Abstract
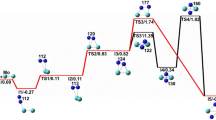
In this work, the structure, stability and reactivity of neutral bimetallic manganese oxide clusters MnMdO2–4 (Md = Ti, V and Cr) with CO and NO are investigated by means of density functional theory (DFT). The hybrid PBE0 with TZVP basis set is employed to obtain MnMdO2–4 minimum energy structures, reactants, reaction intermediates, transition states and products. Our calculations show that the structural parameters are significantly altered when replacing Mn with Ti, V or Cr. All the clusters considered in this study have higher binding energy per atom, which implies that they are highly stable. Further, these clusters have smaller oxygen dissociation energy than CO2 and NO2. This indicates that the O atom can easily dissociate from these clusters and form CO2 and NO2. Our reactivity study shows that the CO and NO oxidation by MnTiO4 cluster is a favourable reaction. These oxidation reactions are barrierless and thermodynamically and kinetically favourable. Therefore, MnTiO4 can be used as a suitable catalyst for CO and NO oxidation. From this study, it is concluded that the titanium-supported manganese oxide will be a suitable catalyst for the CO and NO oxidation in the condensed phase.













Similar content being viewed by others
References
Fiero GJL (2006) Metal oxides chemistry and applications. Taylor & Francis, London
Liu C, Shi J-W, Gao C, Niu C (2016) Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: a review. Appl Catal A Gen 522:54–69
Gao J, Jia C, Zhang L, Wang H, Yang Y, Hung S-F, Hsu Y-Y, Liu B (2016) Tuning chemical bonding of MnO2 through transition-metal doping for enhanced CO oxidation. J Catal 341:82–90
Keav S, Matam S, Ferri D, Weidenkaff A (2014) Structured perovskite-based catalysts and their application as three-way catalytic converters—a review. Catalysts 4(3):226–255
Trovarelli A, de Leitenburg C, Boaro M, Dolcetti G (1999) The utilization of ceria in industrial catalysis. Catal Today 50(2):353–367
Ko C, Kerman K, Ramanathan S (2012) Ultra-thin film solid oxide fuel cells utilizing un-doped nanostructured zirconia electrolytes. J Power Sources 213:343–349
Shao Z, Haile SM (2011) A high-performance cathode for the next generation of solid-oxide fuel cells. Materials for Sustainable Energy, pp 255–258
Zhao C, Wachs IE (2006) Selective oxidation of propylene to acrolein over supported V2O5/Nb2O5 catalysts: an in situ Raman, IR, TPSR and kinetic study. Catal Today 118(3–4):332–343
Gu D, Jia C-J, Weidenthaler C, Bongard H-J, Spliethoff B, Schmidt W, Schüth F (2015) Highly ordered mesoporous cobalt-containing oxides: structure, catalytic properties, and active sites in oxidation of carbon monoxide. J Am Chem Soc 137(35):11407–11418
Resasco J, Dai S, Graham G, Pan X, Christopher P (2018) Combining in-situ transmission electron microscopy and infrared spectroscopy for understanding dynamic and atomic-scale features of supported metal catalysts. J Phys Chem C 122(44):25143–25157
Chen X, Wu C, Guo Z (2018) Synthesis of efficient Cu/CoFe2O4 catalysts for low temperature CO oxidation. Catal Lett, pp 1–11
Yang Z, Li H, Liu X, Li P, Yang J, Lee P-H, Shih K (2018) Promotional effect of CuO loading on the catalytic activity and SO2 resistance of MnOx/TiO2 catalyst for simultaneous NO reduction and Hg0 oxidation. Fuel 227:79–88
