Abstract
Cherry virus A, a capillovirus, can infect different Prunus species and be present as a latent infection in orchards. CVA infection was detected in a Hungarian stock collection of Prunus domestica ‘Besztercei Bt. 2’. In our study, different in vitro virus elimination techniques (thermotherapy combined with shoot tip culture and chemotherapy alone or combined with thermotherapy) were used and compared for their efficiency in eliminating CVA from ‘Besztercei Bt. 2’ plum cultivar. Thermotherapy was carried out at 38/36°C (day/night) in a heat chamber followed by the excision of 1–2 mm long shoot tips for plant regeneration. As a chemotherapy agent, ribavirin or zidovudine was added to the multiplication medium at two different concentrations with or without two weeks of thermotherapy pretreatment. The plum shoots tolerated 14–18 days of heat treatment, 64% of the plants regenerated from shoot tips, and 75% of the tested regenerated plants were confirmed by RT‒PCR to be CVA-free. Ribavirin and zidovudine did not negatively affect the survival of the plum shoots at any applied concentrations. Zidovudine alone was not able to efficiently eradicate virus from the treated plantlets, but when zidovudine was combined with heat treatment, the number of CVA-positive plants decreased to 60%. In contrast, ribavirin alone was very efficient at eliminating CVA from all the tested plants when used at a concentration of 25 mg L− 1. To our knowledge, this is the first report of eliminating CVA from Prunus sp. via in vitro techniques.
Key message
The aim was to compare and evaluate the effectiveness of well-known in vitro techniques for eradicating CVA from plum shoot cultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cherry virus A (CVA) – a virus belonging to the genus Capillovirus, family Betaflexiviridae – has been increasingly reported worldwide since its first discovery. CVA was first reported in Germany in sweet cherry (Jelkmann 1995). Since then, it has been detected in several Prunus hosts not only in sweet and sour cherry (Komorowska and Cieślińska 2004; Grimová et al. 2010) but also in apricot, peach, plum, Japanese apricot (Prunus mume) and myrobalan (James and Jelkmann 1997; Barone et al. 2006; Marais et al. 2008; Kesanakurti et al. 2017; Baráth et al. 2018; Ben Mansour et al. 2023; Wani et al. 2024). CVA is graft-transmissible, and although its vector is still unknown, its presence has been reported in pollen (Beaver-Kanuya and Harper 2019). The virus does not appear to cause any obvious symptoms in host plants but is very widespread and is often reported to be present in mixed infections. Together with other viruses e.g., little cherry virus-1, Prunus necrotic ringspot virus, cherry green ring mottle virus, cherry necrotic rusty mottle virus, it may affect the severity of symptoms, or it may have some influence on graft incompatibility in susceptible combinations of scions and rootstocks (James and Jelkmann 1997; Marais et al. 2012).
To create and maintain healthy fruit tree plantations, it is important to use certified virus-free propagation material. In Prunus species, the most widespread methods for eliminating viruses are based on in vitro shoot cultures (Szabó et al. 2024). Meristem culture has been used for a long time to produce virus-free plants (Knapp et al. 1998), but the molecular mechanism of the virus exclusion from the meristem has been only investigated recently. In their research, Wu and coworkers have shown in the Arabidopsis model plant that a transcription factor WUSCHEL controls the invasion of the meristem by several viruses and block their vertical transmission into the shoot apical meristem (SAM) (Wu et al. 2020). There is evidence that nepoviruses and tobraviruses can enter into the meristem in different hosts, but it seems that the viral movement into the SAM could happen through several different mechanisms which can be different in different host/virus interactions ((Bradamante et al. 2021). During thermotherapy, the replication of the virus is inhibited and the degradation of the RNA increases, so the movement of the virus towards the meristematic cells can slow down, and the newly formed shoot tips can remain virus-free, allowing the excision of a larger portion of the shoot tips, which can increase the probability of successful plant regeneration (Bettoni et al. 2022). In general, the size of excised shoot tips is positively correlated with survival and shoot regeneration but negatively correlated with successful virus eradication (Wang 2018).
