Abstract
Several viruses are known to infect stone fruit trees and cause serious problems in their cultivation; hence, it is essential to use virus-free and healthy propagation material to establish a plantation. As stone fruit trees are propagated vegetatively, both the scion and the rootstock should be pathogen-free. The traditional method for plant virus eradication is meristem culture after in vivo thermotherapy. Prunus species are extremely sensitive to high temperature and have low regeneration capacity from meristem explants which makes it difficult to apply the conventional method. To avoid the application of meristem culture it has become necessary to search for additional methods. The most widespread elimination methods are based on in vitro shoot cultures. It is a challenge to find an optimal method with high efficacy in virus elimination that has little harmful effect on plantlets. In the present study, we collected the elements of current knowledge about viruses and viroids that are able to infect Prunus species and reviewed recent methods that have been used efficiently to eliminate them from Prunus species, applying thermotherapy alone or in combination with shoot tip excision, chemotherapy, cryotherapy or electrotherapy. The key factors influencing the effectivity of virus elimination procedure and in vitro culture survival are also discussed.
Similar content being viewed by others
Introduction
Viruses infecting stone fruits cause serious problems in cultivation and fruit production, reducing quality and quantity. Biotic stresses caused by viruses and viroids can induce symptoms on the leaves and fruits of Prunus species, frequently affecting the economic value of the fruit, decreasing the yield, deteriorating the health of the trees, and eventually leading to the decline of the tree (Rubio et al. 2017). The symptoms and severity of the infection depend on the host plant species and on the virus itself. There is no conventional plant protection method available against viruses; therefore, the use of healthy propagating material and plant protection against vectors are essential. The maintenance of healthy fruit tree propagation materials is fundamental to keeping plantations healthy.
All Prunus viruses and viroids are transmitted by vegetative propagation techniques or by a vector (usually an insect, mite, or nematode) (Rubio et al. 2017). Horizontal transmission by pollen and vertical transmission by seeds have been described for Prune dwarf virus (PDV) and Prunus necrotic ringspot virus (PNRSV) (Mink 1993; Card et al. 2007; Amari et al. 2009). Two comprehensive studies of virus and viroid situations in Prunus species were published a few years ago (Hadidi et al. 2011; Rubio et al. 2017). The present review completes the data with recent significant detections in new host species or new viruses in the last several years (Baráth et al. 2018; Matic et al. 2007; Zhang et al. 2021; Jo et al. 2019; Zheng et al. 2020; Kinoti et al. 2020; Candresse et al. 2017) in Table 1. As detection methods are developing quickly, sensitive techniques (nucleic acid‒based PCR and high-throughput sequencing) enable the identification of viruses from plant species never detected before. Hou et al. (2020) described the discovery of 22 new viruses for stone fruit species between 2011 and 2020 that calls attention to a serious threat still without a clear solution.
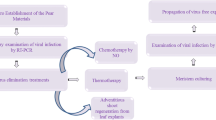
Several methods can be used to produce virus-free plants. In this review, we collected reports about the elimination of viruses from Prunus species using in vitro techniques, presented in Table 2, which offer the availability of healthy non-infected stone fruit plant material. The production of virus-free plants from infected trees is a time- and labor-consuming procedure, as shown in Fig. 1.
Traditional method – in vivo heat treatment and shoot tip grafting or culturing
Correlation between temperature and virulence has been observed for many years. Virus symptoms are the most noticeable at lower temperatures, typically in early spring. In hot summers, the symptoms may even disappear because the titre of virus decreases in plants (Kassanis 1952). RNA silencing, a conserved defense system operating in eukaryotic cells, is also present in plants and is inhibited at low temperature, which could explain the periodic appearance or disappearance of symptoms (Szittya et al. 2003). During heat treatment, the plant’s own defense mechanism is induced by high temperature to eliminate viral nucleic acids. The traditional method for virus elimination is heat treatment or thermotherapy. During heat treatment, the potted plants were kept in a heat chamber at 36 to 38 °C at least 2 weeks (Minoiu 1975, Koubouris et al. 2007, Polak and Hauptmanova 2009, Howell et al. 2000). The choice of a thermotherapy regime should allow the treated plant to survive and simultaneously inactivate the virus, thus resulting in growing virus-free shoot tips.
Kunkel was one of the first to use heat treatment in peaches to cure from “peach yellow” infection (Kunkel 1936). After immersing the bud sticks in a water tank at 34° to 35° for 4 to 5 days, the shoot tips of the treated plants were grafted onto virus-free rootstock (Kunkel 1936). Later, Polak and Hauptmanova (2009) carried out an experiment where after heat treatment at 37 °C lasting for 15 or 22 days in a thermal room whole potted plants were found to be free from viruses.
