Abstract
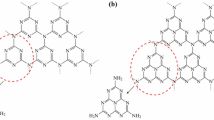
In the present research work, two films of Chitosan-ZnO-α-Fe2O3 nanocatalyst were synthesized by sol gel method using ZnO and Fe2O3 in equimolar concentration along with chitosan. The synthesized catalysts were characterized by fourier-transform infrared spectroscopy, ultraviolet–visible spectroscopy (UV–Vis), X-ray diffraction, field emission scanning electron microscopy and energy dispersive X-ray spectroscopic methods. Morphological studies revealed spongy spherical shaped nanoparticles in both film (A and B) having nanoparticle size 25 nm and 39.5 nm. The synthesized films were employed as a catalyst for reduction of nitrothiophene acetate along with Fe/FeSO4 and the reaction completion was monitored by TLC and UV–Visible spectral analysis. The reduction yield was 79% and 88% respectively for both the film A and film B. Further, the cyclization of formed aminothiophene with trimethylaluminum lead to the formation of dihydrotheinopyrrolone, which was confirmed by liquid chromatography with mass spectral detector and proton nuclear magnetic resonance (1H-NMR) spectroscopic characterization techniques. Thus, the result indicates that the synthesized Chitosan-ZnO-α-Fe2O3 nanocatalysts are efficient over other traditional catalysts for effective reduction of nitro group.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Tan Q, Du C, Sun Y, Yin G, Gao Y (2014) Pd-around-CeO 2–x hybrid nanostructure catalyst: three-phase-transfer synthesis, electrocatalytic properties and dual promoting mechanism. J Mater Chem A 2(5):1429–1435
Tan L, Wu X, Chen D, Liu H, Meng X, Tang F (2013) Confining alloy or core–shell Au–Pd bimetallic nanocrystals in silica nanorattles for enhanced catalytic performance. J Mater Chem A 1(35):10382–10388
Chng LL, Erathodiyil N, Ying JY (2013) Nanostructured catalysts for organic transformations. Acc Chem Res 46(8):1825–1837
Shylesh S, Schünemann V, Thiel WR (2010) Magnetically separable nanocatalysts: bridges between homogeneous and heterogeneous catalysis. Angew Chem Int Ed 49(20):3428–3459
Baig RN, Nadagouda MN, Varma RS (2014) Ruthenium on chitosan: a recyclable heterogeneous catalyst for aqueous hydration of nitriles to amides. Green Chem 16(4):2122–2127
Lamblin M, Nassar-Hardy L, Hierso JC, Fouquet E, Felpin FX (2010) Recyclable heterogeneous palladium catalysts in pure water: sustainable developments in Suzuki, Heck, Sonogashira and Tsuji-Trost reactions. Adv Synth Catal 352(1):33–79
Sarkar S, Ponce NT, Banerjee A, Bandopadhyay R, Rajendran S, Lichtfouse E (2020) Green polymeric nanomaterials for the photocatalytic degradation of dyes: a review. Environ Chem Lett 1–12
Kumar MNR (2000) A review of chitin and chitosan applications. React Funct Polym 46(1):1–27
Jana S, Jana S (eds) (2020) Functional chitosan: drug delivery and biomedical applications. Springer Nature. ISBN:978-981-15-0263-7
AbdElhady MM (2012) Preparation and characterization of chitosan/zinc oxide nanoparticles for imparting antimicrobial and UV protection to cotton fabric. Int J Carbohydr Chem 2012:1–6
Sobahi TR, Abdelaal MY, Makki MS (2014) Chemical modification of chitosan for metal ion removal. Arab J Chem 7(5):741–746
Chen K, Shen Z, Luo J, Wang X, Sun R (2015) Quaternized chitosan/silver nanoparticles composite as a SERS substrate for detecting tricyclazole and Sudan I. Appl Surf Sci 351:466–473
Krishnan SK, Prokhorov E, Bahena D, Esparza R, Meyyappan M (2017) Chitosan-covered Pd@ Pt core–shell nanocubes for direct electron transfer in electrochemical enzymatic glucose biosensor. ACS Omega 2(5):1896–1904
