Abstract
The thermodynamic aspects of keto-enol tautomerism of hyperforin were investigated theoretically using density functional theory methods. At the B3LYP/aug-cc-pVTZ//B3LYP/aug-cc-pVDZ level of theory the enol tautomer dominates the tautomeric mixture and the second enol tautomer 1OH-HB has Gibbs free energy higher by 1.2 kcal/mol, despite possessing an intramolecular hydrogen bond. The purely keto tautomer is less stable by 3.3 kcal/mol compared with the 1OH tautomer, which means that the percentage of the keto tautomer in the tautomeric mixture is only about 0.4%. This is a different picture than in the parent compound of hyperforin—the phloroglucinol, where the keto tautomer is more stable than corresponding enol 1OH tautomer by 0.6 kcal/mol. To explain this difference, several in-between model molecules reflecting gradual transformation from phloroglucinol to hyperforin were build, and all the tautomeric forms were optimized for each molecule. It turned out that the addition of an aliphatic three-carbon bridge to phloroglucinol ring is crucial for the reversal of the tautomer stability order to that for hyperforin. The probable reason is the unfavorable strain in the keto tautomer introduced by the carbon bridge, which forces a specific geometric configuration which destabilizes in consequence the keto tautomer. This picture of hyperforin tautomerism underlines the dominance of enol tautomers, which can be important when studying the antidepressant activity of hyperforin—its interactions with neurotransmitters receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is a disease of affluence which becomes recently a major problem in modern societies. Its pharmacological treatment is one of the biggest challenges in medicine and pharmacy. The widely used selective serotonin reuptake inhibitors (SSRI) present adverse effects; on the other hand, anti-anxiolytic drugs can result in severe addiction problems. In comparison, the alcohol extracts from St. John’s wort (Hypericum perforatum) has the efficiency similar to SSRI drugs but are free from problems with adverse effects or addiction, thus, can be used in therapy of mild to moderate depression [5, 22]. Recent studies suggest that this antidepressant activity can be mainly attributed to hyperforin, a prenylated phloroglucinol derivative which is the reuptake inhibitor of various neurotransmitters, including serotonin, dopamine, norepinephrine, GABA, and glutamic acid [4].
The phloroglucinol (PG), which is the core of the hyperforin molecule, is a polyphenol which undergoes the keto-enol tautomerism and can exist in 5 tautomeric forms (Scheme 1).
We decided to use the following naming convention. The purely enol tautomer, we call it 3OH because of 3 hydroxyl groups. Next, there are two tautomeric forms with 2 hydroxyl groups and 1 keto group. The form where the tautomeric proton goes to more distant carbon atom, we call it 2OH-a, and the form where the proton goes to the proximal carbon atom, we call it 2OH-b. Next, we have the tautomer with 1 hydroxyl group and 2 keto groups which we call 1OH and finally the pure keto tautomer designed keto.
The order of stability of PG tautomers is since a long time a topic of interest. The 3OH enol form is expected to gain stability (lower energy) from the aromatic stabilization of the benzene ring; however, the keto form benefits from great strength of the double carbon-oxygen bonds [24]. Wheland, using the experimental results such as heats of hydrogenation or combustion, estimated that the keto form is more stable by 3 kcal/mol [6, 26, 27]. The well-known reactions where PG reacts readily as the keto form [2, 9, 17] seem to support the dominance of the keto tautomer. However, the alkylation can lead to O- or C-substituted products indicating that both 3OH enol and keto form of PG exist [16]. A [19]C NMR and UV study claimed that PG exists mainly as the benzene form [25]. Quantitatively, during a detailed UV-VIS pH-dependent study, it was established that the 3OH form dominates in water solution with the Gibbs free energy lower than keto form by 8.2 kcal/mol [19]. Reproducing this experimental result by theoretical calculations is not easy, due to large dependence of the keto-enol energy gap on the approximations of the theoretical model. According to an early calculation at the Hartree-Fock level of theory, the 3OH enol tautomer was predicted to be more stable than keto by as much as 35 kcal/mol [20]. On the other hand, some recent calculation using semi-empirical PM3, DFT and MP2 Single Point calculations resulted in predicting keto tautomer to be more stable by several kcal/mol [14]. The problem was thoroughly analyzed by Hatanaka which showed that the experimental result is well reproduced by density functional theory calculations, provided a large enough basis set is employed [15]. Thus, it can be concluded that in bare phloroglucinol the 3OH enol form fully dominates the tautomeric mixture, and the other forms are present only in minute amounts. This picture changes rapidly when we move on to the hyperforin molecule, where the 3OH tautomer is absent, and the more subtle differences between stability of other existing tautomeric forms turn out to be essential. The schematic representation of hyperforin is shown in Scheme 2. The core of this molecule is a phloroglucinol ring. Two carbon atoms separated by carbonyl group are additionally connected by an aliphatic three-carbon bridge. The next feature is an external carbonyl group with n-propyl substituent, and it is connected to one of PG ring carbon atom. Next, there are four prenyl (3-methylbut-2-en-1-yl) groups, two of them connected to PG ring, and two to the bridge. Last, there is a methyl group added to the bridge.
