Abstract
Wastewater-based epidemiology (WBE) is a potential approach for determining the viral prevalence in a community. In the wake of the COVID-19 pandemic, researchers have begun to pay close attention to the presence of SARS-COV-2 RNA in various wastewaters. The potential for detecting SARS-CoV-2 RNA in hospital sewage could make it an invaluable resource for epidemiological studies. In this regard, two specialized hospitals dedicated to COVID-19 patients were chosen for this investigation. Both hospitals utilize the same wastewater treatment systems. The influent and effluents of the two hospitals were sampled in May and June of 2021, and the samples were evaluated for their chemical properties. According to the findings of this study, the wastewater qualities of the two studied hospitals were within the standard ranges. The sewage samples were concentrated using ultrafiltration and PEG precipitation techniques. The E and S genes were studied with RT-qPCR commercial kits. We found E gene of SARS-CoV-2 in 83.3% (5/6) and 66.6% (4/6) of wastewater samples from hospital 1 and hospital 2, respectively, using ultrafiltration concentration method. Wastewater samples taken after chlorine treatment accounted for 16.6% of all positive results. In addition, due to the small sample size, there was no significant correlation (p > 0.05) between the presence of SARS-CoV-2 in wastewater and the number of COVID-19 cases. Hospitals may be a source of SARS-CoV-2 pollution, thus it is important to monitor and enhance wastewater treatment systems to prevent the spread of the virus and safeguard the surrounding environment.
Similar content being viewed by others
1 Introduction
The new betacoronavirus, responsible for the COVID-19 (chronic respiratory infection disease 2019) and SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2) pandemics, has been intensively researched around the globe (Qu et al., 2020). Known by their crown-like spikes, coronaviruses are enveloped viruses with single-stranded positive sense RNA genomes from the Coronaviridae family. This family has approximately 30 distinct viruses and the biggest documented genome size of all RNA viruses (~ 30 Kb) (Chan et al., 2020). In recently published studies on SARS-CoV-2 transmission, multiple transmission pathways have been explored. The most common routes of spreading the disease are by airborne droplets and close patient contact (Mandal et al., 2020; Morawska & Cao, 2020). Nonetheless, the detection of the viral RNA in human feces indicates that the GI tract can be colonized by SARS-CoV-2 (Wu et al., 2020; Xiao et al., 2020). Sewage can therefore become contaminated with SARS-CoV-2 RNA from human feces, saliva, and sputum (Lahrich et al., 2021). Since individuals infected with COVID-19 excrete SARS-CoV-2 in their feces, wastewater-based epidemiology (WBE) is a crucial tool for monitoring outbreaks (Kitajima et al., 2020; Nghiem et al., 2020). WBE is an effective epidemiologic tool for tracking the spread of viruses in communities and learning about outbreaks through the study of viral loads in restricted areas (Arora et al., 2020). Thus, the spread of SARS-CoV-2 can be monitored using effluent and WBE environmental surveillance.
Several studies have reported the incidence and persistence of SARS-CoV-2 in aquatic environments such as water, sewage, and streams (Ibrahim et al., 2021; Lahrich et al., 2021; Mandal et al., 2020; Sangkham, 2021). Despite the fact that viruses represent major health hazards, only few studies have investigated the presence of SARS-CoV-2 in hospital wastewater (Gonçalves et al., 2021; Zhang et al., 2020; Kumar et al., 2021). Macropollutants and micropollutants such as bacteria and viruses are released by hospital units into the sewage systems (Achak et al., 2021; Corpuz et al., 2020; Majumder et al., 2021). Since the start of the COVID-19 pandemic and owing to serious health and environmental concerns, most studies have focused on eradicating viruses from hospital wastewater.
