Abstract
Methanolic extracts of microshoots from agar cultures and of microshoots and media from agitated cultures of Scutellaria lateriflora grown on identical five variants of the Murashige-Skoog (MS) and Linsmaier-Skoog (LS) media with different 6-benzyladenine (BA) and 1-naphthaleneacetic acid (NAA) concentrations (0.5–3.0 mg/l) were analyzed for flavonoids (27 compounds), phenylpropanoid glycosides (2 compounds) and phenolic acids (19 compounds) using the DAD-HPLC method. The amounts of individual compounds and total amounts of the above mentioned metabolite groups in the biomass from both types of the tested cultures were dependent on the composition of basal media and concentrations of BA and NAA in the media variants. In media extracts, no metabolites were confirmed. Higher total amounts of flavonoids were obtained in agar cultures (max. 722.04 and 2989.55 mg/100 g DW on MS and LS media variants, respectively), which were 1.1 and 1.7 times higher, respectively, than in agitated cultures. In both types of cultures, glycosidic conjugates: baicalin and wogonoside were the main flavonoids (max. amounts: 513.93 and 1838.18 mg/100 g DW, and 305.92 and 700.85 mg/100 g DW in agar cultures on MS and LS variants, respectively). High amounts of verbascoside in agar cultures were also confirmed (max. 384.99 and 543.17 mg/100 g DW on MS and LS media variants, respectively). The cells grown in agitated cultures converted exogenous precursors, i.e. hydroquinone (HQ) and 4-hydroxybenzoic acid (4-HBAc) into arbutin (β-d-glucoside of HQ). The highest amount of this product reached 5.63 and 1.45 g/100 g DW after the addition of these two precursors, respectively. This is the first large-scale report documenting in vitro biosynthetic potential of Scutellaria lateriflora microshoots cultivated in two tested culture.
Key message
After testing of 10 media variants in two types of in vitro cultures high amounts of flavonoids (app. 3 g%) and verbascoside (0.54 g%) were obtained. Additionally, 5.6 g% of arbutin as hydroquinone β-d-glucosylation product was confirmed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Scutellaria lateriflora L. (American skullcap) is a species that has long been used in traditional North American medicine (Millspaugh 1974). At present, it has an important position in the official USA therapy (Upton et al. 2011).
The main ingredients of the raw material, which is the herb of this species, include flavonoids specific for the genus Scutellaria, aglycones (e.g. baicalein, wogonin, oroxylin A and scutellarein) and glucuronides (baicalin, wogonoside, scutellarin) and other flavonoid compounds common in the plant kingdom (e.g. luteolin and apigenin) (Choi et al. 2002; Barnes et al. 2007; Shang et al. 2010). The raw material also contains iridoid compounds (e.g. catalpol), phenylpropanoid glycosides (e.g. verbascoside), phenolic acids (caffeic, chlorogenic, p-coumaric, ferulic, protocatechuic and rosmarinic acids) and cinnamic acid (Kawka et al. 2017; Upton and DAyu 2012). Chemical components found in this species determine its sedative and anticonvulsant properties (Kim et al. 2014; Zhang et al. 2009). Herb of S. lateriflora is used for the treatment of epilepsy (grand mal), hysteria and various neurotic states (Awad et al. 2003, Upton and DAyu 2012).
The biotechnological studies of this species are modest. They were mainly focused on evaluation of the usefulness of “hairy root” cultures as a source of secondary metabolites (Marsh et al. 2014; Wilczańska-Barska et al. 2012). The protocols for the micropropagation are also available (Tascan et al. 2010; Cole et al. 2007, 2009).
Having successfully established Scutellaria lateriflora microshoot cultures, our team had begun research on the endogenous accumulation of bioactive metabolites: flavonoids, phenylpropanoid glycosides and phenolic acids. The influence of the light conditions (monochromatic lights, white light and darkness) on the accumulation of these three groups of metabolites in agar culture was investigated earlier. That study showed the greatest effect of blue light on the accumulation of metabolites (Kawka et al. 2017).
Apart from light conditions, other factors influence the production of bioactive compounds (Ramawat and Mathur 2007; Murthy et al. 2014). The present work evaluated the influence of basal media composition (Murashige-Skoog—MS, Linsmaier-Skoog—LS) and concentration of selected plant growth regulators (PGRs) (6-benzyladenine—BA and 1-naphthaleneacetic acid—NAA) on the accumulation of flavonoids, phenylpropanoid glycosides and phenolic acids in two types of culture, namely agar and agitated cultures. Methanolic extracts of the biomass from both types of culture and media from agitated cultures harvested after 4-week culturing cycles were subjected to quantitative determinations using DAD-HPLC. The aim of the experiment was to identify the best conditions for biomass growth and accumulation of metabolites.
Additionally, bioconversion capability of the cells of microshoots maintained in agitated culture on MS medium supplemented with BA + NAA (1.0 + 0.5 mg/l) was investigated, with special focus on the bioconversion of the exogenous hydroquinone (HQ) and 4-hydroxybenzoic acid (4-HBAc) into arbutin (β-D-glucoside of HQ), which is important for medicinal applications and in cosmetology. In in vitro cultures of some plant species, it was possible to obtain via biotransformation high amounts of this product (Piekoszewska et al. 2010; Kwiecień et al. 2013; Szopa et al. 2018).
Materials and methods
Origin of in vitro cultures
The in vitro microshoot cultures of the S. lateriflora were established from seeds acquired in 2015 from W. J. Beal Botanical Garden, Michigan State University. For details see Kawka et al. (2017).
Endogenous accumulation of metabolites
Experimental cultures
The agar cultures were maintained on solid (Phyto agar, Duchefa Biochemie, Haarlem, The Netherlands) MS medium (Murashige and Skoog 1962) and LS medium (Linsmaier and Skoog 1965) variants with different concentrations of BA and NAA (pH 5.7): BA (1.0 mg/l) + NAA (1.0 mg/l); BA (2.0 mg/l) + NAA (2.0 mg/l); BA (3.0 mg/l) + NAA (1.0 mg/l); BA (1.0 mg/l) + NAA (0.5 mg/l); BA (0.5 mg/l) + NAA (2.0 mg/l). The cultures (0.5 g inoculum of fresh biomass—FM) were grown in Erlenmeyer flasks (100 ml) under constant (24/24 h), artificial light (16 μmol/m s, LF-40 W lamp, daylight, Piła, Poland) at 25 ± 2 °C and were subcultured every 4 weeks (three series). Agitated cultures were maintained on the same five different variants of MS and LS media as the agar cultures, under the same light conditions, in Erlenmeyer flask (500 ml) containing 150 ml of medium and 0.5 g of FM inoculum, on a rotary shaker (Altel, Łódź, Poland), operating at 140 rpm with an amplitude of 35 mm, for 4 weeks (three series). In both types of cultures, the biomass was collected after 4-weeks and dried. Media from agitated cultures were lyophilized. The biomass increments were calculated by dividing the sample DW by the DW of inoculum.