The disadvantage of this method is that stone fruits are especially sensitive to high temperature. Thermosensitivity means that only a few plants survive until the end of the treatment, while the eradication itself seems inefficient as the shoot tips of the treated plants were detected in several studies to remain virus infected (Polak and Hauptmanova 2009, Manganaris et al. 2003, Koubouris et al. 2007). In experiments on peach or nectarine cultivars, most of the peach trees died during heat treatment at 37 °C lasting for 15 or 22 days, nectarine plants subjected to 38 °C heat treatment collapsed and died, due to desiccation but with preconditioning (increasing temperature from 28 to 35 °C gradually over 1 week) potted plants were maintained at 35 °C for 2 more weeks until new growth was produced (Polak and Hauptmanova 2009, Manganaris et al. 2003). The sweet cherry cultivars tested did not survive after the 6th day of treatment with thermotherapy; thus, a specific method was described using a modified atmosphere in a heat chamber to allow plants to survive 40 °C for more than 3 weeks (Lenz et al. 1983). Cherries grown in a hydroponic culture system were able to tolerate the heat treatment (alternating temperatures between 40 °C and 32 °C for 14–77 days) sufficient to reduce virus levels in growing shoots. (Howell et al. 2000). In general, peach and cherry cultivars are known for their thermosensibility (Polak and Hauptmanova 2009, Lenz et al. 1983), while plum can tolerate long-term high temperatures (Dziedzic 2008), even at alternating temperatures of 38 °C for two weeks, 38/46°C 16/8 h for three weeks and 50 °C for two hours/day for three days for a total of 37 days thermotherapy which proved to be sufficient to inactivate Plum pox virus (PPV) (Minoiu 1975).
In vitro techniques
The advantage of in vitro tissue culture is that a large amount of homogeneous plant material is available with a limited space requirement as well as year-round availability. It gives the opportunity to carry out experiments in sterile conditions independently of weather and external influences and can speed up the elimination procedure compared to conventional heat treatment (Abdullahi and Lawrence 2022; Koubouris et al. 2007). Tissue cultures are widely used in plant pathogen eradication. Somatic embryos can be produced from induced non-vascular tissues. As most viruses are limited to vascular tissues, it is possible to produce virus-free plants by somatic embryogenesis, but the mechanism is not clear and seems genotype dependent (Panattoni et al. 2013). The disadvantage of the method is that it involves an increased risk of genetic instability and somaclonal variability (Etienne et al. 2016). Somatic embryogenesis can be used with high efficiency and safety, as was shown in the case of grapevine, where it was used to eliminate various viruses and viroids from different cultivars (Goussard et al. 1991; Gambino et al. 2006; Olah et al. 2022; Turcsan et al. 2020). This method was successfully used for almonds (Ebrahimi et al. 2022), but no report has been written yet regarding other stone fruits.
In Prunus species, the most widespread elimination methods are based on in vitro shoot cultures. In the 1980s, in vitro techniques began to be used in stone fruit species to eliminate viruses more efficiently (Deogratias et al. 1989). The application of meristem culture is based on the concept that the concentration of viruses in the shoot tip region is very low or even free of virus infection since most viruses cannot enter the meristem (Mochizuki and Ohki 2015). Therefore, after sterile isolation of the meristem, virus-free plants can be regenerated. Meristem isolation and plant regeneration in vitro may result in virus-free plants in other plant species (Kartha and Gamborg 1975), but in the case of Prunus species, it is extremely difficult because of the low regeneration capacity of the plants after isolation of tinny plant tissues. Micrografting after shoot tip isolation, i.e., grafting shoot tip on virus-free rootstocks under in vitro conditions, is a very elegant but technically a challenging procedure for virus elimination (Chilukamarri et al. 2021; Wang et al. 2022a). It has become widespread as a general practice for maintaining virus-free plants in citrus species (Navarro et al. 1975; Singh et al. 2019). Micrografting was shown to be effective for some peach varieties (Shu and Timon 1996, Navarro et al. 1982) and with optimization to simplifying the technique PPV- and PNRSV-free shoot-tip grafting almonds were produced (Rizqi et al. 2000).
Thermotherapy-based methods- advantages and limitations
In the case of Prunus species, the most common in vitro virus eradication method is thermotherapy combined with shoot tip (a bigger portion, contains meristem and some leaf primordia) isolation as shown in Table 2. Shoot tip culture alone is not an effective method for eliminate PNRSV (Spiegel et al. 1999), but as a result of heat treatment, viruses are inactivated, and a larger shoot tip section can remain virus-free. Shoot tip cultivation and plant regeneration after in vitro heat treatment made it possible to shorten the time of heat treatment compared to in vivo heat therapy and an increase in regeneration capacity compared to meristem isolation, thus resulting in the generation of more virus-free plants (Stein et al. 1991; Koubouris et al. 2007). In general, size of excised shoot tip is positively related to survival and shoot regeneration, while it is negatively related to the success of virus eradication (Wang et al. 2018). Heat treatment can be more effective by varying temperatures, by setting a higher temperature (38 °C) during the day and a lower temperature (28 °C) at night or by gradually raising the temperature from the beginning of the treatment (Spiegel et al. 1999; Dziedzic 2008; Abdullahi and Lawrence 2022; Hesari et al. 2022). Cycling 37 °C (light) and 35 °C (dark) for a 45-day period was sufficient to eliminate PPV, PNRSV and PDV from apricot cultivars (Křižan and Ondrušiková 2009). The heat tolerance of individual plant species and varieties can be very different, and it also varies how efficiently the specific virus is eliminated from different plants as a result of thermotherapy (Gella and Errea 1998; Cieślińska 2007); therefore, the duration of the heat treatment and the temperature must be optimized for each genotype (Wang et al. 2018). The survival rate of peach explants can decline drastically with increasing temperature (24 to 39 °C) during thermotherapy (Zarghami and Ahmadi2022).