Wastewater surveillance is a valuable approach for collecting health data from communities, especially during COVID-19 epidemic. The purpose of this research was to examine the treated and untreated wastewaters from two hospitals for the presence of the RNA genome of SARS-CoV-2. Using RT-qPCR, the probable routes of SARS-CoV-2 RNA were investigated in hospital wastewater samples collected at intervals between May and June 2021. A significant challenge in detecting SARS-CoV-2 in wastewater is the need for efficient concentration procedures prior to RNA extraction and RT-qPCR detection (Ahmed et al., 2020). Most commonly used techniques for concentrating viruses include polyethylene glycol (PEG) precipitation (Bar Or et al., 2020; LaRosa et al., 2020; Wu et al., 2020; Zhang et al., 2020; Nasseri et al., 2021), ultrafiltration (Balboa et al., 2021; Gonçalves et al., 2021; Medema et al., 2020; Rimoldi et al., 2020; Abu Ali et al., 2021), electronegative membrane (Agrawal et al., 2021; Ali et al., 2018; Haramoto et al., 2020; Tanhaei et al., 2021), and ultracentrifugation (Kocamemi et al., 2020; Wurtzer et al., 2020). A comparison of these concentration techniques experimentally led us to favor ultrafiltration and PEG precipitation.
2 Material and Methods
2.1 Sampling and Chemical Analysis
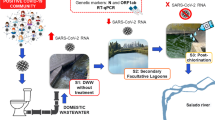
The wastewater treatment facilities of two distinct hospitals in Tehran, Iran, were sampled. These hospitals are the main hospitals dedicated to treatment for COVID-19-infected patients. However, a confidentiality agreement prevents the disclosure of the specific location and names of the two hospitals. Both hospitals employed an aerobic treatment procedure involving activated sludge and prolonged aeration. The volume of wastewater produced at each hospital is 100 m3. The effluents of each hospital are treated with 100 mg/L chlorine solution for 2 h. The schematic of the treatment processes employed in both hospital and sampling points are shown in Fig. 1. On each sampling date, approximately 1 L of grab samples was collected in the morning from the influent and final effluent (around 9 a.m.). Samples were taken thrice from each facility between May 18 and June 11, 2021 (Table 1). It was not feasible to collect multiple samples due to limitations imposed by the hospitals where the original samples were collected. We kept the samples at 4 °C and delivered them to the lab as soon as possible. These 12 hospital wastewater samples were analyzed for their temperature, acidity, turbidity, biochemical oxygen demand (BOD), chemical oxygen demand (COD), and total suspended solid (TSS) through established procedures.
2.2 Concentration of Wastewater Samples
To remove coarse particles, 500 mL of hospital wastewater samples was gently centrifuged at 4600 × g for 30 min. Viral load was concentrated using two methods. In the first method, 100 mL supernatants was concentrated with a 10 kDa Vivaspin® 20 Ultrafiltration Unit (Sartorius Company, Germany). Accordingly, 20 mL of supernatant was added to the filter unit and centrifuged for 20 min at 4000 × g, and the concentrated supernatant was harvested. This was repeated until 100 mL of wastewater was completely concentrated (final volume of concentrated supernatant approximately 1 mL) (Westhaus et al., 2021). In the second method, PEG was used to concentrate wastewater. Accordingly, PEG 6000 (10%) and NaCl (0.3 mol/L) were mixed with 100 mL of wastewater supernatant, and incubated during the night at 4 °C. The prepared mixture was centrifuged the following day at 10,000 × g for 90 min (Fig. 2). We rejected the supernatant and resuspended the pellet in 300 μL of RNase-free water. Finally, the suspension was utilized for RNA extraction (Zhang et al., 2020).
2.3 RNA Extraction and RT-qPCR Assay
The RNJia Virus Kit (ROJE Technologies, Yazd, Iran) was used to extract RNA according to the provided instructions. A total of 140 μL of specimen was combined with the lysis buffer and RNA carrier. The addition of ethanol to the lysate provided a suitable condition for binding the RNA to the silica membrane. The membrane was then selectively bounded to RNA. Two specific washing buffers were used to remove contaminants. Pure viral RNA was finally eluted in RNase-free water and stored at − 70 °C.
RT-PCR quantitative tests were carried out using COVID-19 One-Step RT-PCR kit (COVITECH, Iran). In the PCR assay, a primer/probe set is used to amplify FAM-labeled E genes and ROX-labeled S genes. The negative control consists of nuclease-free water. The positive control consists of a double-strand DNA amplified with the SARS-CoV-2 primers/probes mix. Finally, an internal control targeting RNase P was used to verify the nucleic acid presence in every sample and processed sample. The tests were performed using the StepOne Real-time PCR system (Applied Biosystems, Thermo Fisher Scientific). RT-qPCR assays were performed in 20 μL reactions containing 10 μL TaqMan One-Step RT-qPCR Master Mix, 1 μL of primer and probe set, and 5 μL of extracted RNA. The thermal cycling conditions of one-step RT-qPCR were reverse transcription at 55 °C for 10 min, initial denaturation at 95 °C for 3 min, 45 cycles of amplification at 95 °C for 15 s, and 60 °C for 1 min. RT-qPCR was conducted on duplicate samples of isolated RNA. Following the instructions provided, the samples with cycle threshold value of Ct < 40 were considered positive for both E and S specific genes of SARS-CoV-2.