Honary S, Ghajar K, Khazaeli P, Shalchian P (2011) Preparation, characterization and antibacterial properties of silver-chitosan nanocomposites using different molecular weight grades of chitosan. Trop J Pharm Res 10(1)
Huang H, Yuan Q, Yang X (2004) Preparation and characterization of metal–chitosan nanocomposites. Colloids Surf B 39(1–2):31–37
Wei D, Sun W, Qian W, Ye Y, Ma X (2009) The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr Res 344(17):2375–2382
Phan TTV, Phan DT, Cao XT, Huynh TC, Oh J (2021) Roles of chitosan in green synthesis of metal nanoparticles for biomedical applications. Nanomaterials 11(2):273
Preethi S, Sangaranarayanan MV (2020) Shape-controlled electrodeposition of silver using chitosan as structure-directing agent on disposable pencil graphite electrodes: low-cost electrocatalysts for the detection of hydrogen peroxide and hydrazine hydrate. J Solid State Electrochem 24(11):2773–2788
Hu H, Xin JH, Hu H, Wang X, Miao D, Liu Y (2015) Synthesis and stabilization of metal nanocatalysts for reduction reactions—a review. J Mater Chem A 3(21):11157–11182
Adimule V, Vageesha P, Bagihalli G, Bowmik D, Adarsha HJ (2019) Synthesis, characterization of hybrid nanomaterials of strontium, yttrium, copper doped with indole schiff base derivatives possessing dielectric and semiconductor properties. In: Emerging research in electronics, computer science and technology Springer, Singapore, pp 1131–1140
Adimule V, Suryavanshi A, BC Y, Nandi SS (2020) A facile synthesis of poly (3‐octyl thiophene): Ni0. 4Sr0. 6TiO3 hybrid nanocomposites for solar cell applications. In: Macromolecular symposia, vol 392, no. 1, p 2000001
Wang X, Li Z, Qu Y, Yuan T, Wang W, Wu Y, Li Y (2019) Review of metal catalysts for oxygen reduction reaction: from nanoscale engineering to atomic design. Chemistry 5(6):1486–1511
Gowda S, Gowda C (2003) Zinc/hydrazine: a low cost-facile system for the reduction of nitro compounds. Indian J Chem 42B:180–183
Kumar PS, Rai KML (2012) Reduction of aromatic nitro compounds to amines using zinc and aqueous chelating ethers: mild and efficient method for zinc activation. Chem Pap 66(8):772–778
Zhou Y, Li J, Liu H, Zhao L, Jiang H (2006) An unusual de-nitro reduction of 2-substituted-4-nitroquinolines. Tetrahedron Lett 47(48):8511–8514
Chen XL, Ai BR, Dong Y, Zhang XM, Wang JY (2017) Hexafluoro-2-propanol-assisted quick and chemoselective nitro reduction using iron powder as catalyst under mild conditions. Tetrahedron Lett 58(37):3646–3649
Gamble AB, Garner J, Gordon CP, O’Conner SM, Keller PA (2007) Aryl nitro reduction with iron powder or stannous chloride under ultrasonic irradiation. Synth Commun 37(16):2777–2786
Oun AA, Shankar S, Rhim JW (2020) Multifunctional nanocellulose/metal and metal oxide nanoparticle hybrid nanomaterials. Crit Rev Food Sci Nutr 60(3):435–460
Adimule V, Revaigh MG, Adarsha HJ (2020) Synthesis and fabrication of Y-doped ZnO nanoparticles and their application as a gas sensor for the detection of ammonia. J Mater Eng Perform 29(7):4586–4596
Das K, Maiti S, Liu D (2014) Morphological, mechanical and thermal study of ZnO nanoparticle reinforced chitosan based transparent biocomposite films. J Inst Eng 95(1):35–41
Mahdavi H, Tamami B (2005) Reduction of nitro-aryl compounds with zinc in the presence of poly [N-(2-aminoethyl) acrylamido] trimethylammonium chloride as a phase-transfer catalyst. Synth Commun 35(8):1121–1127
Ghorai TK (2015) Synthesis of spherical mesoporous titania modified iron-niobate nanoclusters for photocatalytic reduction of 4-nitrophenol. J Market Res 4(2):133–143