Dziedzic (2008) investigated factors affected the survival rate of plum shoots during thermotherapy. In addition to temperature treatment, they authors found that the light intensity during heat therapy greatly affects the survival rate. Under a stronger photon flux density (PFD) (54.3 µmol s− 1 m− 2) the rate of shoots survival was higher than under a lower PFD (17.3 µmol s− 1 m− 2), suggesting a minimum light requirement for the survival of shoot culture. Under lower light intensity the higher temperature range (38 °C/36°C) resulted in 100% of dead plum shoots.
In general, the plant cultivar specific proliferation medium is used during thermotherapy (Knapp et al. 1998, Cieślińska 2007, Abdullahi and Lawrence 2022), but it seems the nutrient media also affects the survival of the cultures during heat treatment (Dziedzic 2008, Stein et al. 1991). Higher shoot survival rate, even after longer time of therapy, was obtained on media which contained less mineral components (Lloyd and McCown 1980 (WPM), modified WPM media or distilled water was supplemented with 6% agar only in contrast to Murashige and Skoog (1962) (MS) or modified MS media) and were plant grow regulator-free (Dziedzic 2008). Stein et al. (1991) found that reducing the concentration of 6-benzylamino purine (BA) from 6 mg/L to 0,2 mg/L in the medium improves survival rates from 0% to more than 90% and also observed that the tolerance of plants to heat treatment was influenced by the age of the shoot cultures; the optimal age of peach cultures prior to treatment was 18 or 15 days.
The size of the isolated shoot tip after heat treatment greatly influences the effectiveness of virus eradication (Zarghami and Ahmadi 2022). Due to their small size (0.2–0.5 mm), meristems often die or hardly regenerate, but large shoot tips containing several leaf primordia are more likely to carry the viruses. The use of a larger (1–2 mm) shoot tip in combination with heat treatment was effective for PPV-infected ‘Bebecou’ apricots, and it is found that almost 6 times more virus-free apricots could be produced in a shorter time by using the in vitro method compared to the conventional in vivo heat treatment (Koubouris et al. 2007). A Greek research group applied in vivo heat treatment with preconditioning (increasing temperature from 28 to 35 °C gradually over 1 week (1 °C/day) and were then maintained at 35 °C for 2 more weeks until new growth was produced) on nectarines infected with PPV and PNRSV. They compared the efficiency of the methods when 1.3-2.0 mm long shoot tips were isolated after the treatment or smaller, 0.8–1.3 mm shoot tips were isolated without the treatment and placed on culture medium. A significant portion of successfully regenerated plants were free from viruses; while, the small tips could not regenerate, and they became calloused or brown, the isolation of larger (1.3-2.0 mm) shoot tips after heat treatment can be applied more efficiently to produce virus-free nectarine plants (Manganaris et al. 2003).
Manganaris et al. (2003) observed the effect of the source of explants on shoot tip survival. Meristems (0.8–1.3 mm) derived from vegetative buds of virus-infected shoots, which had been collected either in winter or in spring, produced poor results for plant regeneration rate because of explant contamination, necrosis or undesired callus formation. Only very small percentages (1.7 or 5%) of meristem-tip explants grew satisfactorily and established as transplanted rooted cuttings. Larger size (1.3–2.0 mm) meristem tips from newly developed shoots of plants exposed to thermotherapy resulted in a higher survival and growth rate. The shoot tip explants were cultured on medium without plant growth regulators, and those that survived after 3–4 weeks were transferred to medium containing 8 mM BA and 0.8 mM indoleacetic acid (IAA) for shoot formation.
The difference between the host and virus in their tolerance to the applied method is the basis for the successful inactivation of the virus. The key factors influenced on the effectiveness of virus elimination procedure and in vitro culture survival are shown in Fig. 2.
Chemotherapy
To increase the success rate of virus elimination other strategies has been employed. During chemotherapy, the aim is to inhibit the replication of plant viruses by adding antiviral agents, known from medical science, to the culture medium. The disadvantage of chemotherapy is the phytotoxic effect taken on the in vitro plants which can be manifested in several symptoms. Decreased survival and regeneration rates were most frequently reported, followed by the inhibition of shoot growth, while root development, hyperhydricity, necrosis, chlorosis and discoloration of green parts, especially leaves, and dwarfing can be also observed (Magyar-Tábori et al. 2021).
The most common antiviral agent in plant virus eradication is ribavirin (Virazole), which is a synthetic guanosine analog, and by being integrated into RNA, it inhibits viral RNA synthesis. It appears that ribavirin does not act on a universal mechanism, but rather inhibits different viruses in different ways (Lerch 1987, Parker 2005).