Stone fruits are known to be especially sensitive to high temperatures and have a low regeneration capacity from meristems (Lenz et al. 1983; Manganaris et al. 2003; Polák and Hauptmanová 2009; Şekerz et al. 2015). In vitro thermotherapy followed by shoot tip excision and culture can shorten the treatment time compared to in vivo thermotherapy and increase the regeneration capacity compared to meristem isolation, thereby increasing the efficiency of the elimination process (Stein et al. 1991; Koubouris et al. 2007). Peach and cherry cultivars are extremely heat sensitive (Lenz et al. 1983; Polák and Hauptmanová 2009), while plums can tolerate long-term high temperatures (Dziedzic 2008). The duration and degree of heat treatment should be optimized for each genotype because of the different heat tolerances of individual plant species and cultivars and the resilience of virus species (Gella and Errea 1998; Cieślińska 2007).
Chemotherapy is also used for plant virus elimination. The addition of antiviral agents to culture media can inhibit virus replication in host plants. The most common antiviral agent for plant virus eradication is ribavirin (Virazol). It is a synthetic analogue of guanosine and inhibits viral RNA synthesis by integrating it into newly synthesized RNA (Thomas et al. 2012). Ribavirin has been employed to eliminate the most common viruses from infected sweet cherry, myrobalan, plum, apricot, pear and apple cultivars (Deogratias et al. 1989; Cieślińska 2007; Hauptmanová and Polák 2011; Hu et al. 2012, 2015; Şekerz et al. 2015; Mazeikiene et al. 2019; Zare Khafri et al. 2024). Zidovudine (azidothymidine) has been proven to be a more effective chemotherapeutic agent than ribavirin for virus eradication in peach plants, without causing phytotoxic effects (Pavelkova et al. 2015). No phytotoxic effects of zidovudine were visible on shoot growth of apple cultures even in a wide concentration range up to 1280 mg L− 1 (Sedlák et al. 2023). As a thymidine analogue, zidovudine inhibits reverse transcriptase and prevents the reproduction of retroviruses in the host genome (Veal and Back 1995). Chemotherapy alone is often ineffective or only eliminates viruses with little efficacy, but after preliminary heat treatment, it results in many virus-free plants (Spiegel et al. 1999; Cieślińska 2007; Hu et al. 2012, 2015). The combination of in vitro heat treatment, meristem culture and ribavirin has been reported to be effective against plum pox virus (PPV) despite the reduction in the regeneration capacity of plum plants (Jakab-Ilyefalvi and Pamfil 2011).
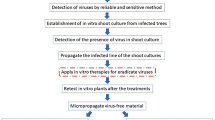
In this study, we used different methods to eliminate latent CVA from the plum cultivar ‘Besztercei Bt. 2’ investigating the effects of heat treatment length, different concentrations of ribavirin and zidovudine and the combinations of such factors. The objective of this study was to compare and evaluate the effectiveness of well-known in vitro techniques for successfully eradicating CVA from plum shoots.
Materials and methods
Plant material and culture conditions
CVA-infected (preliminarily tested by RT‒PCR) Prunus domestica cultivar ‘Besztercei Bt. 2’ was selected from the stock plantation of the Research Centre for Fruit Growing, Hungarian University of Agriculture and Life Sciences (MATE), Érd, Hungary, to establish in vitro shoot cultures. Shoots were surface sterilized in HgCl2 solution (4 g L− 1, 6 min) and then rinsed in sterile distilled water three times. Two-node-long shoot segments were cultured on multiplication media: modified MS media (Murashige and Skoog 1962) supplemented with MS macroelements, FeNaEDTA (20 mg L− 1), microelements according to Balla and Vertesy (2001), myo-inositol (500 mg L-1), Jacquiot vitamins (Jacquiot 1955), phloroglucinol (10 mg L− 1), 6-benzylaminopurine (BAP, 0.25 mg L− 1), indole-3-butyric acid (IBA, 0.1 mg L− 1), gibberellic acid (GA3, 0.3 mg L− 1), sucrose (30 g L− 1) and agar (6 g L− 1). pH of the medium was adjusted to 5.2 before autoclaving at 121 °C for 20 min. In vitro plants were kept in a growth room at 24 ± 1 °C under 16 h photoperiod with a light intensity of 60 μM m− 2 s− 1 provided by warm and cool white fluorescent lamps, and subcultured every four weeks. After successful shoot culture establishment, the virus presence was confirmed by RT‒PCR, and virus-infected lines were selected and propagated for virus elimination treatments.