Tadic M, Panjan M, Damnjanovic V, Milosevic I (2014) Magnetic properties of hematite (α-Fe2O3) nanoparticles prepared by hydrothermal synthesis method. Appl Surf Sci 320:183–187
Wu W, He Q, Jiang C (2008) Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. Nanoscale Res Lett 3(11):397–415
Baker C, Shah SI, Hasanain SK (2004) Magnetic behavior of iron and iron-oxide nanoparticle/polymer composites. J Magn Magn Mater 280(2–3):412–418
Kavitha AL, Prabu HG, Babu SA, Suja SK (2013) Magnetite nanoparticles-chitosan composite containing carbon paste electrode for glucose biosensor application. J Nanosci Nanotechnol 13(1):98–104
Tian F, Niu L, Chen B, Gao X, Lan X, Huo L, Bai G (2017) A novel magnetic core-shell nanocomposite Fe3O4@ chitosan@ ZnO for the green synthesis of 2-benzimidazoles. J Nanopart Res 19(10):1–12
Giri S, Samanta S, Maji S, Ganguli S, Bhaumik A (2005) Magnetic properties of α-Fe2O3 nanoparticle synthesized by a new hydrothermal method. J Magn Magn Mater 285(1–2):296–302
Gill AL, Harris W (2002) Thienopyrrolidinones, US 2002/0028841A1
File PD (1986) JCPDS Joint Committee on Powder Diffraction Standards (now: International Centre for Diffraction Data, www. icdd. com), Pennsylvania
Mansour H, Omri K, Ammar S (2019) Structural, optical and magnetic properties of cobalt doped hematite nanoparticles. Chem Phys 525:110400
Adimule V, Nandi SS, Yallur BC, Bhowmik D, Jagadeesha AH (2021) Optical, structural and photoluminescence properties of Gd x SrO: CdO nanostructures synthesized by Co precipitation method. J Fluoresc 31(2):487–499
Lopez T, Mendez J, Zamudio T, Villa M (1992) Spectroscopic study of sol-gel silica doped with iron ions. Mater Chem Phys 30(3):161–167
Divya K, Vijayan S, George TK, Jisha MS (2017) Antimicrobial properties of chitosan nanoparticles: mode of action and factors affecting activity. Fibers Polym 18(2):221–230
da Trindade LG, Hata GY, Souza JC et al (2020) Preparation and characterization of hematite nanoparticles-decorated zinc oxide particles (ZnO/Fe2O3) as photoelectrodes for solar cell applications. J Mater Sci 55:2923–2936
Ba-Abbad MM, Takriff MS, Benamor A, Mohammad AW (2017) Size and shape controlled of α-Fe 2 O 3 nanoparticles prepared via sol–gel technique and their photocatalytic activity. J Sol-Gel Sci Technol 81(3):880–893
El-Shishtawy RM, Ahmed NS, Almulaiky YQ (2021) Immobilization of catalase on chitosan/zno and chitosan/zno/fe2o3 nanocomposites: a comparative study. Catalysts 11(7):820
Acknowledgements
Authors are thankful to M. S. Ramaiah University of Applied Sciences, Bangalore for providing basic facilities. Authors also acknowledge their thankfulness to Centre for Nano and Soft Materials, Bangalore, Ramaiah Institute of Technology, Bangalore and University of Mysore for providing the characterization facilities.
Funding
Authors did not receive any funding from institution or organization.
Author information
Authors and Affiliations
Contributions
Dr. SB has designed the work and contributed in writing the manuscript. Mrs. MMP has carried the synthesis of compounds, catalysts and characterization of compounds. Dr. BCY has contributed for the characterization of the nanocatalyst. Dr. PK involved in manuscript preparation.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declared that they do not have any conflict.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pai, M.M., Yallur, B.C., Batakurki, S. et al. Facile Synthesis of Chitosan-ZnO-α-Fe2O3 as Hybrid Nanocatalyst and Their Application in Nitrothiopheneacetate Reduction and Cyclization of Aminothiopheneacetate. Top Catal (2022). https://doi.org/10.1007/s11244-021-01544-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s11244-021-01544-8