As the hyperforin incorporates the PG ring, this molecule exhibits also the keto-enol tautomerism. However, due to multiple substitution of PG ring in hyperforin, the number of types of tautomeric forms is limited to only two: 1OH and keto. On the other hand, the presence of additional carbonyl substituent in hyperforin results in the possibility of existence of a second enol tautomer 1OH-HB, where a hydrogen bond between hydroxyl and external carbonyl group is present. These three possible tautomeric forms of hyperforin are depicted in Scheme 3.
According to recent theoretical calculations at the B3LYP/D95** level by Abramova et al., the Gibbs free energies relative to the most stable 1OH form are 0.2 kcal/mol for keto form and 1.4 kcal/mol for 1OH-HB form [1]. This suggests that the keto and 1OH enol forms are present in approximately similar quantities in tautomeric mixture. However, the theoretical model employed uses a limited basis set (D95**). For accurate energy prediction, a more extended basis set should be used. The coexistence of two tautomeric forms of similar Gibbs free energy is rather a rare case and should be treated with caution. Most often, one of the tautomers dominates the tautomeric mixture. It is important to establish which tautomeric form is dominant. It can be especially important for further studies of the mechanism of biological action of a molecule by molecular docking to receptors or enzymes [24].
Therefore, the main aim of the present study is to calculate the Gibbs free energies of three possible forms of hyperforin with largest possible precision. This will allow us to conclude which tautomeric form of hyperforin dominates the tautomeric mixture and is responsible for the biological activity of this molecule.
Computational details
All calculations were performed by using the Gaussian 16 software package [12] except for optimization of the best hyperforin conformers, which were performed by using Gaussian 09 version [11] and B3LYP [3, 23]/D95** [8] model chemistry to ensure proper comparisons with conformer literature data of Abramova et al. All geometry optimizations were followed by frequency calculations to ensure that the obtained stationary points of the potential energy surface are true minima and to obtain the thermochemical corrections to energy. If not stated otherwise, all molecules were optimized first at the B3LYP/6-31G(d,p) [10] level and then reoptimized at the B3LYP/aug-cc-pVDZ [7] level. The benchmark optimizations for phloroglucinol were also performed at the B3LYP/6-311++G(d,p) [18] level. The final Single Point energy calculations of hyperforin were performed at the B3LYP/aug-cc-pVTZ level using geometric parameters optimized at the B3LYP/aug-cc-pVDZ level which is denoted as B3LYP/aug-cc-pVTZ//B3LYP/aug-cc-pVDZ. For the limited systematic rotor search conformational analysis of hyperforin the freely available Avogadro [13] software was used along with the UFF (Universal Force Field). For further Monte Carlo conformer search, the online version (http://bioserv.rpbs.univ-paris-diderot.fr/services/Frog2/) of freely available Frog2 software [21] was used. The maximal number of Monte Carlo steps was set to maximum number of 1000, and the option to obtain the multiple conformations was chosen. The Cartesian coordinates of all tautomeric forms of studied compounds optimized at the B3LYP/aug-cc-pVDZ levels are available as the Supplementary Information.
