Abstract
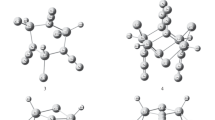
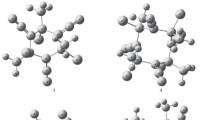
The density functional theory (DFT) method has been employed to systematically investigate the geometrical structures, stabilities, IR spectrum and thermodynamic properties of small asymmetric clusters (HClBN3) n (n = 1–6). When n ≥ 2, the optimized results suggest that the (BNα)2n cyclic structures with alternating boron and α-nitrogen atoms are observed in clusters. The influences of cluster size on the structures of clusters were discussed. The second-order difference in energies show that the (HClBN3)3 isomer is the most stable among the asymmetric clusters (HClBN3) n . Four main characteristic regions are obtained and assigned for the calculated IR spectra. A study of their thermodynamic properties suggests that monomer 1 forms clusters (2–6) thermodynamically favorable by the enthalpies at 298.2 K.
Similar content being viewed by others
References
E. Wiberg and H. Michaud, Z. Naturforsch. 96, 497 (1954).
R. L. Mulinax, G. S. Okin, and R. D. Coombe, J. Phys. Chem. 99, 6294 (1995).
P. I. Paetzold, Z. Anorg. Allg. Chem. 326, 47 (1963).
J. Müller and P. I. Paetzold, Heteroat. Chem. 1, 461 (1990).
P. I. Paetzold, P. P. Habereder, and R. Müllbauer, J. Organomet. Chem. 7, 45 (1967).
R. Hausser-Wallis, H. Oberhammer, W. Einholz, et al., Inorg. Chem. 29, 3286 (1990).
P. I. Paetzold and H. J. Hansen, Z. Anorg. Allg. Chem. 345, 79 (1966).
N. Wiberg, W. C. Joo, and K. H. Schmid, Z. Anorg. Allg. Chem. 394, 197 (1972).
W. Fraenk, T. M. Klapötke, B. Krumm, et al., Chem.Commun., 667 (2000).
W. Fraenk, T. M. Klapötke, B. Krumm, et al., J. Chem. Soc., Dalton Trans., 4635 (2000).
W. Fraenk, T. Habereder, A. Hammerl, et al., Inorg. Chem. 40, 1334 (2001).
J. Müller, Z. Anorg. Allg. Chem. 382, 110 (1971).
P. I. Paetzold, Fortsch. Chem. Forsch. 8, 437 (1967).
P. I. Paetzold, M. Gayoso, and K. Dehnicke, Chem. Ber. 98, 1173 (1965).
L. A. Johnson, S. A. Sturgis, I. A. Al-Jihad, et al., J. Phys. Chem. A 103, 686 (1999).
M. J. Travers and J. V. Gilbert, J. Phys. Chem. A 104, 3780 (2000).
W. Fraenk and T. M. Klapötke, J. Fluorine Chem. 111, 45 (2001).
D. X. Ma, Q. Y. Xia, and C. Zhang, J. At. Mol. Phys. 26, 361 (2009).
D. X. Ma, Q. Y. Xia, W. W. Zhao, et al., Comput. Appl. Chem. 26, 1583 (2009).
J. McMurran, J. Kouvetakis, D. C. Nesting, et al., J. Am. Chem. Soc. 120, 5233 (1998).
J. Kouvetakis, J. McMurran, C. Steffek, et al., Inorg. Chem. 39, 3805 (2000).
J. Kouvetakis, J. McMurran, C. Steffek, et al., Main Group Met. Chem. 24, 77 (2001).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 03, Revision B.03 (Gaussian Inc., Pittsburgh PA, 2003).
A. D. Becke, J. Chem. Phys. 98, 5648 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B 37, 785 (1988).
A. P. Scott and L. Radom, J. Phys. Chem. 100, 16502 (1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Wang, A., Chen, Z., Ma, D. et al. Search for the structures, stabilities, IR spectra, and thermodynamic properties of the asymmetric clusters (HClBN3) n (n = 1–6). Russ. J. Phys. Chem. 90, 2541–2549 (2016). https://doi.org/10.1134/S0036024416130021
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024416130021