In the case of sweet cherry varieties, ribavirin was completely ineffective when applied at a low concentration. Above 50 mg/L, it resulted in an increasing number of virus-free plants, but it strongly inhibited the development of in vitro plants. At concentrations higher than 80 mg/L ribavirin caused shoot tip necrosis in sweet cherry (Deogratias et al. 1989). An experiment on myrobalan (Prunus cerasifera) showed that the effectiveness of chemotherapy was related to the virus species. The efficiency of ribavirin treatment used at four different concentrations was tested on plants that were simultaneously infected with PNRSV and Apple chlorotic leaf spot virus (ACLSV). All treatments were ineffective against PNRSV, while the number of ACLSV-free plants increased with increasing ribavirin concentration (Cieślińska 2007). In the case of ‘Blufree’ and ‘Hanita’ plums, the use of ribavirin even at a low concentration (10 mg/L) was efficient in eliminating PPV (Polak and Hauptmanova 2009).
During ribavirin chemotherapy of plum microshoots, a low concentrations ranging of 10 or 30 mg/L were ineffective for ACLSV and PNRSV elimination, but at higher concentrations of 40 and 50 mg/L, it successfully eliminated both pathogens after two weeks of treatment followed by meristem culture. However, at higher concentrations, ribavirin exhibited some signs of phytotoxicity on microshoots, and the appearance of new amplified fragment length polymorphism (AFLP) bands and the disappearance of others indicated that plant genome stability was challenged and genomic rearrangements were induced in treated plants (Mazeikiene et al. 2019).
There are also studies in which the applied chemotherapy alone was ineffective or had poor efficacy in eliminating viruses, but after preliminary heat treatment, it resulted in many virus-free plants (Spiegel et al. 1999; Cieślińska 2007; Hu et al. 2012, 2015). Compared to traditional in vivo heat treatment, the use of a combination of in vitro heat treatment, meristem culture and ribavirin was found to be more effective in the case of PPV despite the reduction in the regeneration capacity of plum plants (Jakab-Ilyefalvi and Pamfil 2012).
Another chemotherapeutic agent, zidovudine (azidothymidine), proved to be a gentler and more effective reagent than ribavirin in peach virus eradication, which did not have negative outcomes on plants (Pavelkova et al. 2015). As a thymidine analog, zidovudine inhibits the enzyme reverse transcriptase and prevents the reproduction of retroviruses in the host organization. The use of acyclovir, an acyclic purine nucleoside analog, or rimantadine (a-mehyl‐1‐adamantane methylamine hydrochloride), an amantadine analog, was suitable to eliminate PDV, PNRSV and PPV from Prunus persica ‘Redhaven’ and ‘Suncrest’ during a three-week treatment at the tested concentrations (25 or 50 mg/L) and had no damaging effect on the treated shoots during chemotherapy (Pavelkova et al. 2015).
Cryotherapy
In addition to long-term storage, cryopreservation can also be used as a virus elimination method (Wang et al. 2022b). Wang et al. (2006) introduced for the first time the term cryotherapy for the use of cryogenic procedures for pathogen eradication. Cryotherapy refers to exposing infected shoot tips to liquid nitrogen (LN; -196 oC) to produce pathogen-negative plants. During cryotherapy, smaller and highly cytoplasmic meristem cells usually limited to the apical dome of the meristem and the youngest leaf primordia survive the ultra-low temperature treatments at a higher rate than differentiated, larger and more vacuolated cells with high water content. Since, viruses are often unevenly distributed in infected plants such that meristems contain either low virus titres or are free of virus infection, regenerated plants from surviving cells have a good chance of being virus-free (Magyar-Tábori et al. 2021; Wang et al. 2022b).
Brison et al. (1997) studied for the first time the effect of cryopreservation on the production of virus-free Prunus. During a cryotherapy experiment on PPV-infected interspecific Prunus rootstock, shoot tips of 0.3–0.5 mm showed very poorly regrowth (11%) after freezing in LN; however, larger shoot tips of 0.5-2.0 mm in length regenerated over 50% and showed that cryotherapy resulted in twice as many virus-free plants as shoot tip regeneration without freezing (Brison et al. 1997). Brison et al. (1997) concluded that the size of excised shoot tip is not a key for virus eradication in cryotherapy. Ultimately, Şekerz et al. (2015) successfully eliminated PPV from infected apricots using the Brison cryotherapy method; however, the majority of the samples did not survive the treatment, requiring protocol optimization for these sensitive materials.
Since then, cryotherapy has been used to eradicate viruses in several plant species (Wang et al. 2022b). In some cases, cryotherapy combined with other techniques was essential for effective virus eradication, especially for those pathogens that have the ability to infect meristematic cells. Some examples of the successful application of cryotherapy combined with thermotherapy or chemotherapy were applied to eradicate viruses in apple (Bettoni et al. 2022a), raspberry (Mathew et al. 2021), garlic (Vieira et al. 2015), and potatoes (Kushnarenko et al. 2017; Bettoni et al. 2022b). Further virus eradication applications in Prunus spp. may use cryotherapy combined with other in vitro techniques to enhance virus eradication in those species that are difficult to eradicate.