Guo Z, Liang Q-H, Yang Z, Liu S, Huang Z-H, Kang F (2016) Modifying porous carbon nanofibers with MnOx–CeO2–Al2O3 mixed oxides for NO catalytic oxidation at room temperature. Catal. Sci. Technol 6(2):422–425
Wu Z, Tang N, Xiao L, Liu Y, Wang H (2010) MnOx/TiO2 composite nanoxides synthesized by deposition-precipitation method as a superior catalyst for NO oxidation. J Colloid Interface Sci 352(1):143–148
Wang LN, Li XN, Jiang LX, Yang B, Liu QY, Xu HG, Zheng WJ, He SG (2018) Catalytic CO oxidation by O2 mediated by noble-metal-free cluster anions Cu2VO3–5 −. Angew Chem 130(13):3407–3411
Yuan C, Wu HB, Xie Y, Lou XW (2014) Mixed transition-metal oxides: design, synthesis, and energy-related applications. Angew Chem Int Ed 53(6):1488–1504
Sakuma K, Miyajima K, Mafuné F (2013) Oxidation of CO by nickel oxide clusters revealed by post heating. J Phys Chem A 117(16):3260–3265
Acikgoz M, Harrell J, Pavanello M (2018) Seeking a structure–function relationship for γ-Al2O3 surfaces. J Phys Chem C 122(44):25314–25330
Yuan H, Chen J, Guo Y, Wang H, Hu P (2018) Insight into the superior catalytic activity of MnO2 for low-content NO oxidation at room temperature. J Phys Chem C 122(44):25365–25373
Tian LH, Zhao YX, Wu XN, Ding XL, He SG, Ma TM (2012) Structures and reactivity of oxygen-rich scandium cluster anions ScO3–5 −. ChemPhysChem 13(5):1282–1288
Zhong H, Er D, Wen L (2017) Theoretical study on influence of CaO and MgO on the reduction of FeO by CO. Appl Surf Sci 399:630–637
Wang Z-C, Yin S, Bernstein ER (2012) Gas-phase neutral binary oxide clusters: distribution, structure, and reactivity toward CO. J. Phys. Chem. Lett 3(17):2415–2419
Kumavat S, Deshpande M (2014) Alkali metal doped nickel oxide clusters: a density functional study. Comput. Theor. Chem 1035:19–27
Li J, González-Navarrete P, Schlangen M, Schwarz H (2015) Activation of methane and carbon dioxide mediated by transition-metal doped magnesium oxide clusters [MMgO]+/0/− (M = Sc–Zn). Chem Eur J 21(21):7780–7789
Fang H-L, Xu L, Li J, Wang B, Zhang Y-F, Huang X (2015) Catalytic oxidation of CO by N2O on neutral Y2MO5 (M = Y, Al) clusters: a density functional theory study. RSC Adv 5(93):76651–76659
Liu H, Liew KM, Pan C (2014) The role of F-dopants in adsorption of gases on anatase TiO2 (001) surface: a first-principles study. RSC Adv 4(68):35928–35942
Xiong G, Yang C, Zhu W, Xiao H (2016) Density functional theory study of high-energy metal (Al, Mg, Ti, and Zr)/CuO composites. RSC Adv 6(93):90206–90211
Johnson GE, Tyo EC, Castleman Jr AW (2008) Oxidation of CO by aluminum oxide cluster ions in the gas phase. J Phys Chem A 112(21):4732–4735
Xie Y, Dong F, Heinbuch S, Rocca JJ, Bernstein ER (2010) Oxidation reactions on neutral cobalt oxide clusters: experimental and theoretical studies. Phys Chem Chem Phys 12(4):947–959
Matsuda Y, Bernstein ER (2005) Identification, structure, and spectroscopy of neutral vanadium oxide clusters. J Phys Chem A 109(17):3803–3811
Matsuda Y, Bernstein ER (2005) On the titanium oxide neutral cluster distribution in the gas phase: detection through 118 nm single-photon and 193 nm multiphoton ionization. J Phys Chem A 109(2):314–319
Yin S, Bernstein ER (2012) Gas phase chemistry of neutral metal clusters: distribution, reactivity and catalysis. Int J Mass Spectrom 321:49–65
Wang Z-C, Yin S, Bernstein ER (2013) Catalytic oxidation of CO by N2O conducted via the neutral oxide cluster couple VO2/VO3. Phys Chem Chem Phys 15(25):10429–10434
