Abstract
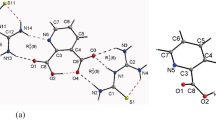
The vibrational spectra of the p-tetrasulfonatothiacalix[4]arene pentasodium salt (TCAS) and tert-butylthiacalix[4]arene (BuTCA) were studied. Comparison of the TCAS and BuTCA IR spectra allows us to isolate the bands of tert-butyl and sulfonate groups. Geometry, IR and Raman spectra were calculated for conformation cone, partial cone, 1,2-, and 1,3-alternate. The most stable conformation of the TCAS is the cone. Characteristic bands were determined for each of the possible conformations. In the case of the TCAS molecule, four ions of sodium are coordinated with the oxygen atoms of sulfonate groups, and the fifth ion interacts with the oxygen and sulfur atoms of the macrocycle. Under the influence of sodium ions, the distribution of electron density in the TCAS molecule and its ability to supramolecular interactions change.
Graphical abstract









Similar content being viewed by others
Data availability
We allow the journal to review all the data, and we confirm the validity of results.
Code availability
NA.
References
Vicens J, Harrowfield J, Baklouti L (eds) (2007) Calixarenes in the nanoworld. Springer, Dordrecht
Neri P, Sessler J, Wang M-X (eds) (2016) Calixarenes and beyond. Springer International Publishing, Switzerland
Ovsyannikov AS, Solovieva SE, Antipin IS, Ferlay S (2017) Coordination polymers based on calixarene derivatives: structure and properties. Coord Chem Rev 352:151–186
Asfari Z, Bohmer V, Harrowfield J, Vincens J (2001) Calixarenes. Kluwer, Netherlands
Solovieva SE, Burilov VA, Antipin IS (2017) Thiacalix[4]arene’s lower rim derivatives: synthesis and supramolecular properties. Macroheterocycles 10:134–146
Baldini L, Sansone F, Casnati A, Ungaro R (2012) Calixarenes in molecular recognition. In Supramolecular chemistry: from molecules to nanomaterials. In Steed JW, Gale PA, (eds.). Wiley, New York pp. 863–878
Perret F, Coleman AW (2011) Biochemistry of anionic calix[n]arenes. Chem Commun 47:7303–7319
Perret F, Lazar AN, Coleman AW (2006) Biochemistry of the para-sulfonato-calix[n]arenes. Chem Commun 42:2425–2438
Ludwig R (2005) Calixarenes for biochemical recognition and separation. Microchim Acta 152:1–19
Paclet MN, Rousseau CF, Yannick C, Morel F, Coleman AW (2006) An absence of non-specific immune response towards para-sulphonato-calixarenes. J Inclusion Phenom Macrocyclic Chem 55:353–357
Atwood JL, Barbour LJ, Hardie MJ, Raston CL (2001) Metal sulfonatocalix[4,5]arene complexes: bi-layers, capsules, spheres, tubular arrays and beond. Coord Chem Rev 222:3–22
Kushch LA, Yagubskii EB, Dmitriev AI, Morgunov RB, Emelyanov VA, Mustafina AR, Gubaidullin AT, Burilov VA, Solovieva SE, Shaniel D, Woike Th (2010) Bifunctional supramolecular systems on the platform of p-sufonatothiacalix[4]arene containing photochromic mononitrosyl Ru(II) and paramagnetic aqua Gd or Dy complexes. Physica B 405:30–33
Mustafina AR, Skripacheva VV, Burilov VA, Yanilkin VV, Amirov RR, Stepanov AS, Solovieva SE, Antipin IS, Konovalov AI (2009) Heterometallic complex formation on p-sulfonatothiacalix[4]arene platform resulting in pH- and redox-modification of [Ru(bpy)3]2+ luminescence. Inorg Chim Acta 362:3279–3284
Kovalenko VI, Chernova AV, Borisoglebskaya EI, Katsyuba SA, Zverev VV, Shagidullin RR, Antipin IS, Solovyeva SE, Stoikov II, Konovalov AI (2002) Cooperative intramolecular hydrogen bond and conformations of thiacalix[4]arene molecules. Russ Chem Bull Int Ed 51:825–827
Schatz J, Schildbach F, Lentz A, Rastatter S, Schilling J, Dormann J, Ruoff A, Debaerdemaeker TZ (2000) The inclusion of carbon disulfide in p-tert-butylcalix[4]- and [6]arene—a combined crystallographic and vibrational spectroscopic study. Naturforsch Teil B 55:213–221
Billes F, Mohammed-Ziegler I (2002) Ab initio equilibrium geometry and vibrational spectroscopic study of 25,26,27,28-tetrahydroxycalix[4]arene. Supramol Chem 14:451–459
Katsyuba SA, Kovalenko VI, Chernova AV, Vandyukova EE, Zverev VV, Shagidullin RR, Antipin IS, Solovieva SE, Stoikov I, Konovalov AI (2005) Vibrational spectra, co-operative intramolecular hydrogen bonding and conformations of calix[4]arene and thiacalix[4]arene molecules and their para-tert-butyl derivatives. Org Biomol Chem 3:2558–2565
Furer VL, Vandyukov AE, Kleshnina SR, Solovieva SE, Antipin IS, Kovalenko VI (2020) FT-IR and FT-Raman study of p-sulfonatocalix[8]arene. J Mol Struc 1203:127474
Furer VL, Potapova LI, Vatsouro IM, Kovalev VV, Shokova EA, Kovalenko VI (2018) Investigation of the conformation and hydrogen bonds in adamantylthiacalix[4]arene by IR spectroscopy and DFT. J Mol Struc 1171:207–213
Furer VL, Potapova LI, Vatsouro IM, Kovalev VV, Shokova EA, Kovalenko VI (2019) Study of conformation and hydrogen bonds in the p-1-adamantylcalix[8]arene by IR spectroscopy and DFT. J Incl Phenom 95:63–71