Extraction and DAD-HPLC analysis
Ellnain-Wojtaszek and Zgórka method with our modifications (Sułkowska-Ziaja et al. 2017) was used to quantify 27 flavonoids, 19 phenolic acids, benzoic and cinnamic acids, 2 phenylpropanoid glycosides. For details see Supplementary data.
Biotransformation experiments
Agitated cultures were maintained in 500 ml Erlenmeyer flasks, with 100 ml of the MS medium with BA (1.0 mg/l) + NAA (0.5 mg/l) (pH 5.7) and the inoculum 1 g of FM under the same culture conditions as in the above-mentioned experiment.
Substrates: HQ (Merck, Darmstadt, Germany) or 4-HBAc (Sigma-Aldrich, St. Louis, USA) were introduced as aqueous solutions (1 or 2 g/l) 14 days after inoculation. The solutions were aseptically administered into the culture flasks using a 0.22 μm membrane filter (Merck-Millipore, Darmstadt, Germany). At the same time, 100 ml of fresh medium was added to each flask. The precursors were administered in a single dose or two and three portions were added at 24 h intervals. The final concentrations of each precursor were: 96, 144, 192, 288, and 384 mg/l of the medium. The biomass and culture media were harvested separately 24 h after addition of the last dose of the precursors. The biomass was dried while the media were lyophilized. The experiment comprised three independent series. The method by Štambergová et al. (1985) was used to quantify arbutin, HQ and 4-HBAc determination. For details see supplementary data.
Statistical analysis
The results were expressed as the means ± SE of three independent experiments. The analysis of variance (ANOVA) and the Scheffé post-hoc test were conducted for each metabolite content against culture medium variant (STATISTICA™ ver.13.3 software, TIBCO® Software Co., Palo Alto, CA).
Results
Endogenous accumulation of metabolites
Out of 27 flavonoids tested, almost all analyzed extracts were evidenced to contain 6 specific Scutellaria flavonoids (baicalein, baicalin, wogonin, wogonoside, scutellarin, oroxylin A), but no flavonoids common in the plant kingdom were detected. None of the extracts was found to contain scutellarein. Out of 2 analyzed phenylpropanoid glycosides, the presence of verbascoside was confirmed. With regard to phenolic acids, out of 19 estimated compounds, only one phenolic acid 3,4-dihydroxyphenylacetic acid (3,4-DAc) was documented to be accumulated in biomass grown on LS media in agar cultures and on MS and LS media in agitated cultures. The analyses of the lyophilized culture media did not show the presence of metabolites.
Agar cultures—Murashige and Skoog media
The concentrations of the applied PGRs: BA and NAA did not have a marked effect on the appearance and degree of differentiation of the cultured biomasses. Microshoots were green with large, delicate leaves (Fig. 1a). Dry biomass increased by from 2.22 to 3.69 times. The highest, 3.69- and 3.43-fold increases were obtained on the MS variants containing BA (0.5 mg/l) + NAA (2.0 mg/l) and 2.0 mg/l of each PGR, respectively (Supplementary Fig. 1).
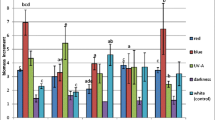
All the biomass extracts were found to contain five flavonoids. No baicalein was found. The contents of individual compounds varied from 1.43 to 17.04-fold, depending on the concentration of PGRs in the medium variants. Two glycosidic conjugates: baicalin and wogonoside were the main compounds. The highest baicalin content of 513.93 mg/100 g DW was found on the MS medium enriched in BA (0.5 mg/l) + NAA (2.0 mg/l). The amounts of baicalin accumulated on four remaining tested MS variants exceeded 320 mg/100 g dry weight (DW) (Table 1). The highest amounts of wogonoside (122.50 and 305.92 mg/100 g DW) were found on the medium variants containing BA (1.0 mg/l) + NAA (1.0 mg/l) and BA (1.0 mg/l) + NAA (0.5 mg/l) (Table 2). In turn, the maximal amounts of wogonin, scutellarin and oroxylin A, did not exceed 5.6, 15.6 and 38.7 mg/100 g DW, respectively (Supplementary Table 1). The total amount of flavonoids ranged from 382.78 to 722.04 mg/100 g DW. It was high, exceeding 500 mg/100 g DW on three variants (1.0 mg/l BA + 1.0 mg/l NAA, 1.0 mg/l BA + 0.5 mg/l NAA, 0.5 mg/l BA + 2.0 mg/l NAA). The highest production was confirmed on the MS medium containing BA (1.0 mg/l) + NAA (0.5 mg/l) (Fig. 2).
Total content of flavonoids [mg/100 g DW ± SE] in methanolic extracts from biomass of S. lateriflora microshoots cultured in 2 types of in vitro cultures, agar and agitated, maintained on MS and LS media variants with different concentration of plant growth regulators (BA/NAA) after 4 weeks of growth. Statistically higher (significant diff. p < 0.05) than: a 1.0/1.0, b vs. 2.0/2.0, c vs. 3.0/1.0, d vs. 1.0/0.5, e vs. 0.5/2.0 medium variant
The amounts of verbascoside varied 2.91-fold depending on the MS medium variant, and ranged from 163.79 mg/100 g DW to 381.73 mg/100 g DW. The amounts of this metabolite were greater than 270 mg/100 g DW on three MS variants. The highest content was confirmed on the medium containing BA (1.0 mg/l) + NAA (1.0 mg/l) (Table 3).
Agar cultures—Linsmaier and Skoog media
Independently of the concentration of PGRs in the medium variants, the biomass was composed of only a few green shoots with large delicate leaves. The increases in dry biomass were low from 0.81 to max. 1.89 times. The highest increase was shown on the LS medium variant containing BA + NAA (3.0 and 1.0 mg/l) (Supplementary Fig. 1).