Virus elimination methods
Combination of in vitro thermotherapy and shoot tip culture
Two-week-old shoot cultures were transferred into a climate chamber for heat treatment at 38/36°C (day/night) and 12 h of light (160 μmol m− 2 s− 1). After 14–23 days of heat treatment, 1–2 mm long shoot tips (containing 2–4 leaf primordia) were cut off under a stereomicroscope under sterile conditions and maintained on multiplication media in Petri dishes for regeneration. Shoot tips were subcultured to fresh medium every 4 weeks.
Chemotherapy
After autoclaving, ribavirin (Duchefa, The Netherlands) or zidovudine (Sigma‒Aldrich, USA) was added to the multiplication media by filter sterilization (Millipore, 0.22 μm, PES membrane) to a final concentration of 25 or 50 mg L-1. Five in vitro shoots (1 cm long) per vessel were maintained on a multiplication medium containing the antiviral agent for 2 successive subcultures each lasting four weeks (total 8 weeks). After 8 weeks, the apical parts (1 cm long) of the treated shoots were removed and cultured as separated lines on antiviral agent-free media until virus indexing.
As a control, 1 cm long shoots were maintained on multiplication media without antiviral agents for 8 weeks. For each treatment, 15 shoots were treated.
Combinations of thermotherapy and chemotherapy
After two weeks of in vitro thermotherapy, 1 cm long apical shoots were cut and transferred onto multiplication media supplemented with the antiviral agent zidovudine at concentrations of 25 or 50 mg L-1 for 8 weeks with one subculture. After 8 weeks, the shoots were cultured on antiviral agent-free media until virus indexing.
As a control group, shoot cultures were treated with thermotherapy for two weeks without chemotherapy.
Virus detection
After treatment, whole micropropagated shoots without basal calli were collected to determine infection status. RNA was extracted from the in vitro plant samples via the CTAB method (Gambino et al. 2008). For virus detection, the reverse-transcription PCR method (RT‒PCR) was used. cDNA synthesis was performed from the extracted RNA with a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA) using random primers according to the manufacturer’s instructions. PCR was performed using Phire Hot Start II DNA Polymerase (Thermo Fisher Scientific), and virus-specific primers CVAmp_Fm (ATGTCGATCATACCAGTYAAG) and CVAmp_Rm (TTACTTCTCGCAACYAC) were used (Baráth et al. 2018). The PCR results were evaluated via gel electrophoresis of the PCR products.
Results
Virus elimination using thermotherapy combined with shoot tip excision
Forty-nine shoot tips 1–2 mm long were excised after thermotherapy (Fig. 1D), 19 shoot tips (39%) died early (turned brown or undesirable calli had formed and were unable to regenerate). In total, 20 in vitro plants regenerated from shoot tips were tested via RT‒PCR for the presence of CVA. Fifteen plants (75%) were CVA-free, while 25% remained infected (Table 1).
Examining the effect of heat treatment length, plum cultures were able to tolerate heat treatment at 38/36°C even for a long period (23 days),- but when shoot tips were excised after this long treatment period, they died quickly and were unable to regenerate. The plants tolerated the 15-day heat treatment well; they remained green and vigorous, and their shoot tips were intact and suitable for preparation (Fig. 1B). The excised shoot tips could regenerate to proliferable shoots after 6 months (Fig. 1E-F) at a high level (69%). All the excised shoot tips were labelled and the successfully regenerated plantlets were able to be individually tested, and 78% of them became CVA-free. Our small-scale pilot test showed that the length of thermotherapy did not increase or reduce the effectiveness of virus elimination within the time interval used (14–18 days), most of CVA-free plants (78%) resulted after 15 days, but after 14 or 18 days the elimination rates were 75%. However, after 3 weeks of treatment, the harmful consequences of long-term thermotherapy became visible, as the plants significantly weakened, turned yellow, and their tips died (Fig. 1C).