3 Results
3.1 Physicochemical Characterization of Hospital Wastewater Samples
Several parameters determine the infectious potential of wastewater, including suspended solids, organic matter, and temperature. Hence, it is of great importance to effectively treat wastewater and lower its organic load (BOD, COD) and TSS content before disinfection. Although the two hospitals had similar wastewater treatment processes in this research, the results showed that the wastewater treatment process of hospital 1 was more satisfactory and had a higher performance in terms of turbidity (91.23%), TSS (94.85%), COD (82.25%), and BOD (84.85%) (Table 2).
3.2 Detection of SARS-CoV-2 Genome in Hospital Wastewater
A total of twelve wastewater samples, comprising raw influent and post-chlorination effluent, were collected from two hospitals. According to the measured Ct values, SARS-CoV-2 RNA was effectively detected in hospital wastewater samples. The Ct values for both target genes ranged from 31.3 to 36.9. As shown in Table 3, the ultrafiltration concentration method yielded a positive rate of 83.3% for the E gene and 16.6% for the S gene for hospital 1, while the PEG concentration method yielded a positive rate of 66.6% for the E gene and 33.3% for the S gene. The results of hospital 2 showed that the ultrafiltration concentration method had a 66.6% positive rate for E and 50% for S genes, whereas the PEG concentration method had a 33.3% positive rate for E and 16.6% for S genes. The E gene was found to be more efficient for detecting SARS-CoV-2 in both hospital wastewater samples. The E gene positive rate was also elevated when samples of both hospitals were concentrated using ultrafiltration.
3.3 Comparison of Hospitalized COVID-19 Cases with the Detected Frequency of SARS-CoV-2 RNA
The daily number of COVID-19-infected individuals who were hospitalized at hospital 2 during sampling is shown in Fig. 3. As it can be seen, the number of patients began to decrease on June 11. From there, it was assumed that SARS-CoV-2 would become less common in all samples. The relationship between the number of COVID-19 hospitalized patients and the median Ct values obtained from influent and effluent wastewater samples concentrated using ultrafiltration and PEG precipitation was not found to be significant (p > 0.05) (Table 4). No data on the number of COVID-19-positive patients was made available by hospital 1 during the study period.
4 Discussion
Several studies published after the initial reports of the COVID-19 pandemic attest to the usefulness of wastewater surveillance in monitoring the spread of the disease and the overall health of communities. Despite the limitation of individual-level Ct values, population-level Ct values are valuable indicators for COVID-19. Also, the Ct values may differ depending on the target genes used in RT-PCR or the method utilized to detect the same genes (Tso et al., 2021). In this work, the Ct values for the E and S specific genes of SARS-CoV-2 ranged from 31.3 to 36.9 for both target genes, which is consistent with earlier studies yielding Ct values ranging from 34 to 40 (Medema et al., 2020; Randazzo et al., 2020; Wu et al., 2020).
In this study, RNA of SARS-CoV-2 was detected in hospital wastewater through analysis of the E and S genes. SARS-CoV-2 RNA has been detected in sewage systems and streams utilizing E and S genes in a number of investigations (Gonçalves et al., 2021; Kumar et al., 2020; LaRosa, et al. 2020; Medema, et al., 2020; Wu, et al., 2020; Kumar, et al., 2021). Our findings suggested the E gene to be more efficient for detecting SARS-CoV-2 in wastewater from both hospitals. Ultrafiltration concentration method revealed the traces of the E gene in 83.3% (5/6) and 66.6% (4/6) of wastewater samples from hospital 1 and hospital 2, respectively. Similar findings were found by Gonçalves et al. (2021), who found that after ultrafiltration with 10 kDa filters, 60% (6/15) of wastewater samples remained E gene positive (Gonçalves et al., 2021).