The contents of individual flavonoids varied from 4.23 to 45.10-fold, depending on concentration of PGRs in the medium variants. Two glucuronic conjugates: baicalin and wogonoside, were present in large amounts. The concentration of baicalin varied from 223.34 to 1838.18 mg/100 g DW. The highest content was found in the microshoots growing on the LS medium with BA (1.0 mg/l) + NAA (0.5 mg/l). On two other LS variants, the baicalin content was high, 407.54 and 408.69 mg/100 g DW (Table 1). The concentrations of wogonoside also varied widely, from app. 30 to app. 700 mg/100 g DW. The highest contents of 700.85 and 638.78 mg/100 g DW were found on the two variants: containing BA (1.0 mg/l) + NAA (0.5 mg/l) and BA (2.0 mg/l) + NAA (2.0 mg/l), respectively (Table 2). On the medium containing BA (1.0 mg/l) + NAA (0.5 mg/l), considerable amounts of baicalein (280.53 mg/100 g DW) were found. The concentrations of this compound were highly variable, from 6.22 to 280.53 mg/100 g DW. The confirmed amounts of wogonin differed on the tested LS variants from min 22.16 to max 121.82 mg/100 g DW on the medium containing BA (2.0 mg/l) + NAA (2.0 mg/l). The amounts of scutellarin and oroxylin A, found in biomass extracts varied widely from 4.6 to 128.99 and from 14.04 to 59.44 mg/100 g DW, respectively (Supplementary Table 1).
The total concentration of flavonoids varied within very wide limits from 368.08 mg/100 g DW to about 3 g/100 g DW. The LS variant supplemented with BA (1.0 mg/l) + NAA (0.5 mg/l) was the most favorable for accumulation. On two other variants, also high total concentrations of flavonoids were obtained: 1181.29 and 995.43 mg/100 g DW (2.0 mg/l BA + 2.0 mg/l NAA, and 0.5 mg/l BA + 2.0 mg/l NAA), respectively (Fig. 2).
The amounts of verbascoside were generally highly variable by 3.83-fold, and ranging from 141.67 to 543.17 mg/100 g DW. The highest content was proven in extracts from the microshoots from the medium containing BA (2.0 mg/l) + NAA (2.0 mg/l). High amounts of 241.24 and 254.41 mg/100 g DW were confirmed in the case of media supplemented with BA (1.0 mg/l) + NAA (1.0 mg/l), and BA (1.0 mg/l) + NAA (0.5 mg/l) (Table 3).
The amounts of 3,4-DAc on five LS variants reached max 70.47 mg/100 g DW (medium containing BA + NAA, 2.0 mg/l each) (Supplementary Table 1).
Agitated cultures—Murashige and Skoog media
The applied BA and NAA concentrations in the tested media variants did not significantly affect the appearance and the degree of differentiation of the cultured biomasses. The microshoots were light green and compact. They were shorter and had smaller leaves in comparison with agar culture cultivated on MS media (Fig. 1b). The increases in the dry biomass were higher than in agar cultures and ranged from 3.43- to 5.10-fold, with the highest increase on the medium containing BA (1.0 mg/l) + NAA (1.0 mg/l) (Supplementary Fig 1).
None of the extracts was confirmed to contain oroxylin A. The presence of baicalein, was proven only in extracts from the microshoots cultivated on two variants of the MS medium. The contents of individual flavonoids varied from 2.80 to 58.06-fold, depending on PGRs concentration. The main compounds produced by microshoots included also glucuronic conjugates—baicalin and wogonoside. The amounts of baicalin varied in a very wide range from 28 to 490 mg/100 g DW, with the highest amounts in the microshoots growing on the MS medium containing BA (1.0 mg/l) + NAA (0.5 mg/l). On the variant containing BA (1.0 mg/l) + NAA (1.0 mg/l), the amount of baicalin reach about 167 mg/100 g DW, but was lower on the remaining variants (Table 1). The amounts of wogonoside were also extremely varied from 2.42 to 140.5 mg/100 g DW. The highest amount was confirmed, as in the case of baicalin, on the MS variants containing BA (1.0 mg/l) + NAA (0.5 mg/l) (Table 2). The maximum confirmed amount of scutellarin was lower, equal to about 80 mg/100 g DW (medium containing 1.0 mg/l BA + 1.0 mg/l NAA) (Supplementary Table 1). The amount of wogonin was no more than 33 mg/100 g DW only on one MS medium variant (Table 2). The amounts of baicalein (confirmed only on two MS variants) were low, equal to 4.64 and 13.01 mg/100 g DW, respectively (Table 1). Consequently, the total amounts of flavonoids were varied in a very wide range from about 30 to 660 mg/100 g DW. The highest content was confirmed for extracts from the microshoots growing on the medium containing BA (1.0 mg/l) + NAA (0.5 mg/l) (Fig. 2).
The amounts of verbascoside varied 13-fold, from about 6.5 to 84.5 mg/100 g DW. The highest content was confirmed for the microshoots cultivated on the medium with BA (1.0 mg/l) + NAA (1.0 mg/l) (Table 3).
The amounts of 3,4-DAc ranged from 3.29 to max 26.52 mg/100 g DW reached on the medium containing BA (1.0 mg/l) + NAA (1.0 mg/l) (Supplementary Table 1).
Agitated cultures—Linsmaier and Skoog media
The microshoots in the agitated cultures were shorter and more densely leaved than in the agar cultures cultivated on LS media. The increases in dry biomass were higher than in the agar cultures (3.61- to 4.30-fold). The highest increases in microshoot biomass by 4.25- and 4.30-fold was found on the media enriched in BA (2.0 mg/l) + NAA (2.0 mg/l) and with BA (1.0 mg/l) + NAA (0.5 mg/l) (Supplementary Fig. 1).
The contents of individual flavonoids varied 1.44–87.16-fold, depending on PGRs concentration. Two glucosides, baicalin and wogonoside were also the quantitatively dominant compounds. The amounts of baicalin ranging from 37.47 to max 842.75 mg/100 g DW were reached on the LS variant containing BA (1.0 mg/l) + NAA (0.5 mg/l). A high content of 440.96 mg/100 g DW was also found in extracts from the microshoots growing on the LS variant with BA (1.0 mg/l) + NAA (1.0 mg/l) (Table 1). Extracts from the microshoots growing on two variants of the LS medium were characterized by a high concentration of wogonoside: 438.26 mg/100 g DW (1.0 mg/l BA + 1.0 mg/l NAA) and 368.60 mg/100 g DW (1.0 mg/l BA + 0.5 mg/l NAA) (Table 2). High amount of baicalein of 180.42 mg/100 g DW was proven in extracts from the microshoots growing on the LS variant enriched in BA (1.0 mg/l) + NAA (1.0 mg/l) (Table 1). The proven amounts of wogonin varied widely from 13.82 to 713.53 mg/100 g DW. The LS variant containing BA (1.0 mg/l) + NAA (1.0 mg/l) was the most favorable medium for the accumulation of this compound (Table 2). The estimated amounts of scutellarin were low on most of the tested variants of the LS medium (0.39–31.57 mg/100 g DW). The presence of oroxylin A was recorded only in extracts from the microshoots growing on two LS variants. The amounts of this compound did not exceed 10 mg/100 g DW (Supplementary Table 1). The total content of flavonoids was also highly varied depending on PGR concentration in the media variants, from only 78.29 mg/100 g DW to about 1.78 g/100 g DW. The highest amounts were found in the microshoots growing on the LS variants containing BA (1.0 mg/l) + NAA (1.0 mg/l), and BA (1.0 mg/l) + NAA (0.5 mg/l) (1775.19 and 1371.28 mg/100 g DW, respectively) (Fig. 2).
