Abstract
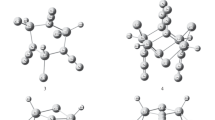
HF cluster is a typical hydrogen bond system. (HF)1–16 clusters have been studied by MP2/aug-cc-pvdz//B3LYP/6-311++G(d,p) method. The global minimum structures of them have been obtained. (HF)2 cluster is a chain structure. As n = 3–10, (HF)n clusters present cyclic conformations, whereas the structures are twisted cyclic structures when n = 13–16. When n = 11 and 12, the structures are 7 + 4 complex and dual-ring structures, respectively. Radial distribution functions of the cyclic conformation (HF)n (n = 3–16) clusters reveal that radiuses of the rings change as n linearly. The structural parameters of the clusters have been analyzed. Hydrogen bond energies of (HF)n (n = 2–16) are calculated, which manifests that they increase first as n in the range of 2–10, and then do not change much as size when n = 11–16. The maximum value of hydrogen energy is − 0.049 a.u., existing in (HF)10 cluster. Stability analysis reveals that (HF)4 and (HF)12 are magic number clusters.




Similar content being viewed by others
References
Rincón L, Almeida R, Garcı́a-Aldea D, Diez y Riega H (2001) Hydrogen bond cooperativity and electron delocalization in hydrogen fluoride clusters. J Chem Phys 114(13):5552–5561. https://doi.org/10.1063/1.1351878
Kozuch S, Martin JML (2013) Halogen bonds: benchmarks and theoretical analysis. J Chem Theory Comput 9(4):1918–1931. https://doi.org/10.1021/ct301064t
Guedes R, Do Couto P, Costa Cabral B (2003) Binding energy, structure, and vibrational spectra of (HCl)2–6 and (HF)2–10 clusters by density functional theory. J Chem Phys 118(3):1272–1281. https://doi.org/10.1063/1.1528952
Avilés MW, McCandless ML, Curotto E (2008) Stereographic projection path integral simulations of (HCl)n clusters (n = 2-5): evidence of quantum induced melting in small hydrogen bonded networks. J Chem Phys 128(12):124517. https://doi.org/10.1063/1.2192773
Douberly G, Miller R (2003) The growth of HF polymers in helium nanodroplets: probing the barriers to ring insertion. J Phys Chem B 107(19):4500–4507. https://doi.org/10.1021/jp022360+
Abu-Awwad FM (2002) Ab initio study of molecular surface electrostatic potential of hydrogen fluoride clusters (HF)n, (n = 2-15). Chem Phys Lett 360(3–4):340–348. https://doi.org/10.1016/s0009-2614(02)00855-2
Kolenbrander KD, Dykstra CE, Lisy JM (1988) Torsional vibrational modes of (HF)3: IR-IR double resonance spectroscopy and electrical interaction theory. J Chem Phys 88(10):5995–6012. https://doi.org/10.1063/1.454492
Klopper W, Quack M, Suhm MA (1998) Explicitly correlated coupled cluster calculations of the dissociation energies and barriers to concerted hydrogen exchange of (HF)n oligomers (n = 2, 3, 4, 5). Mol Phys 94(1):105–119. https://doi.org/10.1080/00268979809482299
Volobuev Y, Necoechea WC, Truhlar DG (1997) Tunneling splittings in predissociated HF dimer. J Phys Chem A 101(17):3045–3048. https://doi.org/10.1021/jp963328g
Li Y, Li Z-R, Wu D, Li R-Y, Hao X-Y, Sun C-C (2004) An ab initio prediction of the extraordinary static first hyperpolarizability for the electron-solvated cluster (FH)2{e}(HF). J Phys Chem B 108(10):3145–3148. https://doi.org/10.1002/chin.200419001
McGrath MJ, Kuo IFW, Siepmann JI (2011) Liquid structures of water, methanol, and hydrogen fluoride at ambient conditions from first principles molecular dynamics simulations with a dispersion corrected density functional. Phys Chem Chem Phys 13(44):19943–19950. https://doi.org/10.1039/c1cp21890e
Howard JC, Gray JL, Hardwick AJ, Nguyen LT, Tschumper GS (2014) Getting down to the fundamentals of hydrogen bonding: anharmonic vibrational frequencies of (HF)2 and (H2O)2 from Ab initio electronic structure computations. J Chem Theory Comput 10(12):5426–5435. https://doi.org/10.1021/ct500860v
Tao Y, Zou W, Kraka E (2017) Strengthening of hydrogen bonding with the push-pull effect. Chem Phys Lett 685:251–258. https://doi.org/10.1016/j.cplett.2017.07.065
Karpfen A, Yanovitskii O (1994) Structure and vibrational spectra of neutral, protonated and deprotonated hydrogen bonded polymers: an ab initio SCF study on chain-like hydrogen fluoride clusters. THEOCHEM J Mol Struct 307:81–97. https://doi.org/10.1016/0166-1280(94)80120-7
Jelil M, Abaydulla A (2015) Graph theoretical enumeration of topology-distinct structures for hydrogen fluoride clusters (HF)n (n ≤ 6). J Chem Phys 143(4):044301. https://doi.org/10.1063/1.4926939
Kucherov SY, Bureiko S, Denisov G (2016) Anticooperativity of FHF hydrogen bonds in clusters of the type F− × (HF)n, RF × (HF)n and XF × (HF)n, R = alkyl and X = H, Br, Cl, F. J Mol Struct 1105:246–255. https://doi.org/10.1016/j.molstruc.2015.10.066