Both concentration detection methods were able to identify SARS-CoV-2 in hospital wastewater. However, based on the obtained positive rates of E and S genes, the difference in viral detection is greater when ultrafiltration concentration is utilized. A number of recent investigations have shown that ultrafiltration method is effective for detecting trace levels of SARS-CoV-2 RNA in a wide range of wastewater types (Baldovin et al., 2021; Sangkham, 2021). PEG-based concentration, however, continues to be the method of choice for COVID-19 wastewater epidemiological studies (Lu et al., 2020; Sangkham, 2021). The disadvantage of the PEG precipitation method is the precipitation of non-viral proteins and non-nucleic acid components associated with the extracellular nucleic acids, which can limit or even prevent subsequent PCR-based identification of viral genomes (Corpuz et al., 2020; McNamara & Dittmer, 2020). Measurement of the virus concentration in wastewater is dependent on the concentration method’s efficiency. Spiking a surrogate virus is the standard method for gathering information on efficacy of concentration methods. Murine hepatitis virus (MHV), MS2 phage, F-specific RNA phage, hepatitis A virus, and bovine coronaviruses (BCoVs) are examples of such viruses (Ali et al., 2016; LaRosa et al., 2020). Due to the potential of infection transmission to laboratory personnel, we did not calculate the concentration method’s efficiency in this investigation.
The Iranian Environmental Protection Agency (Iranian EPA) specifies the maximum concentrations of COD, BOD, TSS, and turbidity for effluent discharge to surface waters as 60 mg/L, 30 mg/L, 40 mg/L, and 50 NTU, respectively (Jamialahmadi et al., 2021). According to the findings of this study, samples collected from the hospitals under investigation were within the standard range. Nevertheless, 16.6% of all positive samples belong to post-chlorination effluents. Similar to prior research (Balboa et al., 2021; Haramoto et al., 2020; Randazzo et al., 2020a, 2020b), our results demonstrated that insufficient treatment of wastewater can introduce SARS-CoV-2 RNA into the environment, which may lead to spread of SARS-CoV-2 (Ali & Ali, 2014; Haramoto et al., 2020; Wang et al., 2020; Zhang et al., 2020). Further studies have shown that viruses in chlorinated effluents are resistant to disinfectants (Carducci et al., 2008; Prado et al., 2011), which may be explained by the presence of organic materials in our samples (Petrinca et al., 2009). Numerous parameters, such as acidity, temperature, wastewater characteristics, humidity level, total suspended particles, and virus structure, affect the persistence of coronaviruses in sewage (Sangkham, 2021). According to reports, suspended solids shield the virus and may necessitate higher disinfectant concentrations. There may be byproducts and environmental concerns associated with the increased usage of disinfectants (Mandal et al., 2020). So, it is suggested to utilize other physicochemical methods such as peracetic acid, ozone, and UV radiation or a combination thereof. Yamamoto et al. (2023) demonstrated that peracetic acid reduces the viral load of SARS-CoV-2 through cleaving disulfide bridges in viral receptors (Yamamoto et al., 2023). Despite some disadvantages such as natural degradation and lower efficiency, peracetic acid can be used in wastewater treatment along with chlorination. However, the optimum concentration and causes of lower efficiency in organic-material-rich wastewaters need to be further explored (Thakur et al., 2021).
The presence of SARS-CoV-2 in wastewater and the number of COVID-19 cases did not appear to be correlated (p > 0.05). SARS-CoV-2 load in sewage is related to the number of vulnerable patients in a catchment area, according to previous research (Hong et al., 2021; Wong et al., 2022; Wurtzer et al., 2020). The absence of a correlation between the number of the daily COVID-19 patients diagnosed in hospital 2 and the SARS-CoV-2 RNA detection in hospital wastewater could be attributable to the degradation of the genetic material, the presence of the suspended solids (Mandal et al., 2020), and the small sample size. During the lockdown, only grab sampling was possible, which is one of the main limitations of our work. Detection of SARS-CoV-2 in wastewater samples has only been the subject of a small number of studies. Grab sampling has been suggested as a viable alternative to the more conventional composite sampling procedure for SARS-CoV-2 sampling in wastewater (Augusto et al., 2022). However, the grab sampling method may show lower population than a composite sample over a period of 24 h. Considering the fact that wastewater surveillance is becoming crucial to track the spread of SARS-CoV-2, these results can help the local authorities to improve the public health.
