASN.2016010093
Xu, F., Cao, J., Luo, M., et al. (2018). Early growth response gene 1 is essential for urban particulate matter-induced inflammation and mucus hyperproduction in airway epithelium. Toxicology Letters, 294, 145–155. https://doi.org/10.1016/j.toxlet.2018.05.003
Xu Z, Li Z, Liao Z, Gao S, Hua L, Ye X, ... & Deng X. (2019). PM2. 5 induced pulmonary fibrosis in vivo and in vitro. Ecotoxicology and environmental safety, 171, 112–121. https://doi.org/10.1016/j.ecoenv.2018.12.061
Xu F, Shi X, Qiu X, Jiang X, Fang Y, Wang J, ... & Zhu T (2020). Investigation of the chemical components of ambient fine particulate matter (PM2. 5) associated with in vitro cellular responses to oxidative stress and inflammation. Environment international, 136:105475. https://doi.org/10.1016/j.envint.2020.105475
Yang, Y. R., Chen, Y. M., Chen, S. Y., & Chan, C. C. (2017). Associations between long-term particulate matter exposure and adult renal function in the taipei metropolis. Environmental Health Perspectives, 125, 602–607. https://doi.org/10.1289/EHP302
Yang, B., Guo, J., & Xiao, C. (2018). Effect of PM2. 5 environmental pollution on rat lung. Environmental Science and Pollution Research, 25, 36136–36146. https://doi.org/10.1007/s11356-018-3492-y
Yang, J., Chen, Y., Yu, Z., et al. (2019). The influence of PM25 on lung injury and cytokines in mice. Experimental Therapeutic Medecine, 18, 2503. https://doi.org/10.3892/ETM.2019.7839
Yang, M., Zhou, R., Qiu, X., et al. (2020). Artificial intelligence-assisted analysis on the association between exposure to ambient fine particulate matter and incidence of arrhythmias in outpatients of cheghai community hospitals. Environmental International, 139, 105745. https://doi.org/10.1016/j.envint.2020.105745
Yao, Y., Pan, J., Liu, Z., et al. (2020a). Temporal association between particulate matter pollution and case fatality rate of COVID-19 in Wuhan. Environmental Research, 189, 13–15. https://doi.org/10.1016/j.envres.2020.109941
Yao Y, Pan J, Wang W, et al (2020b) Spatial Correlation of Particulate Matter Pollution and Death Rate of COVID-19. https://doi.org/10.1101/2020.04.07.20052142
Ye, Z., Lu, X., Deng, Y., et al. (2018). In Utero Exposure to Fine Particulate Matter Causes Hypertension Due to Impaired Renal Dopamine D 1 Receptor in Offspring. Cellular Physiology and Biochemistry, 46, 148–159. https://doi.org/10.1159/000488418
Yoshizaki, K., Brito, J. M., Silva, L. F., et al. (2017). The effects of particulate matter on inflammation of respiratory system: Differences between male and female. Science of the Total Environment, 586, 284–295. https://doi.org/10.1016/j.scitotenv.2017.01.221
Younan, D., Petkus, A. J., Widaman, K. F., et al. (2020). Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain, 143, 289–302. https://doi.org/10.1093/brain/awz348
Yu, N., Shu, S., Lin, Y., et al. (2017). High efficiency cabin air filter in vehicles reduces drivers’ roadway particulate matter exposures and associated lipid peroxidation. PLoS ONE, 12, 1–13. https://doi.org/10.1371/journal.pone.0188498
Yu, H., Wei, J., Cheng, Y., et al. (2018). Synergistic and Antagonistic Interactions among the Particulate Matter Components in Generating Reactive Oxygen Species Based on the Dithiothreitol Assay. Environmental Science and Technology, 52, 2261–2270. https://doi.org/10.1021/acs.est.7b04261
Yue W, Tong L, Liu X, Weng X, Chen X, Wang D, ... & Chen Y (2019). Short term Pm2. 5 exposure caused a robust lung inflammation, vascular remodeling, and exacerbated transition from left ventricular failure to right ventricular hypertrophy. Redox biology, 22:101161. https://doi.org/10.1016/j.redox.2019.101161
Yun Wang D, Giuliano M, Yang L, et al (2020) The Impact of PM 2.5 on the Host Defense of Respiratory System. https://doi.org/10.3389/fcell.2020.00091
Zeng X, Liu J, Du X, Zhang J, Pan K, Shan W, ... & Zhao J (2018). The protective effects of selenium supplementation on ambient PM2. 5-induced cardiovascular injury in rats. Environmental Science and Pollution Research, 25: 22153–22162. https://doi.org/10.1007/s11356-018-2292-8
Zhang, Z., Guo, C., Lau, A. K. H., et al. (2018). Long-term exposure to fine particulate matter, blood pressure, and incident hypertension in Taiwanese adults. Environmental Health Perspectives, 126, 1–8. https://doi.org/10.1289/EHP2466
Zhang, Z., Zhu, D., Cui, B., et al. (2020). Association between particulate matter air pollution and lung cancer. Thorax, 75, 85–87. https://doi.org/10.1136/thoraxjnl-2019-213722
Zhang, Z., Hong, Y., & Liu, N. (2017). Association of ambient Particulate matter 2.5 with intensive care unit admission due to pneumonia: a distributed lag non-linear model. Scientific Reports, 7(1), 1–7. https://doi.org/10.1038/s41598-017-08984-x
Zhao, X., Zhang, X., Xu, X., et al. (2009). Seasonal and diurnal variations of ambient PM2.5 concentration in urban and rural environments in Beijing. Atmospheric Environment, 43, 2893–2900. https://doi.org/10.1016/j.atmosenv.2009.03.009
Zhao, Y. X., Zhang, H. R., Yang, X. N., et al. (2018). Fine Particulate Matter-Induced Exacerbation of Allergic Asthma via Activation of T-cell Immunoglobulin and Mucin Domain 1. Chinese Medical Journal (engl), 131, 2461–2473. https://doi.org/10.4103/0366-6999.243551
Zheng, Y., Fan, J., Chen, H. W., et al. (2019). Trametes orientalis polysaccharide alleviates PM 2.5-induced lung injury in mice through its antioxidant and anti-inflammatory activities. Food & Function, 10, 8005–8015. https://doi.org/10.1039/C9FO01777A
Zou W, Wang X, Hong W, He F, Hu J, Sheng Q, ... & Ran P, (2020). PM2. 5 induces the expression of inflammatory cytokines via the Wnt5a/Ror2 pathway in human bronchial epithelial cells. International journal of chronic obstructive pulmonary disease, 15:2653. https://doi.org/10.2147/COPD.S270762
Acknowledgements
This work was carried out with the support of the Higher Education Personnel Improvement Coordination—Brazil (CAPES)—Financing Code 001, Carlos Chagas Filho The authors acknowledge to Foundation for Research Support of the State of Rio de Janeiro (FAPERJ), and the National Council for Scientific and Technological Development (CNPq, Brazil).
Funding
This study was funded by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and the authors thank also to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro).
Author information
Authors and Affiliations
Contributions
Amanda Garcia: major contributor in writing and edition of the manuscript. Eduarda Santa- Helena, Anna De Falco, Adriana Gioda, Joaquim de Paula Ribeiro and Carolina Rosa Gioda: writing, editing and study supervision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garcia, A., Santa-Helena, E., De Falco, A. et al. Toxicological Effects of Fine Particulate Matter (PM2.5): Health Risks and Associated Systemic Injuries—Systematic Review. Water Air Soil Pollut 234, 346 (2023). https://doi.org/10.1007/s11270-023-06278-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06278-9