The concentrations of verbascoside considerably varied, 7.8-fold, from about 37 mg/100 g DW to about 290 mg/100 g DW. The maximum content was found on the medium containing BA (1.0 mg/l) + NAA (1.0 mg/l). On three other LS variants, the amounts of this compound were also high and similar (93.46, 97.84, 101.28 mg/100 g DW) (Table 3).
The accumulation of 3,4-DAc was clearly promoted by the LS variant with BA (0.5 mg/l) + NAA (2.0 mg/l) (max. content of 131.66 mg/100 g DW) (Supplementary Table 1).
Production of arbutin via biotransformation of exogenous substrates
The cells of S. lateriflora microshoot cultures convert exogenous substrates: HQ and 4-HBAc into arbutin (β-d-glucoside of HQ). The results from other research groups and our earlier experiences documented that high concentration of HQ could inhibit biomass growth and even destroy the cells. Therefore, we divided the doses of the prcursosrs into 2–3 portions (Skrzypczak-Pietraszek unpublished). Only at the highest HQ and 4-HBAc concentrations (384 mg/l), inhibition of biomass growth was observed.
When HQ was administered as the prcursor, arbutin was mainly accumulated in the cultured biomass; however, analysis of the media revealed the presence of arbutin of up to 27%. The biotransformation process showed low efficiency of 0.72 to 15.06%. At the lower precursor concentrations (96–192 mg/l), the obtained amount of arbutin reached from 1.34 to max 3.41 g/100 g DW after application of a single dose of HQ. At those concentrations, a positive effect of dividing the precursor’s dose on arbutin production was observed (the production was 1.32–2.64-fold higher at two portions and 1.21–2.45-fold higher at three portions) with max amount of 4.52 g/100 g DW, at 192 mg/l of HQ, added as two portions. Higher concentrations of HQ (288 and 384 mg/l), were favorable to the biotransformation process. The obtained amounts of the product were higher (5.63 and 5.43 g/100 g DW) after application of a single dose of HQ. Dividing these high doses of HQ did not stimulate the production of arbutin. The addition of HQ in a dose of 288 mg/l, administered in one portion was the most beneficial for the production of arbutin (Supplementary Table 1).
The use of 4-HBAc as the precursor resulted in its bioconversion not only into arbutin, but also into HQ. 4-HBAc is an earlier step in the arbutin biosynthetic pathway than HQ, and theoretically may also undergo other biotransformations, but in the current experiment, the biosynthesis follows the pathway, 4-HBAc → HQ → arbutin, shown in Supplementary Fig. 2. The first step of biotransformation of 4-HBAc into HQ showed an efficiency of 1.60 to 16.56%. The obtained amount of HQ ranged from 0.08 to 3.86 g/100 g DW (single dose of 4-HBAc), from 0.54 to 3.69 g/100 g DW (two portions) and from 0.44 to 1.99 g/100 g DW (three portions). The highest obtained value was confirmed after the administration of 384 mg/l of 4-HBAc in a single dose. Extracts from the biomass and the media were confirmed to contain arbutin, too. This means that a part of the resulting HQ had undergone the second step of biotransformation into arbutin. The efficiency of this stage was very low, max. 2.2%. The amount of arbutin ranged from 0.08 to 1.45 g/100 g DW (single dose of 4-HBAc), from 0.07 to 0.45 g/100 g DW (two portions) and from 0.07 to 0.46 g/100 g DW (three portions). The highest obtained value was confirmed after administration of 144 mg/l 4-HBAc in a single dose.
Discussion
Biomass increments
The increases in dry biomass of S. lateriflora microshoots (from 2.22- to 3.69-fold) during 4-week growth cycles in agar cultures maintained on MS media variants were 1.2–4.2-fold higher in comparisons with the increases in agar cultures on LS media variants. The maximum increase was obtained on the medium with BA (0.5 mg/l) + NAA (2.0 mg/l). This MS variant can be proposed as “growth medium” for agar cultures, however, the increases are not satisfactory enough. Over a period of 4 weeks, 4- to 5-fold increases have been obtained on average in in vitro agar cultures of various plant species, e.g. in shoot-differentiating callus cultures of S. chinensis on MS media variants (Szopa and Ekiert 2011). However, in our experiments with agar shoot cultures of A. melanocarpa maintained on MS and LS media variants, the dry biomass increments were higher on MS media and increased during 4-weeks even 17.2- and 15.3-fold, respectively (Szopa and Ekiert 2014; Szopa et al. 2013). Also, in agar shoot cultures of A. arbutifolia and A. × prunifolia during 4-week growth cycles, the dry biomass increments on MS media variants, as documented by us, were higher (max. 7.1- and 8.4-fold) (Szopa et al. 2018).
The increases in dry biomass (3.43–5.10-fold) obtained during 4 weeks in agitated microshoot cultures of S. lateriflora maintained on MS media variants were mostly slightly higher (up to 1.41 times) in comparison with the same LS variants tested in agitated cultures. The maximum increase was obtained on the MS variant with BA (1.0 mg/l) + NAA (1.0 mg/l). This MS variant can be proposed as a good “growth medium” for agitated cultures. Over a period of 4 weeks, 4- to 5-fold increases have been obtained on average in in vitro agitated cultures of various plant species. In our earlier studies with Ruta graveolens microshoot cultures and R. g. ssp. divaricata shoot-differentiating callus in agitated cultures on LS medium (0.1 mg/l BA + 0.1 mg/l NAA), the biomass increments during 6 weeks reached 3.2- and 3.9-fold, respectively (Ekiert and Czygan 2005) and were lower with our current research. However during 60 days of agitated microshoot cultures of Schisandra chinensis, we obtained max. 15-fold biomass growth on MS media (Szopa et al. 2016). By comparison, in microshoot cultures of three cultivars of Hypericum perforatum (cvs Elixir, Helos, Topas) growing on MS and LS media, max. 9.6- and 9.8-fold increases in biomass were obtained in a shorter culture period (3 weeks).