Yin S, Wang Z, Bernstein ER (2013) O-atom transport catalysis by neutral manganese oxide clusters in the gas phase: reactions with CO, C2H4, NO2, and O2. J Chem Phys 139(8):084307
Wang Z-C, Yin S, Bernstein ER (2013) Generation and reactivity of putative support systems, Ce-Al neutral binary oxide nanoclusters: CO oxidation and C–H bond activation. J Chem Phys 139(19):194313
Yin S, Bernstein ER (2016) Ethylene C–H bond activation by neutral Mn2O5 clusters under visible light irradiation. J. Phys. Chem. Lett 7(9):1709–1716
Iyemperumal SK, Pham TD, Bauer J, Deskins NA (2018) Quantifying support interactions and reactivity trends of single metal atom catalysts over TiO2. J Phys Chem C 122(44):25274–25289
McFarland EW, Metiu H (2013) Catalysis by doped oxides. Chem Rev 113(6):4391–4427
Tosoni S, Pacchioni G (2019) Oxide-supported gold clusters and nanoparticles in catalysis: a computational chemistry perspective. ChemCatChem 11:73–89
Thang HV, Pacchioni G (2018) CO oxidation promoted by a Pt4/TiO2 catalyst: role of lattice oxygen at the metal/oxide interface. Catal Lett, pp 1–9
He H, Chen J, Zhang D, Li F, Chen X, Chen Y, Bian L, Wang Q, Duan P, Wen Z (2018) Modulating the electrocatalytic performance of palladium with the electronic metal–support interaction: a case study on oxygen evolution reaction. ACS Catal 8(7):6617–6626
Alizadeh M, Hosseini SA, Nouri SMM, Khalighi Z, Delfarah B (2018) Low-cost nanostructured Fe2O3-based composite catalysts synthesized by mechanical milling for CO oxidation reaction. Chem Eng Commun 205(8):1041–1049
Alphonse P (2016) Co–Mn-oxide spinel catalysts for CO and propane oxidation at mild temperature. Appl Catal B Environ 180:715–725
Wischert R, Laurent P, Copéret C, Fo D, Sautet P (2012) γ-Alumina: the essential and unexpected role of water for the structure, stability, and reactivity of “defect” sites. J Am Chem Soc 134(35):14430–14449
Zhao Y-X, Li Z-Y, Yang Y, He S-G (2018) Methane activation by gas phase atomic clusters. Acc Chem Res 51(11):2603–2610
Nößler M, Mitrić R, Bonačić-Koutecký V, Johnson GE, Tyo EC, Castleman Jr AW (2010) Generation of oxygen radical centers in binary neutral metal oxide clusters for catalytic oxidation reactions. Angew Chem Int Ed 49(2):407–410
Wang X, Lan Z, Zhang K, Chen J, Jiang L, Wang R (2017) Structure–activity relationships of AMn2O4 (A = Cu and Co) spinels in selective catalytic reduction of NOx: experimental and theoretical study. J Phys Chem C 121(6):3339–3349
Chen J-J, Yang Y, Zhao Y-X, He S-G (2018) Vacuum ultraviolet ionization-induced reaction of neutral Au2Al2O3 clusters with methane. J Phys Chem C 122(11):6159–6165
Marks JH, Ward TB, Duncan MA (2018) Photodissociation of manganese oxide cluster cations. J Phys Chem A 122(13):3383–3390
Han Y-F, Chen F, Zhong Z-Y, Ramesh K, Widjaja E, Chen L-W (2006) Synthesis and characterization of Mn3O4 and Mn2O3 nanocrystals on SBA-15: novel combustion catalysts at low reaction temperatures. Catal Commun 7(10):739–744
Han Y-F, Chen F, Zhong Z, Ramesh K, Chen L, Widjaja E (2006) Controlled synthesis, characterization, and catalytic properties of Mn2O3 and Mn3O4 nanoparticles supported on mesoporous silica SBA-15. J Phys Chem B 110(48):24450–24456
Frey K, Iablokov V, Sáfrán G, Osán J, Sajó I, Szukiewicz R, Chenakin S, Kruse N (2012) Nanostructured MnOx as highly active catalyst for CO oxidation. J Catal 287:30–36