Electrotherapy
Although the exact mechanism is not yet understood, during electrotherapy, a continuous electric current is applied to the exposed plant tissues, and as a result, their nucleoprotein is degraded and leads to the elimination of their virulence activity (Gonzalez et al. 2006; Sabry et al. 2009). Electrotherapy can be used on any kind of explant (stem segment, shoot tip, plantlet, sprout, etc.) for virus elimination with high effectiveness (Adil et al. 2022). The process is very simple and inexpensive, does not require any specific equipment, and only takes a few minutes (Magyar-Tábori et al. 2021). Electrotherapy is not a common method for virus removal in Prunus species, but working on in vivo almond plants, Quacquarelli et al. (1979) successfully obtained 90% Almond mosaic virus-free almond shoots when 500 V current was applied to almond cuttings for 5–10 min, then buds of treated cuttings grafted on healthy almond seedlings.
Conclusions and prospects
The long history of plant virus eradication research still does not provide a universally usable, reliable, quick solution for the virus eradication of stone fruits. General methods are very difficult to find, as the levels of tolerance of plant species and cultivars to the applied treatments are different, and diverse viruses also respond differently to the therapies. Co-infections are more difficult to eliminate than infection with a single virus (Gella and Errea 1998; Cieślińska 2007; Pavelkova et al. 2015, Rizqi et al. 2000). Almost every virus eradication process damages the host plants, and plantlets suffer several stresses, which can result in a low rate of survival, inhibited growth, incomplete development, or abnormal morphology and a decrease in regeneration ability. Harmful effects of the methods used were reported and showed possibilities to mitigate such effects (Magyar-Tábori et al. 2021).
Production of virus-free propagation material by in vitro culture requires the use of specific, sensitive and rapid detection methods to screen the resulting explants. It seems ELISA is not enough sensitive for the detection of viruses occur in low concentration in in vitro tissues and the virus titre is often below the threshold of detection. In an attempt to overcome this problem PCR based detection methods are used that have much higher sensitivity compared to ELISA (Candresse et al. 1995; Manganaris et al. 2003, Dovas et al. 2001). Virus titre can be a virus-specific trait, the occurrence of a virus could be extremely limited or highly localised in host tissues or high titre viruses show an irregular distribution in host plants. Virus localization can influence the success of elimination or detection (Knapp et al. 1998; Laimer 2002).
In vitro techniques require special care and precise, sterile work. However, their great advantage is that they can be carried out in the laboratory under controlled conditions all year round, requiring a small space. Somatic embryogenesis, cryotherapy and electrotherapy are widely used in different plant species with high efficiency to remove viruses from infected explants. Optimization of these methods to Prunus spp. are required to enhance virus eradication in those species that are difficult to produce virus-free. Applying and combining the different methods presented above and understanding the factors crucially contributing to the success of virus elimination and plant survival, holds enormous potential to produce pathogen-free stone fruit propagation material.
Data availability
All the data mentioned in this manuscript are publicly available in the cited scientific articles.
References
Abdullahi I, Lawrence T (2022) Accelerated in vitro thermotherapy and indexing against apple chlorotic leaf spot virus in Shiro plum. Can J Plant Pathol 44(1):136–146
Adil S, Singh V, Anjum A, Quraishi A (2022) A mini-review on electrotherapeutic strategy for the plant viral elimination. Plant Cell Tiss Organ Cult 150:41–55. https://doi.org/10.1007/s11240-022-02265-w
Amari K, Burgos L, Pallas V, Sánchez-Pina MA (2009) Vertical transmission of Prunus necrotic ringspot virus: hitch-hiking from gametes to seedling. J Gen Virol 90(7):1767–1774
Baráth D, Jaksa-Czotter N, Molnár J, Varga T, Balássy J, Szabó LK, Várallyay É (2018) Small RNA NGS revealed the presence of Cherry virus A and Little cherry virus 1 on apricots in Hungary. Viruses, 10(6):318
Bettoni JC, Fazio G, Carvalho Costa L, Hurtado-Gonzales OP, Rwahnih MA, Nedrow A, Volk GM (2022a) Thermotherapy followed by shoot tip cryotherapy eradicates latent viruses and apple hammerhead viroid from in vitro apple rootstocks. Plants 11(5):582
Bettoni JC, Mathew L, Pathirana R, Wiedow C, Hunter DA, McLachlan A, Nadarajan J (2022b) Eradication of potato virus s, potato Virus a, and potato virus m from infected in vitro-grown potato shoots using in vitro therapies. Front Plant Sci 13:878733
Brison M, De Boucaud MT, Pierronnet A, Dosba F (1997) Effect of cryopreservation on the sanitary state of a cv Prunus rootstock experimentally contaminated with Plum Pox Potyvirus. Plant Sci 123(1–2):189–196
Candresse T, Macquaire G, Lanne M, Bousalem M, Quiot-Douine L, Quiot JB, Dunez J (1995) Analysis of plum pox virus variability and development of strain-specific assays. Acta Hortic 386:357–369
Candresse T, Faure C, Theil S, Marais A (2017) First report of nectarine stem pitting-associated virus infecting Prunus mume in Japan. Plant Dis 101(2):393–393
Card SD, Pearson MN, Clover GR (2007) Plant pathogens transmitted by pollen. Australas Plant Pathol 36(5):455–461. https://doi.org/10.1071/AP07050
Chilukamarri L, Ashrafzadeh S, Leung DW (2021) In-vitro grafting–current applications and future prospects. Sci Hort 280:109899
Cieślińska M (2007) Application of thermo-and chemotherapy in vitro for eliminating some viruses. J Fruit Ornam Plant Res 15:117–124
Deogratias JM, Dosba F, Lutz A (1989) Eradication of prune dwarf virus, Prunus necrotic ringspot virus, and apple chlorotic leaf spot virus in sweet cherries by a combination of chemotherapy, thermotherapy and in vitro culture. Can J Plant Pathol 11(4):337–342
Dovas CI, Hatziloukas E, Salomon R, Barg E, Shiboleth Y, Katis NI (2001) Incidence of viruses infecting allium spp. in Greece. Eur J Plant Pathol 107:677–684.