In both types of S. lateriflora cultures tested by us (agar and agitated), the MS medium variants rich in components were more conducive to biomass increases in comparison with LS media variants. The presence of vitamins, amino acid-glycine in the media stimulated the growth of shoots. The experiments let us propose MS medium with BA/NAA (0.5/2.0 mg/l) and (1.0/1.0 mg/l) as the best “growth media” for agar and agitated cultures, respectively.
Endogenous accumulation of flavonoids
The results of HPLC analyses of the biomass extracts of S. lateriflora from both tested (agar and agitated) microshoot cultures indicated that the cells produced most of all the flavonoids specific for Scutellaria genus. Glycosidic forms of these compounds: baicalin and wogonoside were produced in high amounts. Aglycones—baicalein, wogonin, oroxylin A were accumulated in smaller amounts. In both types of cultures, LS media were more beneficial for flavonoid production than MS media variants.
In methanolic extracts of biomass from the agar cultures, the total amounts of flavonoids were higher (0.96–5.4 times) on the LS media variants in comparison with the same MS media variants. Different results were obtained in H. perforatum cvs agitated cultures. The MS media were more beneficial for flavonoid accumulation in Elixir, Helos, Topas cvs. in vitro cultures (Kwiecień et al. 2018). The maximum total amount of flavonoids (2989.56 mg/100 g DW) was confirmed now on the LS medium containing BA (1.0 mg/l) + NAA (0.5 mg/l). This amount was about 4 times higher than the maximum amount (722.04 mg/100 g DW) on the MS variant. This LS variant could be proposed as a “productive” medium for total flavonoids. However, very low (1.84-fold) growth increments were confirmed on this variant.
The accumulation of individual compounds was also higher on LS media than on MS media. Glycosidic conjugates, baicalin and wogonoside were the main metabolites. The maximum amounts of baicalin obtained on the MS and LS variants were high, and interesting from a practical point of view (513.93 and 1838.18 mg/100 g DW, respectively). The LS variant with BA (1.0 mg/l) + NAA (0.5 mg/l) was the most beneficial medium variant for baicalin accumulation. The highest amounts of wogonoside on the tested MS and LS media variants were different and reached 305.92 and 700.85 mg/100 g DW, respectively. The LS variant with BA + NAA (1.0 + 0.5 mg/l) can be proposed also as the best “productive” medium for this metabolite. In order to obtain high amounts of both compounds (baicalin and wogonoside), 2-stage cultures can be proposed: first on the MS medium (1.0 mg/l BA + 0.5 mg/l NAA), the best for growth increments, and then on the LS medium (1.0 mg/l BA + 0.5 mg/l NAA), which promotes their accumulation. The obtained maximum amounts of these two bioactive compounds are 5.2 times (baicalin) and 7.1 times (wogonoside) higher than in the S. baicalensis agar microshoot cultures established and cultivated in our laboratory (Kawka et al. 2019).
Only a few researches investigated the biosynthetic potential of untransformed in vitro cultures of S. lateriflora. In shoot cultures, the presence of mainly baicalein (about 0.5% DW) and wogonin (about 0.2% DW) was demonstrated, whereas in callus tissue only trace amounts of flavonoids were found (Nishikawa et al. 1999). The results of our studies broadened the knowledge of production capacity of cells of S. lateriflora microshoot cultured in vitro and documented that optimization of culture condition is a useful approach to find the best conditions for growth and production of metabolites.
In microshoot extracts from the agitated cultures of S. lateriflora grown on the tested MS and LS variants, the qualitative composition of flavonoids was similar to that confirmed in agar cultures. The maximum total amounts of flavonoids were high but 1.68–2.18-times lower in comparison with agar cultures. They reached 659.54 mg/100 g DW (MS variant, 1.0 mg/l BA + 0.5 mg/l NAA), and 1775.19 and 1371.28 mg/100 g DW (LS variants, 1.0 + 1.0 mg/l and 1.0 + 0.5 mg/l, BA + NAA, respectively). These two LS variants could be proposed as good “productive” media for total flavonoids in agitated cultures.
Again flavonoid glycosides, baicalin and wogonoside were the quantitatively dominant compounds. The highest amount of baicalin reached 491.89 and 842.75 mg/100 g DW on the MS and LS variants containing the same concentrations of cytokinin and auxin (1.0 mg/l BA + 0.5 mg/l NAA). On LS medium variant (1.0 mg/l BA + 1.0 mg/l NAA) the amount of baicalin was slightly lower (440.98 mg/100 g DW). LS medium supplemented with BA 1.0 mg/l and NAA 1.0 mg/l and with BA 1.0 mg/l + NAA 0.5 mg/l were the most beneficial media variants for the accumulation of wogonoside (438.26 and 368.60 mg/100 g DW, respectively). The two LS variants presented above were good “productive” media for these two metabolites. On this media variants, satisfactory enough biomass growth of 3,6- and 4.25-fold was confirmed. Therefore these variants can be proposed as a “universal medium” for growth and flavonoid production in agitated cultures.
The maximum amounts of the analyzed compounds obtained in the agitated cultures were lower than in the agar cultures, but this was compensated for by a more intense biomass growth in agitated cultures.
The obtained maximum total amounts of flavonoids in the S. lateriflora microshoot agitated cultures tested by us were 4.0-times higher than those confirmed by us in microshoot agitated cultures of S. baicalensis (Kawka et al. 2019). Tascan et al. (2010), in a study on stationary liquid cultures of S. lateriflora grown on MS medium found greater increases in biomass than in agar cultures. The estimated amounts of the flavonoids produced by S. lateriflora were also, like in our experiment, higher (201.4 mg/100 g DW) than the results obtained for S. baicalensis (55.3 mg/100 g DW). However, our results are much more attractive because we obtained higher amounts of total flavonoids and very high amounts of baicalin and wogonoside, the important compounds in phytotherapy.
In our agitated cultures, the amounts of bioactive compounds produced were lower than in the agar cultures. Similarly, in in vitro cultures of Schisandra chinensis, higher amounts of metabolites (dibenzocyclooctadiene lignans) were found in agar cultures than in agitated cultures (Szopa et al. 2016). However, greater biomass increments in the agitated cultures of S. lateriflora mean that this type of cultures can be proposed as a good source of flavonoids specific for Scutellaria.
These metabolites are of special interest in phytotherapy, as anti-inflammatory, antiallergic, antiviral, hepatoprotective and photoprotective products (Awad et al. 2003; Zhang et al. 2009).
In conclusion, we propose a two-stage agitated culture system: the first stage on MS medium (1.0 mg/l BA + 1.0 mg/l NAA) beneficial for growth increments and the second stage on LS medium (1.0 mg/l BA + 1.0 mg/l NAA) good for total flavonoid production. The LS variant supplemented with BA (1.0 mg/l) + NAA (1.0 mg/l), can be proposed as a “universal”, growth and flavonoid production medium, too, because on this medium the biomass increments were enough satisfactory (3.6-fold).