5 Conclusion
The hospital sewage quality assessment revealed that activated sludge wastewater treatment system with the extended aeration was effective in reducing contamination to the standard limit. As we reported for the treated effluent from the hospitals, inadequate treatment of wastewater may result in releasing SARS-CoV-2 RNA into the environment. However, it is important to keep in mind that the detection of SARS-CoV-2 RNA does not mean infectious SARS-CoV-2 presence. Additionally, our results confirmed the importance of the viral concentration method in the detection of SARS-CoV-2 RNA in wastewater. The concentration method of SARS-CoV-2 RNA from hospital sewage needs further investigation. SARS-CoV-2 concentrations in solid components of raw hospital sewages should also be studied in future investigations. Due to the hospital policies, it was impossible to perform a 24-h sampling which limited our surveillances of COVID-19 outbreak. However, we anticipate that the findings of this study will prove useful in determining the effectiveness of wastewater treatment plants. We also believe that these findings can help the municipal sanitation authorities in improving the public health and forming an efficient collaboration between researchers and management organizations.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Abu Ali, H., Yaniv, K., Bar-Zeev, E., Chaudhury, S., Shagan, M., Lakkakula, S., Ronen, Z., Kushmaro, A., & Nir, O. (2021). Tracking SARS-CoV-2 RNA through the wastewater treatment process. ACS ES&T Water, 1, 1161–1167.
Achak, M., Alaoui Bakri, S., Chhiti, Y., & M’hamdiAlaoui, F.E., Barka, N., & Boumya, W. (2021). SARS-CoV-2 in hospital wastewater during outbreak of COVID-19: A review on detection, survival and disinfection technologies. Science of the Total Environment, 761, 143192.
Agrawal, S., Orschler, L., & Lackner, S. (2021). Long-term monitoring of SARS-CoV-2 RNA in wastewater of the Frankfurt metropolitan area in Southern Germany. Scientific Reports, 11, 5372.
Ahmed, W., Bertsch, P. M., Bivins, A., Bibby, K., Farkas, K., Gathercole, A., Haramoto, E., Gyawali, P., Korajkic, A., McMinn, B. R., Mueller, J. F., Simpson, S. L., Smith, W. J. M., Symonds, E. M., Thomas, K. V., Verhagen, R., & Kitajima, M. (2020). Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Science of the Total Environment, 739, 139960.
Ali, D., & Ali, H. (2014). Assessment of DNA damage and cytotoxicity of palmatine on human skin epithelial carcinoma cells. Environmental Toxicology and Chemistry, 96(6), 941–950.
Ali, H., Dixit, S., Ali, D., Alkahtane, A. A., Alarifi, S., Ali, B. A., & Alkahtani, S. (2016). Isolation and evaluation of biological efficacy of quercetol in human hepatic carcinoma cells. Drug Design, Development and Therapy, 6(10), 155–162.
Ali, D., Ali, H., Alifiri, S., Alkahtani, S., Alkahtane, A. A., & Huasain, S. A. (2018). Detection of oxidative stress and DNA damage in freshwater snail Lymnea leuteola exposed to profenofos. Frontiers of Environmental Science & Engineering, 12(5), 1–7.
Arora, S., Nag, A., Sethi, J., Rajvanshi, J., Saxena, S., Shrivastava, S. K., & Gupta, A. B. (2020). Sewage surveillance for the presence of SARS-CoV-2 genome as a useful wastewater based epidemiology (WBE) tracking tool in India. Water Science and Technology, 82, 2823–2836.
Augusto, M. R., Claro, I. C. M., Siqueira, A. K., Sousa, G. S., Caldereiro, C. R., Duran, A. F. A., de Miranda, T. B., Camillo, L. M. B., Cabral, A. D., & Bueno, R. F. (2022). Sampling strategies for wastewater surveillance: Evaluating the variability of SARS-COV-2 RNA concentration in composite and grab samples. Journal of Environmental Chemical Engineering., 10, 107478.
Balboa, S., Mauricio-Iglesias, M., Rodriguez, S., Martínez-Lamas, L., Vasallo, F. J., Regueiro, B., & Lema, J. M. (2021). The fate of SARS-COV-2 in WWTPS points out the sludge line as a suitable spot for detection of COVID-19. Science of the Total Environment, 772, 145268.