Results and discussion
We started our investigations by finding a proper model chemistry to describe the hyperforin tautomerism. We should keep in mind that this is rather a large molecule for advanced quantum chemical investigations. Thus, we decided to stay within the density functional theory and use the B3LYP hybrid functional which is well tested in thermochemical calculations of organic molecules. The remaining question is the choice of the atomic orbital basis set. It should be large enough to account for subtle energy differences between tautomers but should also allow accomplishing the calculations within a reasonable time frame. Another problem with the hyperforin molecule is that it contains several prenyl groups, which allows for a magnitude of different conformations. Sampling this huge conformational space is not a simple task and requires some careful analysis. Modeling water environment is not necessary as hyperforin is a lipophilic molecule, and gas phase calculations should be appropriate.
The plan of the publication is as follows. First, we decided to make a series of calculations for the bare phloroglucinol and compare it to the experimental results to validate the accuracy of our model chemistry. Next, we gradually build the hyperforin molecule, starting with phloroglucinol, adding consecutive molecular fragments. On each step, we optimize the tautomers and study the tautomeric equilibrium in terms of Gibbs free energy comparisons. The final step is finding the best conformations of hyperforin and optimizing them on highest affordable computational level.
Phloroglucinol
To find the most stable tautomeric forms of phloroglucinol, for each tautomeric form, all possible rotamers with different orientations of OH groups were build and optimized first at the B3LYP/6-31G(d,p) level and then at the B3LYP/aug-cc-pVDZ level of theory. The lowest energy rotamers are showed in Fig. 1.
The Gibbs free energies of phloroglucinol tautomers are gathered in Table 1 below.
It follows from Table 1 that the most stable is 3OH tautomer which is pure enol. The second tautomer in stability order is keto, but the Gibbs free energy difference is so large that it can be assumed that 99.99% of phloroglucinol exist in its 3OH tautomeric form. The 1OH tautomeric form is 0.6 kcal/mol less stable than keto, so the energy gap is very small. At the end, come the less stable by 3.2 kcal/mol 2OH-a and 2OH-b tautomers. The energy gap between last two tautomers is only 0.52 kcal/mol.
First, to make a comparison with experimental data [19], let us focus on the energy difference between the 3OH and keto forms. It was stated in the introduction that according to experimental data, this difference was measured to be 8.2 kcal/mol. To analyze the influence of the size of orbital basis set on the Gibbs free energy of phloroglucinol, we optimized the best rotamers at the following levels of theory: (1) B3LYP/6-31G(d,p), (2) B3LYP/6-311++G(d,p), (3) B3LYP/aug-cc-pVDZ, and (4) B3LYP/aug-cc-pVTZ. In the following table, the difference is Gibbs free energy is presented versus the increasing size of the atomic orbital basis set.
The absolute values of the Gibbs free energy of all tautomeric forms of PG optimized at all theory levels from Table 2 are given in Table S1 in the Supplementary Information.
It follows that using a relatively small Pople’s 6-31G(d,p) basis set produces a result which is not in good agreement with experiment. Expanding this basis set to triple zeta and adding diffuse functions (6-311++G(d,p) basis set) gives much better result. The Dunning basis sets perform even better with aug-cc-pVDZ basis giving 5.65 kcal/mol and aug-cc-pVTZ 6.93 kcal/mol. Thus, the best choice here should be aug-cc-pVTZ basis set. However, this basis set is so large that in the case of hyperforin molecule, the full geometry optimizations would be prohibitively time consuming. For this reason, we decided to use the accurate enough aug-cc-pVDZ basis set for geometry optimizations and aug-cc-pVTZ for Single Point energy calculations on the optimized structures.
It turns out that so large dependence of tautomer energy difference on basis set is characteristic for keto/3OH tautomers pair. But we should keep in mind that in hyperforin, the only two tautomers possible are 1OH and keto. Let us now see how the energy difference between these two tautomers of PG behaves when varying the size of the basis set. The results are gathered in Table 3.