Furer VL, Potapova LI, Kovalenko VI (2017) DFT study of hydrogen bonding and IR spectra of calix[6]arene. J Mol Struct 1128:439–447
Furer VL, Potapova LI, Kovalenko VI (2017) Cyclic cooperative intramolecular hydrogen bond in p-tert-butylcalix[6]arene according to FTIR spectroscopy and DFT studies. Spectrochim Acta 181:98–108
Cerioni G, Biali S, Rappoport Z (1996) Hydrogen bonding in calix[n]arenes. A preliminary 17O NMR study. Tetrahedron Lett 37:5797–5800
Bernardino RJ, Costa Cabral BJ (1999) Structure, conformational equilibrium, and proton affinity of calix[4]arene by density functional theory. J Phys Chem A 103:9080–9085
Kim K, Park SJ, Choe JI (2008) DFT conformational study of calix[5]arene and calix[4]arene hydrogen bond. Bull Korean Chem Soc 29:1893–1897
Abbasi A, Sardroodi JJ (2016) N-doped TiO2 anatase nanoparticles as a highly sensitive gas sensor for NO2 detection: insights from DFT computations. Environ Sci: Nano 3:1153–1164
Abbasi A, Sardroodi JJ (2019) Adsorption of O3, SO2 and SO3 gas molecules on MoS2 monolayers: a computational investigation. Environ Sci: Nano Appl Surface Sci 469:781–791
Bonab PJ, Esrafili MD, Ebrahimzadeh AR, Sardroodi JJ (2021) Molecular dynamic simulations of choline chloride and phenyl propionic acid deep eutectic solvents: investigation of structural and dynamic properties. J Mol Graph Model 106:107908
Iki N, Suzuki T, Koyama K, Kabuto C, Miyano S (2002) Inclusion behaviour of thiacalix[4]arensulfonate toward water-miscible organic molecules studied by salting-out and X-ray crystallography. Org Lett 4:509–512
Atwood JL, Coleman AW, Zhang H, Bott SG (1989) Organic clays. Synthesis and structure of N5[calix[4]arene sulfonate]∙12 H2O, K5[calix[4]arene sulfonate]∙8H2O, Rb5[calix[4]arene sulfonate]∙5H2O, and Cs5[calix[4]arene sulfonate]∙4H2O. J Incl Phenom 7:203–211
Becke AD (1993) Density functional thermochemistry. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Cole-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Schwenke DW, Truhlar DG (1985) Systematic study of basis set superposition errors in the calculated energy of two HF molecules. J Chem Phys 82:2418–2426
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09 Revision C.01. Gaussian Inc., Wallingford
Scott AP, Radom L (1996) Harmonic vibrational frequencies: an evaluation of Hartree-Fock, Moller-Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J Chem Phys 100:16502–16513
Sipachev VA (1985) Calculation of shrinkage corrections in harmonic approximation. J Mol Struct (Theochem) 121:143–151
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516
Akdas H, Bringel L, Graf E, Hosseini MW, Mislin G, Pananel J, Cian AD, Fischer J (1998) Thiacalixarenes: synthesis and structural analysis of thiacalix[4]arene and of p-tert-butylthiacalix[4]arene. Tetr Lett 39:2311–2314
Glendening ED, Landis CR, Weinhold F (2012) Natural bond orbital methods. Comput Mol Sci 2:1–42
Ugozzoli F, Andretti GD (1992) Symbolic representation of the molecular conformation of calixarenes. J Incl Phenom 13:337–348
Acknowledgements
The authors are grateful to the Assigned Spectral-Analytical Center of FRC Kazan Scientific Center of RAS for technical assistance in research.
Funding
Contributions to research were funded by the state assignment to Arbuzov Institute of Organic and Physical Chemistry, FRC Kazan Scientific Center, Russian Academy of Sciences (AAAA-A18-118041760011–2).
Author information
Authors and Affiliations
Contributions
Victor Furer: conceptualization, methodology, software, writing—original draft preparation, and editing
Alexandr Vandyukov: investigation of IR and Raman spectra
Sophia Kleshnina: synthesis of calixarenes
Svetlana Solovieva: conceptualization, methodology, reviewing, and editing
Igor Antipin: conceptualization, methodology, reviewing, and editing
Valery Kovalenko: conceptualization, methodology, reviewing, and editing
Corresponding author
Ethics declarations
Ethics approval
The authors agree with ethical standards. This work was not published previously, and is not submitted to more than one journal. No data have been fabricated or manipulated.
Consent to participate
The authors agree to participate in the article.
Consent for publication
The authors agree to participate in the publication of the article.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Furer, V.L., Vandyukov, A.E., Kleshnina, S.R. et al. Study of the conformation and hydrogen bonds of the p-tetrasulfonatothiacalix[4]arene pentasodium salt by vibrational spectroscopy and DFT. J Mol Model 27, 326 (2021). https://doi.org/10.1007/s00894-021-04905-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04905-y