Baldovin, T., Amoruso, I., Fonzo, M., Buja, A., Baldo, V., Cocchio, S., & Bertoncello, C. (2021). SARS-CoV-2 RNA detection and persistence in wastewater samples: An experimental network for COVID-19 environmental surveillance in Padua, Veneto Region (NE Italy). Science of the Total Environment, 760, 143329.
Bar Or, I., Yaniv, K., Shagan, M., Ozer, E., Erster, O., Mendelson, E., Mannasse, B., Shirazi, R., Kramarsky-Winter, E., Nir, O., Abu-Ali, H., Ronen, Z., Rinott, E., Lewis, Y.E., Friedler, E., Bitkover, E., Paitan, Y., Berchenko, Y., & Kushmaro, A. (2020). Regressing SARS-CoV-2 sewage measurements onto COVID-19 burden in the population A proof-of-concept for quantitative environmental surveillance medRxiv
Carducci, A., Morici, P., Pizzi, F., Battistini, R., Rovini, E., & Verani, M. (2008). Study of the viral removal efficiency in an urban wastewater treatment plant. Water Science and Technology, 58, 893-e897.
Chan, V. W. S., Chiu, P. K. F., Yee, C. H., Yuan, Y., Ng, C. F., & Teoh, J. Y. C. (2020). A systematic review on COVID-19: Urological manifestations, viral RNA detection and special considerations in urological conditions. World Journal of Urology, 27, 1–12.
Corpuz, M. V. A., Buonerba, A., Vigliotta, G., Zarra, T., Ballesteros, F., Campiglia, P., Belgiorno, V., Korshin, G., & Naddeo, V. (2020). Viruses in wastewater: Occurrence, abundance and detection methods. Science of the Total Environment, 745, 140910.
Gonçalves, J., Koritnik, T., Mioč, V., Trkov, M., Bolješič, M., Berginc, N., Prosenc, K., Kotar, T., & Paragi, M. (2021). Detection of SARS-CoV-2 RNA in hospital wastewater from a low COVID-19 disease prevalence area. Science of the Total Environment, 755, 143226.
Haramoto, E., Malla, B., Thakali, O., & Kitajima, M. (2020). First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Science of the Total Environment, 737, 140405.
Hong, P. Y., Rachmadi, A. T., Mantilla-Calderon, D., Alkahtani, M., Bashawri, Y. M., Al Qarni, H., O’Reilly, K. M., & Zhou, J. (2021). Estimating the minimum number of SARS-CoV-2 infected cases needed to detect viral RNA in wastewater: To what extent of the outbreak can surveillance of wastewater tell us? Environmental Research, 195, 110748.
Ibrahim, Y., Ouda, M., Kadadou, D., Banat, F., Naddeo, V., Alsafar, H., Yousef, A. F., & Barcel´o, D., & Hasan, S.W. (2021). Detection and removal of waterborne enteric viruses from wastewater: A comprehensive review. Journal of Environmental Chemical Engineering, 9, 105613.
Jamialahmadi, N., Rahimi, S., & Esmaeili, A. (2021). Hospital wastewater in Iran: A systematic review and challenges for proper management during coronavirus disease (2019) pandemic. Journal of Applied Research in Water and Wastewater, 8(1), 59–65.
Kitajima, M., Ahmed, W., Bibby, K., Carducci, A., Gerba, C. P., Hamilton, K. A., Haramoto, E., & Rose, J. B. (2020). SARS-CoV-2 in wastewater: State of the knowledge and research needs. Science of the Total Environment, 739, 139076.
Kocamemi, B.A., Kurt, H., Hacıoglu, S., Yaralı, C., Saatci, A.M., & Pakdemirli, B. (2020). First data-set on SARS-CoV-2 detection for Istanbul wastewaters in Turkey medRxiv
Kumar, M., Patel, A. K., Shah, A. V., Raval, J., Rajpara, N., Joshi, M., & Joshi, C. G. (2020). First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Science of the Total Environment, 746, 141326.
Kumar, M., Kuroda, K., Patel, A. K., Patel, N., Bhattacharya, P., Joshi, M., & Joshi, C. G. (2021). Decay of SARS-CoV-2 RNA along the wastewater treatment outfitted with Upflow Anaerobic Sludge Blanket (UASB) system evaluated through two sample concentration techniques. Science of the Total Environment, 754, 142329.