Endogenous accumulation of verbascoside
The HPLC analysis of the extracts documented the presence of only one phenylpropanoid glycoside: verbascoside. The maximum amount of this compound on the tested agar LS variants (BA + NAA, 2.0 mg/l each), equal to 543 mg/100 g DW, was 1.4 times higher than the maximum content obtained on the agar MS medium with BA (1.0 mg/l) + NAA (1.0 mg/l) (almost 382 mg/100 g DW).
In the agitated cultures, the maximum amounts of this compound were markedly lower (4.6 and 1.9 times) compared to the agar cultures, on both tested MS and LS variants and amounted to 84.52 (MS, BA + NAA, 1.0 + 1.0 mg/l) and 286.56 mg/100 g DW (LS, BA + NAA, 1.0 + 1.0 mg/l).
In our experiments with S. baicalensis cultures, the highest amounts of this compound in agar cultures were higher than in our S. lateriflora cultures and reached 830.9 and 1003.7 mg/100 g DW on MS and LS media, respectively. In agitated cultures of S. baicalensis, they were also high and reached 810.5 and 1115.4 mg/100 g DW on the MS and LS medium, respectively (Kawka et al. 2019). In microshoot cultures of S. lateriflora established by another team, no verbascoside was found, whereas in callus tissue it was the dominant metabolite (about 2.5% DW) accompanied by trace amounts of flavonoids (Nishikawa et al. 1999). In in vitro shoot cultures of other species of Scutellaria, such as S. pontica, S. ventenatii, S. orientalis, S. taurica and S. alpina presence of verbascoside was confirmed (up to 0.3% DW). In Verbena officinalis callus cultures established in our laboratory, cell metabolism was selectively focused on verbascoside production. In agar cultures, we obtained a very high amount of this metabolite of 2.45 g/100 g DW on the MS medium supplemented with BA (1.0 mg/l) + IBA (1.0 mg/l) (Kubica et al. 2017). In another experiment, we obtained extremely high amounts of this compound 6.71 and 6.02 g/100 g DW under blue and blue/red (30/70%) LED lights, respectively, but quantities of other analyzed metabolites, like phenolic acids were very low (Kubica et al 2020).
Verbascoside is a compound with proven antioxidant, anti-inflammatory, analgesic, immunomodulatory, antineoplastic, hepatoprotective and antiviral properties (Alipieva et al. 2014). Our results (max 543.17 mg/100 g DW in agar cultures and max 286.56 mg/100 g DW in agitated cultures) obtained with S. lateriflora in vitro culture are satisfactory, because they are associated with high production of specific flavonoids. The maximum amounts of verbascoside in agar culture were confirmed on other media variants than those proposed as good for flavonoid production, but in agitated culture, the LS variant with BA (1.0 mg/l) + NAA (1.0 mg/l) was good for production of both baicalin and wogonoside. In this situation, we can propose this variant as “productive medium” for three biologically active metabolites: verbascoside, baicalin and wogonoside.
Endogenous accumulation of 3,4-dihydroxyphenylacetic acid
3,4-DAc, the only one among 19 estimated phenolic acids, was found in biomass from the agar cultures grown only on the tested LS variants. Depending on the concentration of PGRs, its amount increased 9.4-fold. The maximum content reached about 70 mg/100 g DW (2.0 mg/l BA + 2.0 mg/l NAA). The presence of this compound was confirmed in methanolic extracts of biomass from all tested MS and LS variants in agitated cultures and its amount increased, depending on the concentration of PGRs, by 8.0- and 50.3-fold. The maximum content confirmed on the LS medium with BA (0.5 mg/l) + NAA (2.0 mg/l), equal to 131.66 mg/100 g DW, is of practical interest. Interestingly, we also confirmed the presence of this compound in in vitro cultures of S. baicalensis, S. subvetulina, S. albida and S. columnea (51.20, 52.78, 604.06, 50.47 mg/100 g DW, respectively) (Kawka et al. 2019). In addition, we confirmed its presence in our agitated microshoot cultures of H. perforatum cvs. - Elixir, Helos, Topas, (31.66–129.99 mg/100 g DW). Particularly high amounts of this compound were documented in cv. Helos (129.29 mg/100 g DW) on the LS medium containing BA + NAA, 1.0 mg/l each (Kwiecień et al. 2015). This compound has proven antiproliferative and apoptotic properties (Kampa et al. 2004).
Production of arbutin via biotransformation of exogenous substrates
After optimization of conditions for the exogenous HQ bioconversion process, we obtained interesting results from a practical standpoint (5.63 g/100 g DW arbutin), higher than usually obtained 0.43–2.95 g% DW (without continuous precursor supply) (Duškova et al. 1999). This content is higher than results of our experiments with cultures of Origanum majorana (5,26 g/100 g DW) (Skrzypczak-Pietraszek et al. 2017) and Schisandra chinensis (3.90 g/100 g DW) (Szopa et al. 2017) but lower than for Hypericum perforatum (7.21 g/100 g DW), Ruta graveolens (7.80 g/100 g DW) (Piekoszewska et al. 2010), Aronia melanocarpa (8.27 g/100 g DW) (Kwiecień et al. 2013), A. arbutifolia (8.36 g/100 g DW) and A. × prunifolia (7.36 g/100 g DW) (Szopa et al. 2018). Obtained by us maximum content of arbutin exceeds that requires by Polish Pharmacopoeia XI for Vitis idaeae folium national monograph (4.0%).
Our results indicate that cell metabolism in S. lateriflora microshoot culture is selectively focused on biosynthesis of flavonoids with glucuronic acid unit, i.e. baicalin and wogonoside, and verbascoside with another carbohydrate units (glucose and rhamnose).These results documented the presence of glucuronidases and other glucosidases in the cells of tested microshoots cultures. Our results connected with bioconversion indicate that the cells are also rich in β-d-glucosidases. Efficiency of biotransformation of HQ in to arbutin is low (0.72–15.06%), what could indicate that glucuronidases dominate in the cells. Arbutin is a valuable compound because of its use in the treatment of urinary tract infections as a disinfectant, and also in dermatology and cosmetology for removing skin discoloration (Ekiert et al. 2013; Migas and Krauze-Baranowska 2015).
