Abstract
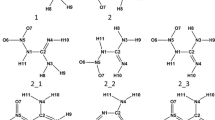
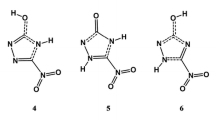
A complete conformational analysis on the isolated and PCM modeled aqueous solution cationic, neutral, and anionic forms of aurantinidin, comprising the diverse tautomers of the latter forms, was carried out at the B3LYP/6–31 + + G(d,p) level. The most stable conformers of isolated cationic and neutral forms of aurantinidin are completely planar, whereas ring B and 3OH are significantly twisted with regard to the AC system in the anion. In contrast, PCM calculations show that the plane of the B ring is slightly rotated with regard to the AC bicycle even in the most stable conformers of the cation and quinonoidal form. The most stable conformers of the cation, in both gas phase and aqueous solution, display anti and syn orientations for hydroxyls at 3 and 5 positions, whereas syn and anti orientation of hydroxyls at 7 and 4' positions are nearly isoenergetic. The most stable tautomer of neutral aurantinidin is obtained by deprotonating hydroxyl at C5 in gas phase, but at C7 in water solution, according to PCM. Also, the most stable tautomer of the anion is different in gas phase (hydrogens are abstracted from hydroxyls at C5 and C4') and PCM calculations for water solution (C3 and C5). Tautomeric equilibria affect substantially the geometries of the AC-B backbone providing bond length variations that basically agree with the predictions of the resonance model. Intramolecular hydrogen bond between O3 and H6' is not present in the most stable anions. Ionization potentials and O–H bond dissociation energies are consistent with an important antioxidant activity.





Similar content being viewed by others
References
Clevenger S (1964) A new anthocyanidin in Impatiens. Can J Biochem 42:154–155. https://doi.org/10.1139/o64-018
Hurst HM, Harborne JB (1967) Plant polyphenols–XVI.: identification of flavonoids by reductive cleavage. Phytochemistry 6:1111–1118. https://doi.org/10.1016/S0031-9422(00)86069-0
Jurd L, Harborne JB (1968) The structure of aurantinidin. Phytochemistry 7:1209–1211. https://doi.org/10.1016/S0031-9422(00)88273-4
Estévez L, Mosquera RA (2009) Conformational and substitution effects on the electron distribution in a series of anthocyanidins. J Phys Chem A 113:9908–9919. https://doi.org/10.1021/jp904298z
Guzmán R, Santiago C, Sánchez M (2009) A density functional study of antioxidant properties on anthocyanidins. J Mol Struct 935:110–114. https://doi.org/10.1016/j.molstruc.2009.06.048
Sakata K, Saito N, Honda T (2006) Ab initio study of molecular structures and excited states in anthocyanidins. Tetrahedron 62:3721–3731. https://doi.org/10.1016/j.tet.2006.01.081
Heredia FJ, Franchia-Aricha EM, Rivas-Gonzalo JC, Vicario IM, Santos-Buelga C (1998) Chromatic characterization of anthocyanins from red grapes-I. pH effect. Food Chem 63:491–498. https://doi.org/10.1016/S0308-8146(98)00051-X
Brouillard R (1982) Chemical structure of anthocyanins. In: Markasis P (ed) Anthocyanins as food colors. Academic Press, New York
Hall CA III, Cuppett SL (1997) Structure-activities of natural antioxidants. In: Cuppett CA, Cuppett SL, Aruoma OI (eds) Antioxidant methodology in vivo and in vitro concepts. AOCS Press, Champaign, IL
Wright JS, Johnson ER, DiLabio GA (2001) Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123:1173–1183. https://doi.org/10.1021/ja002455u
Pratt DA, DiLabio GA, Brigati G, Pedulli GF, Valgimigli L (2001) 5-Pyrimidinols: a novel chain-breaking antioxidants more effective than phenols. J Am Chem Soc 123:4625–4626. https://doi.org/10.1021/ja005679l
Zhang HY, Sun YM, Wang XL (2003) Substituent effects on O-H bond dissociation enthalpies and ionization potentials for catechols: a DFT study and its impliations in the rational design of phenolic antioxidants and elucidation of structure-activity relationships for flavonoid antioxidants. Chem Eur J 9:502–508. https://doi.org/10.1002/chem.200390052
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Lee C, Yang G, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2016) Gaussian 09 revision D. 01. Gaussian Inc, Wallingford CT
Estévez L, Mosquera RA (2007) A density functional theory study on pelargonidin. J Phys Chem A 111:11100–11109. https://doi.org/10.1021/jp074941a