Lahrich, S., Laghrib, F., Farahi, A., Bakasse, M., Saqrane, S., & El Mhammedi, M. A. (2021). Review on the contamination of wastewater by COVID-19 virus: Impact and treatment. Science of the Total Environment, 751, 142325.
LaRosa, G., Bonadonna, L., Lucentini, L., Kenmoe, S., & Suffredini, E. (2020). Coronavirus in water environments: Occurrence, persistence and concentration methods — A scoping review. Water Research, 179, 115899.
Lu, D., Huang, Z., Luo, J., Zhang, X., & Sha, S. (2020). Primary concentration — the critical step in implementing the wastewater based epidemiology for the COVID-19 pandemic: A mini-review. Science of the Total Environment, 747, 141245.
Majumder, A., Gupta, A. K., Ghosal, P. S., & Varma, M. (2021). A review on hospital wastewater treatment: A special emphasis on occurrence and removal of pharmaceutically active compounds, resistant microorganisms, and SARS-CoV-2. Journal of Environmental Chemical Engineering, 7, 104812.
Mandal, P., Gupta, A. K., & Dubey, B. K. (2020). A review on presence, survival, disinfection/removal methods of coronavirus in wastewater and progress of wastewater-based epidemiology. Journal of Environmental Chemical Engineering, 8, 104317.
McNamara, R. P., & Dittmer, D. P. (2020). Modern techniques for the isolation of extracellular vesicles and viruses. Journal of Neuroimmune Pharmacology, 15, 459–472.
Medema, G., Heijnen, L., Elsinga, G., Italiaander, R., & Brouwer, A. (2020). Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environmental Science & Technology Letters, 7, 511–516.
Morawska, L., & Cao, J. (2020). Airborne transmission of SARS-CoV-2: The world should face the reality. Environment International, 139, 105730.
Nasseri, S., Yavarian, J., Baghani, A. N., Azad, T. M., Nejati, A., Nabizadeh, R., Hadi, M., Jandaghi, N. Z. S., Vakili, B., Vaghefi, S. K. A., Baghban, M., Yousefi, S., Nazmara, S., & Alimohammadi, M. (2021). The presence of SARS-CoV-2 in raw and treated wastewater in 3 cities of Iran: Tehran, Qom and Anzali during coronavirus disease 2019 (COVID-19) outbreak. Journal of Environmental Health Science & Engineering, 19, 573–584.
Nghiem, L. D., Morgan, B., Donner, E., & Short, M. D. (2020). The COVID-19 pandemic: Considerations for the waste and wastewater services sector. Case Studies in Chemical and Environmental Engineering, 1, 100006.
Petrinca, A. R., Donia, D., Pierangeli, A., Gabrieli, A. M., Degener, A. M., Bonanni, E., Diaco, L., Cecchini, G., Anastasi, P., & Divizia, M. (2009). Presence and environment circulation of enteric viruses in three different wastewater treatment plants. Journal of Applied Microbiology, 106, 1608-e1617.
Prado, T., Silva, D. M., Guilayn, W. C., Rose, T. L., Gaspar, A. M. C., & Miagostovich, M. P. (2011). Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Research, 45, 1287–1297.
Qu, G., Li, X., Hu, L., & Jiang, G. (2020). An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19). Environmental Science & Technology, 54, 3730–3732.
Randazzo, W., Cuevas-Ferrando, E., & Sanju´ an, R., Domingo-Calap, P., & S´ anchez, G. (2020). Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. International Journal of Hygiene and Environmental Health, 230, 113621.
Rimoldi, S. G., Stefani, F., Gigantiello, A., Polesello, S., Comandatore, F., Mileto, D., Maresca, M., Longobardi, C., Mancon, A., Romeri, F., Pagani, C., Cappelli, F., Roscioli, C., Moja, L., Gismondo, M. R., & Salerno, F. (2020). Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Science of the Total Environment, 744, 140911.
Sangkham, S. (2021). A review on detection of SARS-CoV-2 RNA in wastewater in light of the current knowledge of treatment process for removal of viral fragments. Journal of Environmental Management, 299, 113563.