An interesting result is the confirmation of the bioconversion of exogenous 4-HBAc into arbutin by us. In our earlier researches with in vitro cultures of R. graveolens, R. g. ssp. divaricata, A. melanocarpa and S. chinensis, this precursor was not transformed into arbutin. Surprisingly, in those cultures, we obtained, very interesting results from a theoretical point of view, namely, a mixture of two products: 4-HBAc β-glucopyranosyl ester and 4-HBAc 4-O-β-glucopyranoside in different proportions (Kwiecień et al. 2013; Szopa et al. 2017).
Our results have documented the high biosynthetic potential of the cell of S. lateriflora microshoots cultured in vitro for endogenous accumulation of biologically active metabolites—baicalin, wogonoside and verbascoside and for production of arbutin via β-d-glucosylation of HQ. We proposed the agitated cultures of S. lateriflora as a potential rich source of these important in phytotherepy compounds.
Conclusions
Our results documented the influence of PGR concentration in the MS and LS media on growth increments and biosynthetic potential of S. lateriflora cells cultivated in vitro in two types of culture, i.e. agar and agitated cultures. The MS media were more beneficial for biomass increments, on the other hand, LS media were more favorable for metabolite production. Higher total amounts of Scutellaria specific flavonoids and verbarscoside were obtained in agar cultures, but the biomass increments were low. Cells of S. lateriflora in both tested types of culture produce glycosidic conjugates—baicalin and wogonoside, and also verbascoside as the main compounds in amounts interesting from a practical point of view. The maximum amounts of these metabolites obtained in agar cultures are as follows: baicalin—1838.18, wogonoside—513.93 and verbascoside—543.17 mg/100 g DW. In agitated cultures, the amounts were lower but still high: baicalin—842.75, wogonoside—438.26 and verbascoside—286.56 mg/100 g DW. In order to obtain high amounts of these three metabolites, 2-stage agitated culture system can also be proposed: first stage on the MS “growth” medium, and then the second stage on the LS “productive” medium (the media variants with the same concentrations of PGRs, 1.0 mg/l BA + 1.0 mg/l NAA). Cells of microshoot agitated cultures also convert exogenous HQ into arbutin (max. 5.63 g%). High amounts of glucuronides and confirmation of β-d-glucosylation of HQ indicate that cells of S. lateriflora are rich in glycosidases. Our results documented that the microshoot culture of S. lateriflora, established by us, is a rich source of bioactive metabolites and valuable model for biotechnological experiments. The next stage of the experiment should involve maintaining S. lateriflora in vitro cultures in commercially available bioreactors, RITA and PlantForm, constructed especially for maintaining the microshoot cultures.
Abbreviations
- BA:
-
6-Benzyladenine
- 3,4-DAc:
-
3,4-Dihydroxyphenylacetic acid
- DAD-HPLC:
-
Diode-array detector—high performance liquid chromatography
- DW:
-
Dry weight
- FM:
-
Fresh biomass
- 4-HBAc:
-
4-Hydroxybenzoic acid
- HQ:
-
Hydroquinone
- LS:
-
Linsmaier and Skoog
- MS:
-
Murashige and Skoog
- NAA:
-
1-Naphthaleneacetic acid
- PGRs:
-
Plant growth regulators
- PhAcs:
-
Phenolic acids
References
Alipieva K, Korkina L, Orhan IE, Georgiev MI (2014) Verbascoside—a review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol Adv 32:1065–1076. https://doi.org/10.1016/j.biotechadv.2014.07.001
Awad R, Arnason JT, Trudeau V, Bergeron C, Budzinski JW, Foster BC, Merali Z (2003) Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): a medicinal plant with anxiolytic properties. Phytomedicine 10:640–649. https://doi.org/10.1078/0944-7113-00374
Barnes J, Anderson LA, Phillipson JD (2007) Scullcap. In: Herbal medicines, 3rd edn. The Pharmaceutical Press, New York, pp 530–532
Choi J, Conrad CC, Malakowsky CA, Talent JM, Yuan CS, Gracy RW (2002) Flavones from Scutellaria baicalensis Georgi attenuate apoptosis and protein oxidation in neuronal cell lines. BBA Gen Subjects 1571(3):201–210. https://doi.org/10.1016/S0304-4165(02)00217-9
Cole IB, Saxena PK, Murch SJ (2007) Medicinal biotechnology in the genus Scutellaria. In Vitro Cell Dev 43:318–327. https://doi.org/10.1007/s11627-007-9055-4
Cole IB, Farooq FT, Murch SJ (2009) Protocols for establishment of an in vitro collection of medicinal plants in the genus Scutellaria. In: Jain SM, Saxena PK (eds) Protocols for in vitro cultures and secondary metabolite analysis of aromatic and medicinal plants. Methods in molecular biology, vol 547. Humana Press, Totawa NJ, pp 155–165
Duškova J, Dušek J, Jahodář L (1999) Zur Biotransformation von Hydrochinon zu Arbutin in den in vitro-Kulturen. Herba Pol 45:78–81
Ekiert H, Czygan FCH (2005) Accumulation of biologically active furanocumarins in agitated cultures of Ruta graveolens and Ruta graveolens ssp. divaricata (Tenore) Gams. Pharmazie 60:623–626
Ekiert H, Kwiecień I, Szopa A, Muszyńska B (2013) Possibilities of arbutin production using plant biotechnology methods. Pol J Cosmetol 15:151–162
Kampa M, Alexaki V, Notas G et al (2004) Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res 6:R63–74. https://doi.org/10.1186/bcr752
Kawka B, Kwiecień I, Ekiert H (2017) Influence of culture medium composition and light conditions on the accumulation of bioactive compounds in shoot cultures of Scutellaria lateriflora L. (American Skullcap) grown in vitro. Appl Biochem Biotechnol 183(4):1414–1425
Kawka B, Kwiecień I, Ekiert H (2019) Production of specific flavonoids and verbascoside in shoot cultures of Scutellaria baicalensis. In: Ramawat K, Ekiert H, Goyal S (eds) Plant cell and tissue differentiation and secondary metabolites: Reference series in phytochemistry. Springer, Switzerland, pp 1–24. https://doi.org/10.1007/978-3-030-11253-0_7-1
Kim JK, Kim YS, Kim Y, Uddin MR, Kim YB, Kim HH (2014) Comparative analysis of flavonoids and polar metabolites from hairy roots of Scutellaria baicalensis and Scutellaria lateriflora. World J Microb Biot 30(3):887–892. https://doi.org/10.1007/s11274-013-1498-7