Virus elimination using chemotherapy alone or in combination with heat treatment
The plant survival rate was not negatively affected by any of the antiviral agents used at concentrations of 25 or 50 mg L− 1. No significant phytotoxic effects were observed during these treatments. Zidovudine alone was not able to efficiently eliminate CVA at any concentration; however, treatment with zidovudine (25 mg L− 1) after heat treatment increased the elimination from 7 to 40%. Ribavirin applied at a lower concentration (25 mg L− 1) was very efficient, and no CVA was detected in the tested plants. At a higher concentration (50 mg L− 1) of ribavirin, 40% of the tested plants were virus-free. Surprisingly, some of the control plants (7–25%) tested negative for CVA.
Discussion
In the case of CVA-infected plum cultivar ‘Besztercei Bt. 2’, 1–2 mm long shoot tip isolation following in vitro thermotherapy proved to be a suitable method for eliminating CVA and producing CVA-free plants. The size of the excised tip seemed to be a crucial factor in the elimination procedure. We tried to achieve the smallest possible size that could survive and regenerate but was free from the virus. In our work, we isolated 1- to 2-mm long shoot tips. Even using these relatively large shoot tips, not limited only to the meristematic region, 39% of the shoot tips died early (turned brown or undesirable calli had formed and were unable to regenerate). Zarghami and Ahmadi (2023) reported that the survival rate of peach cultivars decreased from 65 to 20% with decreasing meristem size (from 1.0 to 0.2 mm). The use of a larger (1–2 mm) shoot tip in combination with heat treatment was effective in eliminating viruses from apricots and nectarines (Koubouris et al. 2007; Manganaris et al. 2003). In vitro thermotherapy of plum shoots at 38/36°C for 15 days proved to be an ideal duration, as the plants could tolerate this length of time at increased temperature, and remained green and vigorous, their shoot tips were intact and suitable for excision. The shoot tips that were excised after 15 days of heat treatment and further grown on culture medium resulted in 69% regeneration, and 78% of the tested regenerated plantlets were confirmed to be CVA-free. During the examined time interval (14–18 days), the length of thermotherapy did not change the effectiveness of virus eradication. Abdullahi and Lawrence (2022) reported that 7 days of heat treatment at 37/32°C prior to meristem preparation was sufficient to eliminate apple chlorotic leaf spot virus (ACLSV) from Shiro plum, while Koubouris et al. (2007) applied heat treatment for 20 days at 35–37 °C to eliminate PPV from the apricot cultivar ‘Bebecou’. Hesari et al. (2022) applied heat treatment at 37 °C for 4 weeks and this resulted in 100% PPV-free and Prunus necrotic ring spot virus (PNRSV)-free peach plants. A 45-day-long period at 37/35°C was suitable for eliminating PPV, PNRSV and prune dwarf virus (PDV) from apricot cultivars (Křižan and Ondrušiková 2009).