Ching S, Kriz DA, Luthy KM, Njagi EC, Suib SL (2011) Self-assembly of manganese oxide nanoparticles and hollow spheres. Catalytic activity in carbon monoxide oxidation. Chem Commun 47(29):8286–8288
Xi Y, Ren J-C (2016) Design of a CO oxidation catalyst based on two-dimensional MnO2. J Phys Chem C 120(42):24302–24306
Li K, Chen J, Peng Y, Lin W, Yan T, Li J (2017) The relationship between surface open cells of α-MnO2 and CO oxidation ability from a surface point of view. J Mater Chem A 5(39):20911–20921
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110(13):6158–6170
Schäfer A, Huber C, Ahlrichs R (1994) Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J Chem Phys 100(8):5829–5835
Nößler M, Mitrić R, Bonačić-Koutecký V (2012) Binary neutral metal oxide clusters with oxygen radical centers for catalytic oxidation reactions: from cluster models toward surfaces. J Phys Chem C 116(21):11570–11574
Lousada CM, Johansson AJ, Brinck T, Jonsson M (2013) Reactivity of metal oxide clusters with hydrogen peroxide and water—a DFT study evaluating the performance of different exchange–correlation functionals. Phys Chem Chem Phys 15(15):5539–5552
Frisch M J TGW, Schlegel H B, Scuseria G, E RMA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji, H CM, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg, J L HM, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima, T HY, Kitao O, Nakai H, Vreven T, Montgomery J A, Peralta J E, Ogliaro, F BM, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Kobayashi, R NJ, Raghavachari K, Rendell A, Burant J C, Iyengar S S, Tomasi, J CM, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo, C JJ, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli, C OJW, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador, P DJJ, Dapprich S, Daniels A D, Farkas, Foresman J B, Ortiz, J V CJAFDJ (2009) Gaussian 09, Revision B.01. Wallingford CT
Wang Y, Gong X, Wang J (2010) Comparative DFT study of structure and magnetism of TMnOm (TM = Sc–Mn, n = 1–2, m = 1–6) clusters. Phys Chem Chem Phys 12(10):2471–2477
Valiev M, Bylaska EJ, Govind N, Kowalski K, Straatsma TP, Van Dam HJJ, Wang D, Nieplocha J, Apra E, Windus TL (2010) NWChem: a comprehensive and scalable open-source solution for large scale molecular simulations. Comput Phys Commun 181(9):1477–1489
Li L, Qu L, Cheng J, Li J, Hao Z (2009) Oxidation of nitric oxide to nitrogen dioxide over Ru catalysts. Appl Catal B Environ 88(1–2):224–231
Li L, Shen Q, Cheng J, Hao Z (2010) Catalytic oxidation of NO over TiO2 supported platinum clusters I. Preparation, characterization and catalytic properties. Appl Catal B Environ 93(3–4):259–266
Yuan H, Chen J, Wang H, Hu P (2018) Activity trend for low-concentration NO oxidation at room temperature on rutile-type metal oxides. ACS Catal 8(11):10864–10870
Acknowledgements
The authors (S.S. and S.P.) are thankful to the Science and Engineering Research Board, Govt. of India, for the financial support in the form of the project (YSS/2015/001311).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sampathkumar, S., Subramaniam, V. & Paranthaman, S. Structure, stability and reactivity of neutral bimetallic manganese oxide clusters with CO and NO—a DFT study. Struct Chem 30, 2109–2122 (2019). https://doi.org/10.1007/s11224-019-01319-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01319-8