Kubica P, Szopa A, Ekiert H (2017) Production of verbascoside and phenolic acids in biomass of Verbena officinalis L. (vervain) cultured under different in vitro conditions. Nat Prod Res 31(14):1663–1668. https://doi.org/10.1080/14786419.2017.1286477
Kubica P, Szopa A, Prokopiuk B, Komsta Ł, Pawłowska B, Ekiert H (2020) The influence of light quality on the production of verbascoside, isovervascoside and phenolic acids and the content of photosynthetic pigments in biomass of Verbena officinalis L. cultured in vitro. J Photoch Photobio B 203:111768. https://doi.org/10.1016/j.photobiol.2019.111768
Kwiecień I, Szopa A, Madej K, Ekiert H (2013) Arbutin production via biotransformation of hydroquinone in in vitro cultures of Aronia melanocarpa (Michx.) Elliott. Acta Biochim Pol 60:865–870
Kwiecień I, Szydłowska A, Kawka B, Beerhues L, Ekiert H (2015) Accumulation of biologically active phenolic acids in agitated shoot cultures of three Hypericum perforatum cultivars: ‘Elixir’, ‘Helos’ and ‘Topas’. Plant Cell Tissue Organ Cult 123(2):273–281
Kwiecień I, Smolin J, Ekiert H (2018) The impact of media composition on production of flavonoids in agitated shoot cultures of the three Hypericum perforatum cultivars: ‘Elixir’, ‘Helos’ and ‘Topas’. In Vitro Cell Dev 54:332–340
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Marsh Z, Yang T, Nopo-Olazabal L, Wu S, Ingle T, Joshee N, Medina-Bolivar F (2014) Effect of light, methyl jasmonate and cyclodextrin on production of phenolic compounds in hairy root cultures of Scutellaria lateriflora. Phytochemistry 107:50–60. https://doi.org/10.1016/j.phytochem.2014.08.020
Migas P, Krauze-Baranowska M (2015) The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem Lett 13:35–40. https://doi.org/10.1016/j.phytol.2015.05.015
Millspaugh CF (1974) Scutellaria. American medicinal plants. Dover Publications, New York, pp 469–472
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy HN, Lee EJ, Peak KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult 118:1–16
Nishikawa K, Furukawa H, Fujioka T, Fujii H, Mihashi K, Shimomura K, Ishimaru K (1999) Phenolics in tissue cultures of Scutellaria. Nat Med 53(4):209–213
Piekoszewska A, Ekiert H, Zubek S (2010) Arbutin production in Ruta graveolens and Hypericum perforatum L. in vitro cultures. Acta Physiol Plant 32:223–229
Ramawat KG, Mathur M (2007) Factors affecting the production of secondary metabolites. In: Ramawat KG, Merillon JM (eds) Biotechnology: secondary metabolites. Plants and Microbes. Science Publ Inc., Enfield, pp 59–102
Shang X, He X, He X, Li M, Zhang R, Fan P, Zhang Q, Jia Z (2010) The genus Scutellaria: an ethnopharmacological and phytochemical review. J Ethnopharmacol 128:279–313
Skrzypczak-Pietraszek E, Kwiecień I, Gołdyn A, Pietraszek J (2017) HPLC-DAD analysis of arbutin produced from hydroquinone in a biotransformation process in Origanum majorana L. shoot culture. Phytochem Lett 20:443–448
Štambergová A, Supčiková M, Leifertová I (1985) Hodnoceni fenolických lŕtek v Arctostaphylos uva-ursi. IV. Stanoveni arbutinu, metylarbutinu a hydrochinonu v listech metodou HPLC. Ceskoslov Farm 34:179–183
Sułkowska-Ziaja K, Maślanka A, Szewczyk A, Muszyńska B (2017) Physiologically active compounds in four species of Phellinus. Nat Prod Commun 12:363–366
Szopa A, Ekiert H (2011) Lignans in Schisandra chinensis in vitro cultures. Pharmazie 66:633–634
Szopa A, Ekiert H (2014) Production of biologically active phenolic acids in Aronia melanocarpa (Michx) Elliott in vitro cultures cultivated on different variants of the Murashige and Skoog medium. Plant Growth Regul 72(1):51–58. https://doi.org/10.1007/s10725-013-9835-2
Szopa A, Ekiert H, Muszyńska B (2013) Accumulation of hydroxybenzoic acids and other biologically active phenolic acids in shoot and callus cultures of Aronia melanocarpa (Michx.) Elliott (black chokeberry). Plant Cell Tissue Organ Cult 113:323–329
Szopa A, Kokotkiewicz A, Marzec-Wróblewska U, Buciński A, Luczkiewicz M, Ekiert H (2016) Accumulation of dibenzocyclooctadiene lignans in agar cultures and in stationary and agitated liquid cultures of Schisandra chinensis (Turcz.) Baill. Appl Microbiol Biotechnol 100:3965–3977. https://doi.org/10.1007/s00253-015-7230-9
Szopa A, Kubica P, Ekiert H (2018) Agitated shoot cultures of Aronia arbutifolia and Aronia × prunifolia—biotechnological studies on the accumulation of phenolic compounds and biotransformation capability. Plant Cell Tiss Organ Cult. https://doi.org/10.1007/s11240-018-1436-3
Szopa A, Kwiecień I, Ekiert H (2017) Biotransformation of hydroquinone and 4-hydroxybenzoic acid in Schisandra chinensis (Chinese magnolia vine) in vitro cultures. Acta Sci Pol-Hortoru 16(6):57–66
Tascan A, Adelberg J, Tascan M, Rimando J, Yadav AK (2010) Hyperhydricity and flavonoid content of Scutellaria species in vitro on polyester-supported liquid culture system. HortScience 45(11):1723–1728
Upton R, DAyu RH (2012) Skullcap Scutellaria lateriflora L.: an American nervine. J Herb Med 2(3):76–96
Upton R, Graff A, Jolliffe G, Länger R (2011) Skullcap aerial parts. In: American Herbal Pharmacopoeia: botanical pharmacognosy - microscopic characterization of botanical medicines, pp 599–603
Wilczańska-Barska A, Królicka A, Głód D, Majdan M, Kawiak A, Krauze-Baranowska M (2012) Enhanced accumulation of secondary metabolites in hairy root cultures of Scutellaria lateriflora following elicitation. Biotechnol Lett 34(90):1757–1763
Zhang XY, Lian S, Li S, Stringer JL (2009) Characterization of chemical ingredients and anticonvulsant activity of American skullcap (Scutellaria lateriflora). Phytomedicine 16:485–493. https://doi.org/10.1016/j.phymed.2008.07.011
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Nokwanda Pearl Makunga.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kawka, B., Kwiecień, I. & Ekiert, H. Endogenous production of specific flavonoids and verbascoside in agar and agitated microshoot cultures of Scutellaria lateriflora L. and biotransformation potential. Plant Cell Tiss Organ Cult 142, 471–482 (2020). https://doi.org/10.1007/s11240-020-01837-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01837-y