The plum cultivar ‘Besztercei Bt. 2’ proved to be tolerant to the applied chemical treatments, and no significant phytotoxic effects or plant deaths occurred even at high (50 mg L− 1) concentrations of the agents used. Interestingly, even during treatment with ribavirin or zidovudine, a lower concentration (25 mg L− 1) resulted in more virus-free plants, which is not consistent with previously published results (Cieślińska 2007; Deogratias et al. 1989; Paunovic et al. 2007); however, Cieślińska (2007) obtained more ACLSV-free plants using ribavirin at concentration of 50 mg L− 1 than the concentration of 100 mg L− 1. Hauptmanová and Polák (2011) also obtained similar results when 5 mg L− 1 ribavirin removed PPV from apricot (57%), while using a treatment of 10 mg L− 1 ribavirin, all treated shoots remained infected after 9 weeks of chemotherapy, and hence they concluded that the treatment period should be extended to 12 weeks for effective virus elimination. Cieślińska (2007) showed that ribavirin at concentrations of 10, 25, 50 and 100 mg L− 1 was effective in eliminating ACLSV from 37 to 85% of myrobalan and PNRSV from 38 to 100% of the ‘Empress’ plum, but elimination of PNRSV from myrobalan and PDV from ‘Early Rivers’ sweet cherry shoots was successful only if chemotherapy was combined with thermotherapy. Regenerating the apical part of the axis (approximately 3 mm in length) after 20 mg L− 1 ribavirin treatment for 4 weeks was suitable for obtaining the PDV-free cherry cultivars ‘Tamara’ and ‘Amid’ (Paprstein et al. 2019). Ribavirin at higher concentrations (50–100 mg L− 1) is often phytotoxic to shoots (Cieślińska 2002, 2007; Deogratias et al. 1989; Vescan et al. 2011; Sedlák et al. 2023). Hauptmanová and Polák (2011) successfully eliminated PPV from plum and apricot plants by long-term treatment at lower ribavirin concentrations (5 or 10 mg L− 1). Even a very low concentration (1 mg L− 1) and short treatment time (4 weeks) were suitable for the in vitro elimination of PPV from apricots (Şekerz et al. 2015). Chemotherapy with lower concentrations of antiviral agents in combination with heat treatment was found to be effective in eliminating PPV from plums (Jakab-Ilyefalvi and Pamfil 2011). Zare Khafri et al. (2024) reported results similar to our work on apricot cultivars, where thermotherapy with shoot tip (1–2 mm) culture resulted in the highest eradication rate (90–100%) but lower regeneration rate (60–80%); however, with the use of chemotherapy (25 mg L− 1 ribavirin), more virus-free plants were obtained overall. The differences between reported survival rates and the success of the virus elimination process obtained in these studies may be due to the host species and cultivars as well as the type and strategy of the infecting virus.
The resulting CVA-free untreated control plants indicating the possibility of spontaneous loss of the virus during long-term in vitro maintenance (Nacheva et al. 2002; Chandel 2010). However, the reason could also be a very low concentration of the virus in the shoots, so the RT-PCR method was not sensitive enough to detect the presence of the virus (Jevremović et al. 2023).
In the present study, thermotherapy at 38/36°C for 14–18 days combined with shoot tip (1–2 mm) culture resulted in a high percentage of virus-free plants; however, this could be achieved with significant plant mortality. Chemotherapy (ribavirin at 25 mg L− 1) completely eradicated the virus from the plum shoots without causing plant mortality. Based on our results, chemotherapy can effectively eradicate CVA from plum cultivar ‘Besztercei Bt. 2’ with a high plant survival rate in a shorter treatment time compared to thermotherapy followed by shoot tip culture. To our knowledge, this is the first report of eliminating CVA from in vitro Prunus shoot culture(s). Although CVA infection has not been correlated with any disease, the possibility that it could influence the severity of symptoms in mixed infections must be considered; therefore, our virus elimination study may be useful in the future.