For the smallest basis set, the difference is − 1.52 kcal/mol, and it changes only slightly when the size of the basis set increases. Quantitatively, these changes are much smaller than for keto/3OH tautomers pair. Especially the difference between aug-cc-pVDZ and aug-cc-pVTZ results is now in the range of the computational error, and we can safely assume that the Gibbs free energy is well reproduced even by the aug-cc-pVDZ basis set. All basis sets predict the lower energy of the keto tautomer in PG.
Methylated phloroglucinol
The first step towards hyperforin modeling is the methylation of phloroglucinol: adding two methyl groups to each of two ring carbon atoms and one methyl group to third ring carbon atom. Now, only two tautomeric forms are possible—namely keto and one enol tautomer (1OH)—see Fig. 2 below.
This methylation introduces only subtle structural changes to the six-membered ring of phloroglucinol and the Gibbs free energy difference of the two tautomers is very close to unsubstituted phloroglucinol—compare Tables 3 and 4.
Thus, we can conclude that introduction of methyl groups instead of hydrogen atoms do not change the 1OH and keto tautomers order.
Methylated phloroglucinol + bridge
The next step towards hyperforin is introduction of an aliphatic—(CH2)3—bridge instead of two methyl groups. The resulting structures are shown in Fig. 3 below
The Gibbs free energies of MePG-b are gathered in Table 5. It follows from Table 5 that introduction of the bridge changes the tautomeric order. Now, the keto tautomer is less stable by 2.98 kcal/mol
This is a large change, and the reason behind it is interesting. When analyzing influence of a factor (in this case a carbon bridge) on energy difference of two tautomers, we may ask on which tautomeric form the impact is larger. Here we can propose that the influence of the bridge on the keto tautomer is greater and is the source of the reversal of tautomeric forms stability order. This is because adding the bridge changes geometry of the ring very much. For easier analysis, the impact of adding the bridge, the 1OH, and keto tautomers are shown separately in Figs. 4 and 5.
The molecules are oriented in similar way like in Figs. 2 and 3 but rotated so that the plane of the six-membered ring is as parallel as possible to the plane of the view of the reader. The bridge (for MePG-b) is in the upper right part of the figures.
One characteristic feature is that after adding the bridge, the upper (in Figs. 4 and 5) carbonyl group in the PG ring becomes heavily bent to the left side. As it was already on the left side in 1OH tautomer but on the right side in keto tautomer one can conclude that the deformation has larger impact on keto tautomer. It can be also seen on the lower end of the molecules (in Figs. 4 and 5) that after introducing the bridge the deformation becomes larger for keto tautomer. These observations suggest that due to unfavorable deformations of the ring of keto tautomer introduced by adding the bridge, its Gibbs free energy becomes larger than 1OH tautomer.
Therefore, at this stage of modeling the hyperforin, the 1OH tautomer is already the dominant tautomeric form.
Methylated phloroglucinol + bridge + carbonyl group
The next stage towards hyperforin modeling is the introduction of a carbonyl substituent to the ring.
In this compound, one of the remaining two methyl groups is substituted by COCH3 in a similar way as in the hyperforin molecule.
Two possible enol tautomers emerge: (1) 1OH-HB where there exists a hydrogen bond between new carbonyl group and hydroxyl group connected to the ring and (2) 1OH tautomer where the OH group is more distant from carbonyl substituent, and there is no hydrogen bond present (Fig.6.). In the case of keto tautomer, there is only one isomer possible.
It follows from Table 6 that the Gibbs free energy difference between 1OH and keto tautomers is very similar to the previous compound, but the new 1OH-HB tautomer has even lower energy due to strong hydrogen bond present. To estimate the energy of this hydrogen bond, we performed optimization of a rotamer where the OH bond is rotated to not allow creation of the hydrogen bond with carbonyl group (Fig. 7).