Dziedzic E (2008) Elimination of Prunus necrotic ring spot virus (PNRSV) from plum ‘Earliblue’ shoots through thermotherapy in vitro. J Fruit Ornam Plant Res 16:101–109
Ebrahimi M, Habashi AA, Emadpour M, Kazemi N (2022) Recovery of virus-free almond (Prunus dulcis) cultivars by somatic embryogenesis from meristem undergone thermotherapy. Sci Rep 12(1):14948
Etienne H, Guyot R, Beulé T, Breitler JC, Jaligot E (2016) Plant fidelity in somatic embryogenesis-regenerated plants. Somatic embryogenesis: fundamental aspects and applications, 121–150
Gambino G, Bondaz J, Gribaudo I (2006) Detection and elimination of viruses in callus, somatic embryos and regenerated plantlets of grapevine. Eur J Plant Pathol 114:397–404
Gella R, Errea P (1998) Application of in vitro therapy for ilarvirus elimination in three Prunus species. J Phytopathol 146(8–9):445–449
Gonzalez JE, Sanchez R, Sanchez A (2006) Biophysical analysis of electric current mediated nucleoprotein inactivation process. Cent Agric 2:42–47
Goussard PG, Wiid J, Kasdorf GGF (1991) The effectiveness of in vitro somatic embryogenesis in eliminating fanleaf virus and leafroll associated viruses from grapevines. South Afr J Enol Viticulture 12:77–81
Hadidi A, Barba M, Candresse T, Jelkmann W (2011) Virus and virus-like Diseases of pome and stone fruits. APS Press/American Phytopathological Society
Hesari N, Haji Mohammadi A, Zarghami R, Fakheri B, Kiss-Bába E, Szegő A, Mirmazloum I (2022) Eradication of PPV and PNRSV viruses from three Peach cultivars using Thermotherapy in Vitro, including optimization of Microshoots’ multiplication and rooting medium. Horticulturae 8(10):929
Hou W, Li S, Massart S (2020) Is there a biological desert with the discovery of new plant viruses? A retrospective analysis for new fruit tree viruses. Front Microbiol 11:592816
Howell WE, Eastwell KC, Li TSC (2000) Heat treatment, chemo-therapy and hydroponic culture for obtaining virus-free trees of sweet cherry. In XVIII International Symposium on Virus and Virus-like Diseases of Temperate Fruit Crops-Top Fruit Diseases 550 (pp. 455–458)
Hu GJ, Hong N, Wang LP, Hu HJ, Wang GP (2012) Efficacy of virus elimination from in vitro-cultured sand pear (Pyrus pyrifolia) by chemotherapy combined with thermotherapy. Crop Prot 37:20–25
Hu G, Dong Y, Zhang Z, Fan X, Ren F, Zhou J (2015) Virus elimination from in vitro apple by thermotherapy combined with chemotherapy. Plant Cell Tissue and Organ Culture (PCTOC) 121:435–443
Jakab-Ilyefalvi Z, Pamfil D, Clapa D, Fira A (2012) The effect of heat treatment and in vitro chemotherapy mediated by 2-thiouracil on plum pox virus (PPV) content in meristem regenerated plum plants. Ann Romanian Soc Cell Biol 17(1)
Jo Y, Choi H, Back CK, Chu H, Zhou Y, Lian S et al (2019) First report of peach leaf pitting-associated virus in peach trees exhibiting Calico in Korea. Plant Dis 103(11):2971
Kartha KK, Gamborg OL (1975) Elimination of cassava mosaic Disease by meristem culture. Phytopathology 65(7):826–828
Kassanis B (1952) Some effects of high temperature on the susceptibility of plants to Infection with viruses. Ann Appl Biol 39(3):358–369
Kinoti WM, Nancarrow N, Dann A, Rodoni BC, Constable FE (2020) Updating the quarantine status of Prunus infecting viruses in Australia. Viruses 12(2):246
Knapp E, Hanzer V, Mendonca D, da Câmara Machado A, Katinger H, de Câmara Machado ML (1998) Improved virus detection in rosaceous fruit trees in vitro. Plant Cell Tissue and Organ Culture 52(1–2):3–6
Koubouris GC, Maliogka VI, Efthimiou K, Katis NI, Vasilakakis MD (2007) Elimination of Plum pox virus through in vitro thermotherapy and shoot tip culture compared to conventional heat treatment in apricot cultivar Bebecou. J Gen Plant Pathol 73(5):370–373
Křižan B, Ondrušiková E (2009) Thermotherapy of apricot cultivars. I Int Symp Biotechnol Fruit Species: BIOTECHFRUIT2008 839:269–274
Kudělková M, Pavelková R, Ondrušiková E (2015) Virus elimination in peach using chemotherapy. In VI International Symposium on Production and Establishment of Micropropagated Plants 1155 (pp. 431–438)
Kunkel LO (1936) Heat treatments for the cure of yellows and other virus Diseases of Peach. Phytopathology, 26(9)