Data availability
All the data mentioned are publicly available in this manuscript or in the cited scientific articles.
Abbreviations
- ACLSV:
-
Apple chlorotic leaf spot trichovirus
- CVA:
-
Cherry capillovirus A
- PDV:
-
Prune dwarf ilarvirus
- PNRSV:
-
Prunus necrotic ringspot ilarvirus
- PPV:
-
Plum pox potyvirus
- RT‒PCR:
-
Reverse transcription‒polymerase chain reaction
- SAM:
-
Shoot apical meristem
References
Abdullahi I, Lawrence T (2022) Accelerated in vitro thermotherapy and indexing against apple chlorotic leaf spot virus in Shiro plum. Can J Plant Pathol 44(1):136–146
Balla I, Vértesy J (2001) In vitro culture of Hungarian apricot (Prunus armeniaca L.) varieties. Acta Hortic 560:395–398. https://doi.org/10.17660/ActaHortic.2001.560.75
Baráth D, Jaksa-Czotter N, Molnár J, Varga T, Balássy J, Szabó LK, Várallyay É (2018) Small RNA NGS revealed the presence of cherry virus A and little cherry virus 1 on apricots in Hungary. Viruses 10(6):318
Barone M, Alioto D, Marais A, Candresse T, Ragozzino A (2006) First report and high prevalence in noncherry host of cherry virus A in Italy. Plant Dis 90(11):1459–1459
Beaver-Kanuya E, Harper SJ (2019) Detection and quantification of four viruses in prunus pollen: implications for biosecurity. J Virol Methods 271:113673
Ben Mansour K, Komínek P, Komínková M, Brožová J (2023) Characterization of prunus necrotic ringspot virus and cherry virus a infecting myrobalan rootstock. Viruses 15(8):1723
Bettoni JC, Fazio G, Carvalho Costa L, Hurtado-Gonzales OP, Rwahnih MA, Nedrow A, Volk GM (2022) Thermotherapy followed by shoot tip cryotherapy eradicates latent viruses and apple hammerhead viroid from in vitro apple rootstocks. Plants 11(5):582
Bradamante G, Mittelsten Scheid O, Incarbone M (2021) Under siege: virus control in plant meristems and progeny. Plant Cell 33(8):2523–2537
Chandel V, Rana T, Kumar V, Hallan V, Zaidi AA (2010) Stone fruit viruses: economic importance and biotechnological approaches towards management strategies. Antibodies Plant Virus 3:58–88
Cieślińska M (2002) Elimination of apple chlorotic leaf spot virus (ACLSV) from pear by in vitro thermotherapy and chemotherapy. In VIII International Symposium on Pear 596 (pp. 481–484)
Cieślińska M (2007) Application of thermo-and chemotherapy in vitro for eliminating some viruses. J Fruit Ornam Plant Res 15:117–124
Deogratias JM, Dosba F, Lutz A (1989) Eradication of prune dwarf virus, prunus necrotic ringspot virus, and apple chlorotic leaf spot virus in sweet cherries by a combination of chemotherapy, thermotherapy and in vitro culture. Can J Plant Pathol 11(4):337–342
Dziedzic E (2008) Elimination of prunus necrotic ring spot virus (pnrsv) from plum ‘earliblue’ shoots through thermotherapy in vitro. J Fruit Ornam Plant Res 16:101–109
Gambino G, Perrone I, Gribaudo I (2008) A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal 19(6):520–525
Gella R, Errea P (1998) Application of in vitro therapy for ilarvirus elimination in three Prunus species. J Phytopathol 146(8–9):445–449
Grimová L, Zouhar M, Ryšánek P, Drabešová J, Mazáková J, Paprštein F (2010) First occurrence of cherry virus a (cva) in the Czech Republic. Julius-Kühn-Archiv 427:275
Hauptmanová A, Polák J (2011) The elimination of plum pox virus in plum cv. bluefree and apricot cv. Hanita by chemotherapy of in vitro cultures. Hort Sci (Prague) 38:49–53
Hesari N, Haji Mohammadi A, Zarghami R, Fakheri B, Kiss-Bába E, Szegő A, Mirmazloum I (2022) Eradication of PPV and PNRSV viruses from three peach cultivars using thermotherapy in vitro, including optimization of microshoots’ multiplication and rooting medium. Horticulturae 8(10):929
Hu GJ, Hong N, Wang LP, Hu HJ, Wang GP (2012) Efficacy of virus elimination from in vitro-cultured sand pear (Pyrus pyrifolia) by chemotherapy combined with thermotherapy. Crop Prot 37:20–25