The Gibbs free energy difference between 1OH-nonHB and 1OH-HB equals to 5 kcal/mol and can be used as the estimate of this hydrogen bond strength. It is clear, that the fact that the 1OH-HB tautomer is more stable than 1OH must be attributed to this hydrogen bond. Without it, 1OH-nonHB is much less stable than 1OH. The energy difference between 1OH and 1OH-HB (Table 6) is smaller than the strength of the HB because there are some unfavorable steric interactions in the hydrogen-bonded isomer between the C(O)CH3 group and aliphatic bridge.
Hyperforin
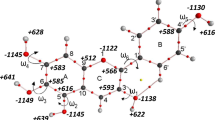
Finally, we arrived at hyperforin. To finish building it from our last model, the Me-PG-b-CO molecule, we must make the following structural changes. The COMe substituent must be replaced by CO(CH2)2CH3, the last two methyl groups in the phloroglucinol ring have to be replaced by prenyl chains, two additional prenyl chains must be added to the bridge, and lastly one methyl group have to be added to the bridge. In practice, we have not followed exactly the process of manually building the molecule but downloaded the sdf file of the 1OH tautomer from PubChem database (CID = 114787) and used it as a starting point for the geometry optimization and building other tautomers (Table 7).
The simple geometry optimization at the B3LYP/6-31G(d,p) level, without any conformational search gave the following results:
In general, addition of several carbon chains is not expected to have a large impact on the energy of tautomeric forms of the molecule, but we must be very careful about conformations here. As there are five long carbon chains, mostly aliphatic, there is a very large conformational space regarding this molecule. There are several double bonds and several heteroatoms, so various interactions are possible: from hydrophobic to hydrogen bonds. Therefore, there is a need for doing a reliable conformational space search. The full systematic search for this molecule would be virtually impossible, due to large number of rotatable bonds. Thus, we need some way of doing an intelligent, reliable, and simplified conformational space search.
Our first step was a simple conformational analysis in the free Avogadro software as it selects only the most important rotatable bonds and thus facilitates faster conformation search. The force field employed was UFF (universal force field). From these results, for each tautomer, we selected 10 conformers of lowest energy and reoptimized them at the B3LYP/6-31G(d,p) level. Absolute values of Gibbs free energies are given in Tables S2–S4, Supplementary Information. At this point, one conformer of 1OH tautomer had lower Gibbs free energy by 0.13 kcal/mol compared with PubChem structure, but no better conformers of 1OH-HB and keto were found.
To have a point of reference whether our conformers are good enough, we took the conformers obtained by Abramova et al. [1] by Monte Carlo search and optimized at the B3LYP/D95** level of theory, and we ensured that we get the same Gibbs free energy by using Gaussian 09 and the same level of theory (Table 8).
We obtained the same values of Gibbs free energies, so we can be sure that if we obtain conformers of lower energy, they will be better.
Next, using the same software and level of theory, we reoptimized our conformations found during Avogadro conformational search. The results are gathered in Table 9 below.
It follows from Tables 8 and 9 that we managed to get a better conformer of 1OH (by 0.9 kcal/mol), but our conformers of 1OH-HB and keto have higher Gibbs free energies than in publication of Abramova et al.
Therefore, we decided to make an additional Monte Carlo conformational search using the free software Frog2 accessible via its web page. As the starting structures, we submitted our best conformers but also the best conformers of Abramova et al. The absolute values of Gibbs free energies of 10 best conformers for each tautomer, obtained by Frog2 program, are listed in the Supplementary Information as Tables S5–S7.
This time we found lower energy conformers of all tautomers, and the results are listed in Table 10.
Comparison of Tables 8 and 10 shows that we obtained 1OH tautomer lower by 2.13 kcal/mol and 1OH-HB lower by 1.29 kcal/mol compared with Abramova et al. The keto tautomer has the same energy.