Estévez L, Mosquera RA (2008) Molecular structure and antioxidant properties of delphinidin. J Phys Chem A 112:10614–10623. https://doi.org/10.1021/jp8043237
Tomasi J, Mennuci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094. https://doi.org/10.1021/cr9904009
Rezabal E, Mercero JM, Lopez X, Ugalde JM (2007) A theoretical study of the principles regulating the specificity for Al(III) against Mg(II) in protein cavities. J Inorg Biochem 101:1192–1200. https://doi.org/10.1016/j.jinorgbio.2007.06.010
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, New York
Bader RFW (2000) AIMPAC: a suite of programs for the AIM theory. Mc Master University, Hamilton, ON, Canada
Pereira GK, Donate PM, Galembeck SE (1997) Effects of substitution for hydroxyl in the B-ring of the flavylium cation. J Mol Struct (THEOCHEM) 392:169–179. https://doi.org/10.1016/S0166-1280(97)90395-X
Pereira GK, Donate PM, Galembeck SE (1996) Electronic structure of hydroxylated flavylium cation derivatives of the flavylium cation. J Mol Struct (THEOCHEM) 363:87–96. https://doi.org/10.1016/0166-1280(95)04423-X
Mandado M, Graña AM, Mosquera RA (2004) Do 1,2-ethanediol and 1,2-dihydroxybenzene present intramolecular hydrogen bond? Phys Chem Chem Phys 6:4391–4396. https://doi.org/10.1039/B406266C
Klein RA (2002) Electron density topological analysis of hydrogen bonding in glucopyranose and hydrated glucopyranose. J Am Chem Soc 124:13931–13937. https://doi.org/10.1021/ja0206947
Klein RA (2002) Hydrogen bonding in diols and binary diol–water systems investigated using DFT methods. II. Calculated infrared OH-stretch frequencies, force constants, and NMR chemical shifts correlate with hydrogen bond geometry and electron density topology. A reevaluation of geometrical criteria for hydrogen bonding. J Comput Chem 24:1120–1131. https://doi.org/10.1002/jcc.10256
Estévez L, Mosquera RA (2008) Where is the positive charge of flavylium cations? Chem Phys Lett 451:121–126. https://doi.org/10.1016/j.cplett.2007.11.065
Vila A, Mosquera RA (2001) Electron density analysis of small ring ethers. Tetrahedron 57:9415–9422. https://doi.org/10.1016/S0040-4020(01)00946-2
Haslam E (1998) Practical polyphenolics. Cambridge University Press, Cambridge
Robbins RR (2003) Phenolic acids in foods: an overview of analytical methodology. J Agric Food Chem 51:2866–2887. https://doi.org/10.1021/jf026182t
Zhang HY, Sun YM, Wang XL (2003) Substituent effects on O-H bond dissociation enthalpies and ionization potentials of catechols: a DFT study and its implications in the rational design of phenolic antioxidants and elucidation of structure–activity relationships for flavonoid antioxidants. Chem Eur J 9:502–508. https://doi.org/10.1002/chem.200390052
Lu L, Qiag M, Li F, Zhang H, Zhang S (2014) Theoretical investigation on the antioxidative activity of anthocyanidins: a DFT/B3LYP study. Dyes Pigm 103:175–182. https://doi.org/10.1016/j.dyepig.2013.12.015
Johansson AJ, Blomberg MRA, Siegbahn PEM (2008) Quantifying the effects of the self-interaction error in density functional theory: when do the delocalized states appear? II. Iron-oxo complexes and closed-shell substrate molecules. J. Chem. Phys. 129:154301. https://doi.org/10.1063/1.2991180
Rienstra-Kiracofe JC, Tschumper GS, Shaefer HF III, Nandi S, Ellison GB (2022) Atomic and molecular electron affinities: photoelectron experiments and theoretical computations. Chem Rev 102:231–282. https://doi.org/10.1021/cr990044u
Leopoldini M, Marino T, Russo N, Toscano M (2004) Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J Phys Chem A 108:4916–4922. https://doi.org/10.1021/jp037247d
De Heer MI, Korth HG, Mulder P (1999) Poly methoxy phenols in solution: O−H bond dissociation enthalpies, structures, and hydrogen bonding. J Org Chem 64:6969–6975. https://doi.org/10.1021/jo9901485
Acknowledgements
We thank "Centro de Supercomputación de Galicia" (CESGA) for free access to its computational facilities. Xunta de Galicia is acknowledged for financial support through GRC 2019/24.
Author information
Authors and Affiliations
Contributions
All the authors have shared all the tasks.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garcìa-Bugarín, M., Peña-Gallego, Á. & Mosquera, R.A. A density functional theory study on aurantinidin. Theor Chem Acc 142, 73 (2023). https://doi.org/10.1007/s00214-023-03021-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03021-9