Hu G, Dong Y, Zhang Z, Fan X, Ren F, Zhou J (2015) Virus elimination from in vitro apple by thermotherapy combined with chemotherapy. Plant Cell Tissue Organ Cult (PCTOC) 121:435–443
Jacquiot C (1955) Sur La culture in vitro de tissue cambiol de Chataignier (Castanea vesca Gaertn). Compt Rend Acad Sci Paris 240:557–558
Jakab-Ilyefalvi Z, Pamfil D (2011) Results regarding the classical and modern pathogen elimination techniques of plum pox virus at plum (Prunus domestica L). Annals Romanian Soc Cell Biology 16(1)
James D, Jelkmann W (1997) Detection of cherry virus A in Canada and Germany. In XVII International Symposium Virus and Virus-Like Diseases of Temperate Fruit Crops 472 (pp. 299–304)
Jelkmann W (1995) Cherry virus A: cDNA cloning of dsRNA, nucleotide sequence analysis and serology reveal a new plant capillovirus in sweet cherry. J Gen Virol 76(8):2015–2024
Jevremović D, Vasilijević B, Anđelić T, Vujović T (2023) Effect of D and V cryo-plate methods for plum pox virus eradication from two plum cultivars. Plant Cell Tissue Organ Cult (PCTOC) 152(3):529–538
Kesanakurti P, Belton M, Saeed H, Rast H, Boyes I, Rott M (2017) Comparative analysis of cherry virus a genome sequences assembled from deep sequencing data. Arch Virol 162:2821–2828
Knapp E, Hanzer V, Mendonca D, da Câmara Machado A, Katinger H, de Câmara Machado ML (1998) Improved virus detection in rosaceous fruit trees in vitro. Plant Cell Tissue Organ Cult 52(1–2):3–6
Komorowska B, Cieślińska M (2004) First report of cherry virus A and little cherry virus-1 in Poland. Plant Dis 88(8):909–909
Koubouris GC, Maliogka VI, Efthimiou K, Katis NI, Vasilakakis MD (2007) Elimination of plum pox virus through in vitro thermotherapy and shoot tip culture compared to conventional heat treatment in apricot cultivar Bebecou. J Gen Plant Pathol 73(5):370–373
Křižan B, Ondrušiková E (2009) Thermotherapy of apricot cultivars. In I International Symposium on Biotechnology of Fruit Species: BIOTECHFRUIT2008 839 (pp. 269–274)
Lenz F, Baumann G, Kornkamhaeng P (1983) High temperature treatment of Prunus avium L.F12/1 for virus elimination. J Phytopathol 106(4):373–375
Manganaris GA, Economou AS, Boubourakas IN, Katis NI (2003) Elimination of PPV and PNRSV through thermotherapy and meristem-tip culture in nectarine. Plant Cell Rep 22(3):195–200. Phytopathology 26(9)
Marais A, Faure C, Svanella-Dumas L, Candresse T (2008) First report of cherry virus A in Prunus mume in China. Plant Dis 92(11):1589–1589
Marais A, Svanella-Dumas L, Barone M, Gentit P, Faure C, Charlot G, Candresse T (2012) Development of a polyvalent RT‐PCR detection assay covering the genetic diversity of cherry capillovirus A. Plant Pathol 61(1):195–204
Mazeikiene I, Kviklys D, Siksnianiene JB, Zinkus D, Stanys V (2019) Influence of ribavirin on Prunus domestica L. regeneration, genome stability and virus eradication in vitro. In Proceedings of the Latvian Academy of Sciences (Vol. 73, No. 3, pp. 238–243). De Gruyter Poland
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nacheva L, Milusheva S, Ivanova K (2002) Elimination of plum pox virus (PPV) in plum (Prunus domestica L.) cvs Kyustendilska Sinya and Veljevka through in vitro techniques. In VII International Symposium on Plum and Prune Genetics, Breeding and Pomology 577 (pp. 289–291)
Paprstein F, Sedlak J, Polak J, Kumari S (2019) Elimination of PDV from sweet cherry cultivars by in vitro chemotherapy. Acta Hortic. 1242, 31–34. https://doi.org/10.17660/ActaHortic.2019.1242.4
Paunovic S, Ruzic D, Vujovic T, Milenkovic S, Jevremovic D (2007) In vitro production of plum pox virus-free plums by chemotherapy with ribavirin. Biotechnol Biotec Eq 21(4):417–421
Pavelkova R, Kudelkova M, Ondrusikova E, Eichmeier A (2015) Virus elimination in Peach Cv. ‘Red haven’by Chemotherapy. Agricultural Commun 3(2):16–20
Polák J, Hauptmanová A (2009) Preliminary results of in vivo thermotherapy of plum, apricot and peach cultivars artificially infected with PPV-M and PPV-D strains of Plum pox virus. Hortic Sci 36:92–96