Thus, we have found the best possible conformations of hyperforin tautomers, and now we are ready to reoptimize them at higher computational level. The structures were reoptimized at the B3LYP/aug-cc-pVDZ level of theory using Gaussian 16 software. The Cartesian coordinates of three optimized tautomers of hyperforin are presented in Supplementary Information in Tables S23–S25. Finally, to use even more extended basis set for accurate energetic prediction for each tautomer a Single Point calculation at the B3LYP/aug-cc-pVTZ was performed. The Gibbs free energy at this level of theory was calculated as the sum of B3LYP/aug-cc-pVTZ total energy and thermal corrections to Gibbs free energy obtained at B3LYP/aug-cc-pVDZ level. The relative energies are gathered in Table 11 below. The absolute values of the energetic data are presented in Supplementary Information (Tables S8–S9). To analyze the influence of thermal corrections on hyperforin tautomerism three kinds of energetic data are provided: the total energy, the energy with thermal correction at 298.15 K, and Gibbs free energy.
It follows that the optimization at higher level of theory affected mostly the relative energy of 1OH-HB tautomer which is now close to 1OH (Tables 10 and 11). The keto tautomer is still the least stable, and the conclusion must be drawn that it is nearly absent in the tautomeric mixture. As follows from Table 11, inclusion of thermal corrections does not change qualitatively the picture, according to total energy the dominance of 1OH tautomer is slightly more pronounced. At the most advanced level of theory, the B3LYP/aug-cc-pVTZ the energetic gaps between 1OH and other tautomers are even larger. This result confirms the B3LYP/aug-cc-pVDZ conclusion about the dominance of 1OH tautomer in the tautomeric mixture.
These results are in contrary to Abramova et al. findings (Table 8) who found that the 1OH and keto are of similar Gibbs free energy. However, our study, augmented by thorough conformational search and higher level of quantum chemical calculations shows the dominance of enol forms of hyperforin in the tautomeric mixture.
A question arises, why the 1OH-HB tautomer is less stable than 1OH despite possessing a hydrogen bond? If we analyze the 3D structures of different tautomers, we can find out that in the 1OH tautomer, there exist a stabilizing interaction of hydrogen atom from OH group with the double bond of prenyl group. This interaction is not possible in 1OH-HB tautomer where the creation of the hydrogen bond introduces some additional unfavorable structural changes which overcome the stabilizing interaction of the hydrogen bond. Therefore, in the contrary to the MePG-b-CO molecule, in hyperforin, the 1OH-HB tautomer has higher Gibbs free energy than 1OH tautomer.
Conclusions
-
1.
According to B3LYP/aug-cc-pVTZ//B3LYP/aug-cc-pVDZ calculations the stability of tautomeric forms of hyperforin are ordered as follows:
- a.
The dominant tautomeric form of hyperforin is 1OH enol with molar percentage of 88%
- b.
The second stable form is 1OH-HB, an enol tautomer with hydrogen bond between external carbonyl group and hydroxyl group. Its Gibbs free energy is higher than 1OH by 1.2 kcal/mol which corresponds to molar percentage of 11.6%
- c.
The least stable tautomeric form is the keto tautomer with 3.3 kcal/mol Gibbs free energy higher than 1OH and which percentage of 0.4%
- a.
-
2.
At the B3LYP/aug-cc-pVDZ level, the relative Gibbs free energies of 1OH, 1OH-HB, and keto tautomers are 0.0, 0.55, and 2.83 kcal/mol respectively, which gives similar picture to higher level calculations and proves reliability of B3LYP/aug-cc-pVDZ model chemistry which will be used to draw the remaining conclusions
-
3.
The differences between relative stability of tautomers measured by different forms of energy (total energy, thermal corrected energy, Gibbs free energy) are similar and stay within 1 kcal/mol frame
-
4.
The higher energy of the hydrogen-bonded tautomer 1OH-HB may be accounted to some structural changes induced by creation of the hydrogen bond which have destabilizing consequences for the molecule, and finally their energetic cost overcome the stabilizing energy of the hydrogen bond
-
5.
In a simplified hyperforin model—a phloroglucinol with carbon bridge, proper methyl substitution, and carbonyl substituent the dominant tautomeric form is 1OH, similar to hyperforin, and the keto form is less stable by 2.78 kcal/mol. In this model compound the 1OH-HB tautomer is actually more stable than 1OH because there are no prenyl groups and steric interactions described in p. 4
-
6.