Tanhaei, M., Mohebbi, S. R., Hosseini, S. M., Rafieepoor, M., Kazemian, S., Ghaemi, A., Shamloei, S., Mirjalali, H., Aghdaei, H. A., & Zali, M. R. (2021). The first detection of SARS-CoV-2 RNA in the wastewater of Tehran. Iran. Environmental Science and Pollution Research, 28, 38629–38636.
Thakur, A. K., Sathyamurthy, R., Velraj, R., Lynch, I., Saidur, R., Pandey, A. K., Sharshir, S. W., Kabeel, A. E., Hwang, J. Y., & GaneshKumari, P. (2021). Secondary transmission of SARS-CoV-2 through wastewater: Concerns and tactics for treatment to effectively control the pandemic. Journal of Environmental Management., 15(290), 112668.
Tso, C. F., Garikipati, A., Green-Saxena, A., Mao, Q., & Das, R. (2021). Correlation of population SARS-CoV-2 cycle threshold values to local disease dynamics: Exploratory observational study. JMIR Public Health and Surveillance., 7, e28265.
Wang, J., Shen, J., Ye, D., Yan, X., Zhang, Y., Yang, W., Li, X., Wang, J., Zhang, L., & Pan, L. (2020). Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus disease 2019 (COVID-19) pandemic in China. Environmental Pollution, 262, 114665.
Westhaus, S., Weber, F. A., Schiwy, S., Linnemann, V., Brinkmann, M., Widera, M., Greve, C., Janke, A., Hollert, H., Wintgens, T., & Ciesek, S. (2021). Detection of SARSCoV-2 in raw and treated wastewater in Germany — Suitability for COVID-19 surveillance and potential transmission risks. Science of the Total Environment, 751, 141750.
Wong, Y. J., Shiu, H. Y., Chang, J. H. H., Ooi, M. C. G., Li, H. H., Homma, R., Shimizu, Y., Chiueh, P. T., Maneechot, L., & Sulaiman, N. M. N. (2022). Spatiotemporal impact of COVID-19 on Taiwan air quality in the absence of a lockdown: Influence of urban public transportation use and meteorological conditions. Journal of Cleaner Production, 365, 132893.
Wu, Y., Guo, C., Tang, L., Hong, Z., Zhou, J., Dong, X., Yin, H., Xiao, Q., Tang, Y., Qu, X., Kuang, L., Fang, X., Mishra, N., Lu, J., Shan, H., Jiang, G., & Huang, X. (2020). Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterology & Hepatology, 5, 434–435.
Wurtzer, S., Marechal, V., Mouchel, J., Maday, Y., Teyssou, R., Richard, E., Almayrac, J. L., & Moulin, L. (2020). Evaluation of lockdown effect on SARS-CoV-2 dynamics through viral genome quantification in waste water, Greater Paris, France, 5 March to 23 April 2020. Eurosurveillance, 25, 2000776.
Xiao, F., Sun, J., Xu, Y., Li, F., Huang, X., Li, H., Zhao, J., Huang, J., & Zhao, J. (2020). Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerging Infectious Diseases, 26, 1920–1922.
Yamamoto, Y., Nakano, Y., Murae, M., Shimizu, Y., Sakai, S., Ogawa, M., Mizukami, T., Inoue, T., Onodera, T., Takahashi, Y., Wakita, T., Fukasawa, M., Miyazaki, S., & Noguchi, K. (2023). Direct inhibition of SARS-CoV-2 spike protein by peracetic acid. International Journal of Molecular Siences, 24, 20.
Zhang, D., Ling, H., Huang, X., Li, J., Li, W., Yi, C., Zhang, T., Jiang, Y., He, Y., Deng, S., Zhang, X., Wang, X., Liu, Y., Li, G., & Qu, J. (2020). Potential spreading risks and disinfection challenges of medical wastewater by the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Science of the Total Environment, 741, 140445.
Acknowledgements
This project was financially supported by Vice Chancellor Office of Alzahra University, which would like to be appreciated.
Author information
Authors and Affiliations
Contributions
The draft of the manuscript was prepared by E.M.Q. The critical revision of the manuscript as well as edition and supervision of the study was performed by P.M. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qamsari, E.M., Mohammadi, P. Evaluation of SARS-CoV-2 RNA Presence in Treated and Untreated Hospital Sewage. Water Air Soil Pollut 234, 273 (2023). https://doi.org/10.1007/s11270-023-06273-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06273-0