Haghmoradi A, Chapman WG (2019) Bond cooperativity and ring formation in hydrogen fluoride thermodynamic properties: a two-density formalism framework. J Chem Phys 150(17):174503. https://doi.org/10.1063/1.5079874
Asselin P, Soulard P, Madebène B, Goubet M, Huet TR, Georges R, Pirali O, Roy P (2014) The cyclic ground state structure of the HF trimer revealed by far infrared jet-cooled Fourier transform spectroscopy. Phys Chem Chem Phys 16(10):4797–4806. https://doi.org/10.1039/c3cp55047h
Grein F (2020) Additivity and non-additivity of dissociation energies in intermolecular interactions. Theoretical studies on (H2)n, n = 2-8, (CO2)n, n = 2-6 and (HF)n, n = 2-8. Mol Phys:1–12. https://doi.org/10.1080/00268976.2020.1753839
Friedrich J, Perlt E, Roatsch M, Spickermann C, Kirchner B (2011) Coupled cluster in condensed phase. Part i: static quantum chemical calculations of hydrogen fluoride clusters. J Chem Theory Comput 7(4):843–851. https://doi.org/10.1021/ct100131c
Johnson M, Sandor E, Arzi E (1975) The crystal structure of deuterium fluoride. Acta Crystallogr B 31(8):1998–2003. https://doi.org/10.1107/s0567740875006711
Janzen J, Bartell L (1969) Electron-diffraction structural study of polymeric gaseous hydrogen fluoride. J Chem Phys 50(8):3611–3618. https://doi.org/10.1063/1.1671593
Duff YL, Holzer W (1974) Raman scattering of HF in the gas state and in liquid solution. J Chem Phys 60(5):2175–2178. https://doi.org/10.1063/1.1681331
Avilés MW, Gray PT, Curotto E (2006) Stereographic projection path-integral simulations of (HF)n clusters. J Chem Phys 124(17):174305. https://doi.org/10.1063/1.2192773
Lee C, Yang W, Parr R (1988) Development of the Colle-Salvetti conelation energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/physrevb.37.785
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Petersson G, Nakatsuji H (2016) Gaussian 16 revision a. 03. 2016; Gaussian Inc. Wallingford CT 2(3):4
Dennington R, Keith TA, Millam JM (2016) GaussView, version 6.0. 16. Semichem Inc, Shawnee
Lu T, Chen FW (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592. https://doi.org/10.1002/jcc.22885
Huber K-P, Herzberg G (1979) Constants of diatomic molecules. Mol spectra mol struct:8–869. https://doi.org/10.1007/978-1-4757-0961-2_2
Dyke TR, Howard BJ, Klemperer W (1972) Radiofrequency and microwave spectrum of the hydrogen fluoride dimer; a nonrigid molecule. J Chem Phys 56(5):2442–2454. https://doi.org/10.1063/1.1677553
Hodges MP, Stone AJ, Cabaleiro Lago E (1998) Analytical potentials for HF dimer and larger HF clusters from ab initio calculations. J Phys Chem A 102(14):2455–2465. https://doi.org/10.1021/jp972148j
Liu SY, Michael DW, Dykstra CE, Lisy JM (1986) The stabilities of the hydrogen fluoride trimer and tetramer. J Chem Phys 84(9):5032–5036. https://doi.org/10.1063/1.450652
Srolovitz D, Egami T, Vitek V (1981) Radial distribution function and structural relaxation in amorphous solids. Phys Rev B 24(12):6936–6944. https://doi.org/10.1103/physrevb.24.6936
Contreras-García J, Johnson ER, Keinan S, Chaudret R, Piquemal J-P, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7(3):625–632. https://doi.org/10.1021/ct100641a
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang WT (2010) Revealing noncovalent interactions. J Am Chem Soc 132(18):6498–6506. https://doi.org/10.1021/ja100936w
Song C, Tian Z (2019) Systematic study on the structures and properties of (Ag2S)n (n = 1-8) clusters. J Mol Model 25(10):310. https://doi.org/10.1007/s00894-019-4191-4
Tian Z, Cheng L (2016) First principles study on the structural evolution and properties of (MCl)n (n = 1-12, M = Cu, Ag) clusters. RSC Adv 6(36):30311–30319. https://doi.org/10.1039/c6ra01258b
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Zhang C, Freeman DL, Doll JD (1989) Monte Carlo studies of hydrogen fluoride clusters: cluster size distributions in hydrogen fluoride vapor. J Chem Phys 91(4):2489–2497. https://doi.org/10.1063/1.457008
Acknowledgments
The calculations were carried out on the Theoretical and Computational Chemistry LAB, School of Chemistry and Materials Engineering, Fuyang Normal University, China.
Funding
This work was supported by 2017 Fuyang municipal government-Fuyang Normal University horizontal cooperation project (XDHX201719, XDHX201739), scientific research starting fund for doctor of Fuyang Normal University, and the natural science project of Anhui province department of education (No. KJ2019A0539).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 4505 kb)
Rights and permissions
About this article
Cite this article
Song, C., Tian, Z., Wang, C. et al. Growth behavior and properties of (HF)1–16 clusters. Struct Chem 32, 395–403 (2021). https://doi.org/10.1007/s11224-020-01637-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01637-2