In a more simplified hyperforin model—a phloroglucinol with carbon bridge and methyl substitution the dominant tautomeric form is still the 1OH form with keto form less stable by 2.98 kcal/mol
-
7.
In methylated phloroglucinol, the tautomer stability order reverses and the dominant form is now keto with Gibbs free energy difference of 1OH higher by 0.45 kcal/mol
-
8.
In pure phloroglucinol, the keto tautomer is still more stable than 1OH, but the Gibbs free energy difference between 1OH and keto forms is only 0.61 kcal/mol
-
9.
It turns out that the reversal of tautomer stability order is induced by introduction of the carbon bridge to methylated phloroglucinol. The probable cause is the severe ring deformation which destabilizes the keto form
References
Abramova I, Rudshteyn B, Liebman JF, Greer A (2017). Photochemistry and photobiology 93:626–631
Baeyer A (1886). Ber 19:159
Becke AD (1993). J Chem Phys 98:5648
Chatterjee SS, Bhattacharya SK, Wonnemann M, Singer A, Mueller WE (1998). Life Sci 63(6):499–510
Di Carlo G, Borrelli F, Ernst E, Izzo AA (2001). Trends Pharmacol Sci 22:292–297
Dudek GO (1963). J Am Chem Soc 85:694–697
Dunning Jr TH (1989). J Chem Phys 90:1007–1023
Dunning Jr TH, Hay PJ (1977) In: Schaefer III HF (ed) Modern Theoretical Chemistry, vol 3. Plenum, New York, pp 1–28
Farmer VC, Thomson RH (1956). Chem Ind (London):86
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, DeFrees DJ, People JA, Gordon MS (1982). J Chem Phys 77:3654–3665
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision D.01. Gaussian Inc, Wallingford
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, Revision A.03. Gaussian, Inc, Wallingford
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012). J Cheminform 4:17
Hatanaka M (2011). Comput Theor Chem 971:58–64
Hatanaka M (2013). Tetrahed Lett 54(11):1452–1455
Herzig J, Erthal B (1910). Monutsh Chem 31:827
Highet RJ, Ekhato IV (1988). J Org Chem 53:2843–2844
Krishnan R, Binkley JS, Seeger R, Pople JA (1980). J Chem Phys 72:650–654
Lohrie M, Knoche W (1993). J Am Chem Soc 115:919–924
Mandix K, Colding A, Elming K, Sunesen L, Shim I (1993). Int J Quantum Chem 46:159–170
Néron B, Ménager H, Maufrais C, Joly N, Maupetit J, Letort S, Carrere S, Tuffery P, Letondal C (2009). Bioinformatics 25(22):3005–3011
Newall CA, Barnes J, Anderson LR (2002) Herbal medicines: a guide for healthcare professionals. Pharmaceutical Press, London
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994). J Phys Chem 98:11623–11810
Taylor PJ, van der Zwan G, Antonov L (2013) In: Antonov L (ed) Tautomerism: methods and theories, Wiley
Wang D, Hildenbrand K, Leitich J, Schuchmann H-P, Clemens v S (1993). Z. Naturforsch 48b:478–482
Wheland GW (1948) Advanced organic chemistry3rd edn. Wiley, New York Section 14, 499
Wheland GW (1955) Resonance in Organic Chemistry. Wiley, New York, Section 7, p 405
Funding
Computational Grant G36-9 from the Interdisciplinary Centre for Mathematical and Computational Modelling at Warsaw University (ICM UW) is gratefully acknowledged. Computational Grant from the Wroclaw Centre for Networking and Supercomputing (WCSS) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 45 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Oziminski, W.P., Wójtowicz, A. New theoretical insights on tautomerism of hyperforin—a prenylated phloroglucinol derivative which may be responsible for St. John’s wort ( Hypericum perforatum ) antidepressant activity . Struct Chem 31, 657–666 (2020). https://doi.org/10.1007/s11224-019-01434-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01434-6