Kushnarenko S, Romadanova N, Aralbayeva M, Zholamanova S, Alexandrova A, Karpova O (2017) Combined Ribavirin treatment and cryotherapy for efficient Potato virus M and Potato virus S eradication in potato (Solanum tuberosum L.) in vitro shoots. Vitro Cell Dev Biology-Plant 53(4):425–432
Laimer M (2002) Detection and elimination of viruses and phytoplasmas from pome and stone fruit trees, vol 28. Horticultural Reviews-Westport Then New York, pp 187–236
Lenz F, Baumann G, Kornkamhaeng P (1983) High temperature treatment of Prunus avium L.F12/1 for virus elimination. J Phytopathol 106(4):373–375
Lerch B (1987) On the inhibition of plant virus multiplication by Ribavirin. Antiviral Res 7(5):257–270
Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia by use of shoot tip culture. Proc. Int. Plant. Propagators Soc 30:421–427
Magyar-Tábori K, Mendler-Drienyovszki N, Hanász A, Zsombik L, Dobránszki J (2021) Phytotoxicity and other adverse effects on the in vitro shoot cultures caused by virus elimination treatments: reasons and solutions. Plants 10(4):670
Manganaris GA, Economou AS, Boubourakas IN, Katis NI (2003) Elimination of PPV and PNRSV through thermotherapy and meristem-tip culture in nectarine. Plant Cell Rep 22(3):195 200. Phytopathology, 26(9)
Mathew L, Tiffin H, Erridge Z, McLachlan A, Hunter D, Pathirana R (2021) Efficiency of eradication of raspberry bushy dwarf virus from infected raspberry (Rubus idaeus) by in vitro chemotherapy, thermotherapy and cryotherapy and their combinations. Plant Cell Tissue and Organ Culture (PCTOC) 144:133–141
Matic S, Myrta A, Minafra A (2007) First report of little cherry virus 1 in cherry, plum, almond and peach in Italy. J Plant Pathol 89(Suppl. 3)
Mazeikiene I, Kviklys D, Siksnianiene JB, Zinkus D, Stanys V (2019) Influence of Ribavirin on Prunus domestica L. regeneration, genome stability and virus eradication in vitro. In Proceedings of the Latvian Academy of Sciences (Vol. 73, No. 3, pp. 238–243). De Gruyter Poland
Mink GI (1993) Pollen and seed-transmitted viruses and viroids. Annu Rev Phytopathol 31(1):375–402. https://doi.org/10.1146/annurev.py.31.090193.002111
MiNoiu N (1975) New investigations on plum pox virus. Archiv fur Phytopathologie und Pflanzenschutz 11(6):389–397
Mochizuki T, Ohki ST (2015) Detection of plant virus in meristem by immunohistochemistry and in situ hybridization. Plant Virology Protocols, pp 275–287
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with Tobacco tissue cultures. Physiol Plant 15:473–479
Naderpour M, Ahmadi B, Mirzaei L (2022) Electrotherapy, thermotherapy, chemotherapy, and cryotherapy to regenerate Prunus armeniaca L. free of ACLSV, ApMV, and TRSV.
Navarro L, Roistacher CN, Murashige T (1975) Improvement of shoot-tip grafting in vitro for virus-free citrus. J Am Soc Hortic Sci 100:471–479
Navarro L, Llácer G, Cambra M, Arregui JM, Juárez J (1982) Shoot-tip grafting in vitro for elimination of viruses in peach plants (prunus persica batsch). In XII International Symposium on Fruit Tree Virus Diseases 130 (pp. 185–192)
Numaguchi K, Takeda T, Tsuchida Y, Nakaune R (2019) Large-scale field survey reveals overall yield loss in Japanese apricot possibly caused by two ampeloviruses. J Gen Plant Pathol 85(2):116–121
Olah R, Turcsan M, Olah K, Farkas E, Deak T, Jahnke G, Sardy DAN (2022) Somatic embryogenesis: a Tool for fast and Reliable Virus and Viroid Elimination for Grapevine and other plant species. Horticulturae 8(6):508
Panattoni A, Luvisi A, Triolo E (2013) Elimination of viruses in plants: twenty years of progress. Spanish Journal of Agricultural Research 11(1):173–188
Parker WB (2005) Metabolism and antiviral activity of ribavirin. Virus Research 107:165–171
Pavelkova R, Kudelkova M, Ondrusikova E, Eichmeier A (2015) Virus elimination in Peach Cv.‘Red haven’by Chemotherapy. Agricultural Commun 3(2):16–20
Polak J, Hauptmanova A (2009) Preliminary results of in vivo thermotherapy of plum, apricot and peach cultivars artificially infected with PPV-M and PPV-D strains of Plum pox virus. Hortic Sci 36:92–96
Quacquarelli A, Gallitelli D, Savino V, Piazzolla P (1979) The use of electric current (RACE)* for obtaining mosaic-free almonds. In XI International Symposium on Fruit Tree Virus Diseases 94:251–256.