Sedlák J, Přibylová J, Koloňuk I, Špak J, Lenz O, Semerák M (2023) Elimination of solanum nigrum ilarvirus 1 and apple hammerhead viroid from apple cultivars using antivirals ribavirin, rimantadine, and zidovudine. Viruses 15(8):1684
Şekerz MG, Süzerer V, Elibuyuk IO, Çiftçi YÖ (2015) In vitro elimination of PPV from infected apricot shoot tips via chemotherapy and cryotherapy. Int J Agriculturebiology 17(5)
Spiegel S, Tam Y, Rosner A, Brison M, Helliot B, Pierronnet A, De Boucaud MT (1999) In vitro elimination of Prunus necrotic ringspot virus in a plum cultivar. Plant Biotechnology and in vitro Biology in the 21st Century. Springer Netherlands, pp 545–547
Stein A, Spiegel S, Faingersh G, Levy S (1991) Responses of micropropaged peach cultivars to thermotherapy for elimination of Prunus necrotic ringspot virus. Ann Appl Biol 119:265–271
Szabó LK, Desiderio F, Kirilla Z, Hegedűs A, Várallyay É, Preininger É (2024) A mini-review on in vitro methods for virus elimination from Prunus sp. fruit trees. Plant Cell Tissue Organ Cult (PCTOC) 156(2):42
Thomas E, Ghany MG, Liang TJ (2012) The application and mechanism of action of ribavirin in therapy of hepatitis C. Antiviral Chem Chemother 23(1):1–12
Veal GJ, Back DJ (1995) Metabolism of zidovudine. Gen Pharmacology: Vascular Syst 26(7):1469–1475
Vescan LA, Pamfil D, Zagrai I, Ioana VB, Clapa D, Ciuzan O, Iulia F (2011) In vitro techniques for plum pox virus elimination from two infected Romanian plum cultivars. Bull UASVM Anim Sci Biotechnologies 68:1–2
Wang MR, Cui ZH, Li JW, Hao XY, Zhao L, Wang QC (2018) In vitro thermotherapy-based methods for plant virus eradication. Plant Method 14:1–18
Wani S, Shah MD, Padder BA et al (2024) Occurrence of Cherry Virus A (CVA) infecting stone fruits in Kashmir province of Northwestern Himalayan Region of India. Indian Phytopathol. https://doi.org/10.1007/s42360-023-00704-y
Wu H, Qu X, Dong Z, Luo L, Shao C, Forner J, Zhao Z (2020) WUSCHEL triggers innate antiviral immunity in plant stem cells. Science 370(6513):227–231
Zare Khafri A, Zarghami R, Naderpour M, Ahmadi B, Mirzaei L (2024) Assessment of virus eradication methods from infected in vitro-grown apricot cultures. Plant Cell Tissue Organ Cult (PCTOC) 156(2):1–13
Zarghami R, Ahmadi B (2023) Production of plum pox virus-free and Prunus necrotic ringspot virus-free regenerants using thermotherapy and meristem-tip culture in Prunus persica L. Erwerbs-Obstbau 65(4):719–727
Acknowledgements
The authors are grateful to all the laboratory workers who helped with the laboratory work in the background. Special thanks to Gábor Radócz and Sámuel Szilágyi for taking the plant photos. LKS and FD are PhD students at Hungarian University of Agriculture and Life Sciences at the Doctoral School of Horticultural Sciences and Doctoral School of Biological Sciences, respectively.
Funding
Open access funding provided by Hungarian University of Agriculture and Life Sciences. This work was financially supported by NKFIH K127951.
Open access funding provided by Hungarian University of Agriculture and Life Sciences.
Author information
Authors and Affiliations
Contributions
ÉP and ÉV conceived the idea and designed the experiments. LKS conducted the research; ZK helped with the maintenance of plant cultures and the implementation of treatments; and DF assisted in virus testing and data analysis. All the authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or nonfinancial interests to disclose.
Additional information
Communicated by Cristian Silvestri.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szabó, L.K., Desiderio, F., Kirilla, Z. et al. Elimination of cherry virus A from Prunus domestica ‘Besztercei Bt. 2’ using in vitro techniques. Plant Cell Tiss Organ Cult 157, 45 (2024). https://doi.org/10.1007/s11240-024-02770-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-024-02770-0