Rizqi A, Zemzami M, Spiegel S (2000) Recovery of virus-free almond plants by improved in vitro shoot-tip grafting. In XVIII International Symposium on Virus and Virus-like Diseases of Temperate Fruit Crops-Top Fruit Diseases 550:447–454
Rubio M, Martínez-Gómez P, Marais A, Sánchez‐Navarro JA, Pallás V, Candresse T (2017) Recent advances and prospects in Prunus virology. Ann Appl Biol 171(2):125–138
Sabry YM, Mahmoud MH, Abdelghaffar MH (2009) Evaluation of some therapies to eliminate Potato Y Potyvirus from potato plant. Inter J Virol 5:64–76
Şekerz MG, Süzerer V, Elibuyuk IO, Çiftçi YÖ (2015) In Vitro Elimination of PPV from Infected Apricot Shoot Tips via Chemotherapy and Cryotherapy. International journal of agriculturebiology, 17(5)
Shu W, Timon B (1996) Preliminary study on the methods of getting virus-free peach plantlets in vitro. Acta Horticulturae 374:191–194
Singh AK, Meetei NT, Kundu S, Salma U, Mandal N (2019) In vitro micrografting using three diverse indigenous rootstocks for the production of Citrus Tristeza virus-free plants of Khasi mandarin. Vitro Cell Dev Biology-Plant 55:180–189
Spiegel S, Tam Y, Rosner A, Brison M, Helliot B, Pierronnet A, De Boucaud MT (1999) In vitro elimination of Prunus necrotic ringspot virus in a plum cultivar. Plant Biotechnology and in vitro Biology in the 21st Century. Springer Netherlands, pp 545–547
Stein A, Spiegel S, Faingersh G, Levy S (1991) Responses of micropropagated peach cultivars to thermotherapy for elimination of Prunus necrotic ringspot virus. Ann Appl Biol 119:265–271
Szittya G, Silhavy D, Molnár A, Havelda Z, Lovas Á, Lakatos L, Burgyán J (2003) Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J 22(3):633–640
Turcsan M, Demian E, Varga T, Jaksa-Czotter N, Szegedi E, Olah R, Varallyay E (2020) Hts-based monitoring of the efficiency of somatic embryogenesis and meristem cultures used for virus elimination in grapevine. Plants 9(12):1782
Vieira RL, da Silva AL, Zaffari GR, Steinmacher DA, de Freitas Fraga HP, Guerra MP (2015) Efficient elimination of virus complex from garlic (Allium sativum L.) by cryotherapy of shoot tips. Acta Physiol Plant 37:1–11
Wang Q, Liu Y, Xie Y, You M (2006) Cryotherapy of potato shoot tips for efficient elimination of potato leafroll virus (PLRV) and potato virus Y (PVY). Potato Res 49:119–129
Wang MR, Cui ZH, Li JW, Hao XY, Zhao L, Wang QC (2018) In vitro thermotherapy-based methods for plant virus eradication. Plant Method 14:1–18
Wang MR, Bettoni JC, Zhang AL, Lu X, Zhang D, Wang QC (2022a) In vitro micrografting of horticultural plants: Method development and the use for micropropagation. Horticulturae 8(7):576
Wang MR, Bi WL, Bettoni JC, Zhang D, Volk GM, Wang QC (2022b) Shoot tip cryotherapy for plant pathogen eradication. Plant Pathol 71(6):1241–1254
Zarghami R, Ahmadi B (2022) Production of Plum Pox Virus-Free and Prunus Necrotic Ringspot Virus-Free Regenerants Using Thermotherapy and Meristem-Tip Culture in Prunus persica L. Erwerbs-Obstbau, pp 1–9
Zhang Y, Zhou J, Zhan B, Li S, Zhang Z (2021) First report of peach leaf pitting-associated virus (PLPaV), plum bark necrosis stem pitting-associated virus (PBNSPaV), and mume virus A (MuVA) from Mei (Prunus mume) in China. Plant Disease. https://doi.org/10.1094/pdis-11-20-2521-pdn. PMID: 33554663
Zheng YY, Bu FD, Wu CJ, Chen JG, Liu Z, Xiang BC, Cui BM (2020) First Report of Mume Virus A Infection of Prunus persica in China. Plant Dis 104(10):2741
Acknowledgements
LKS and FD are PhD students at Hungarian University of Agriculture and Life Sciences at the Doctoral School of Horticultural Sciences and Doctoral School of Biological Sciences, respectively.
Funding
This work was financially supported by NKFIH K127951.
Open access funding provided by Hungarian University of Agriculture and Life Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or nonfinancial interests to disclose.
Additional information
Communicated by Ranjith Pathirana.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szabó, L.K., Desiderio, F., Kirilla, Z. et al. A mini-review on in vitro methods for virus elimination from Prunus sp. fruit trees. Plant Cell Tiss Organ Cult 156, 42 (2024). https://doi.org/10.1007/s11240-023-02670-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-023-02670-9