Abstract
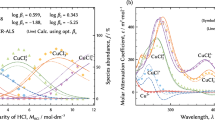
The anion exchange reaction is fundamental to the adsorption and desorption of a specific species from a solution phase to an extracting phase, and it is widely used for separation and waste fluid treatment in industrial fields. However, the details of the anion exchange reaction are poorly understood. Quantitative thermodynamic analysis needs a precise solution condition before and after the exchange reaction. Identification of species adsorbed on the anion exchanger is also necessary because there are multiple species in the solution phase in general. Cobalt is a base metal that is widely used in modern society. One of the authors determined the distribution of cobalt-chloro complexes in hydrochloric acid solutions. It is necessary to know what species are adsorbed on anion exchangers for the thermodynamic analysis of the anion exchange reaction. The comparison in structures between the species in the solution phase and adsorbed on anion exchangers reveals what species are adsorbed. Therefore, the determination of the structures of cobalt-chloro complexes in the solution phase is the next step for quantitative analysis. X-ray absorption spectroscopy (XAS) was used for the structure analysis. Factor analysis can decompose extended X-ray absorption fine structure (EXAFS) and X-ray absorption near-edge structure (XANES) spectra consisting of multiple species into individual spectra of single species using the distribution determined using UV-Vis absorption spectra. Fitting EXAFS theoretical models to the decomposed individual spectra determined the structures of three cobalt-chloro complexes: an octahedron of [CoII(H2O)6]2+, a distorted octahedron of [CoII(H2O)5Cl]+, and a tetrahedron of [CoIICl4]2−. The XANES spectra showed us that the Cl ligand in [CoII(H2O)5Cl]+ was attracted to the center atom of CoII by an electrostatic force, and the bonding system between Cl ligands and CoII in [CoIICl4]2− involved covalency.







Similar content being viewed by others

References
Kraus KA, Nelson F (1955) Anion exchange studies of the fission products. In Proceedings International Conference Peaceful Uses of Atomic Energy. Geneva 7:113–125
Billard I, Švecová L (2018) Metals: from speciation in the aqueous phases to the liquid–liquid extraction mechanism. J Solut Chem, 47:1291–1292. https://doi.org/10.1007/s10953-018-0797-x
Sillén LG, Martell AE (1966) Stability constants of metal-ion complexes Section I: Inorganic Ligands, compiled by Lars Gunnar Sillén; Section II: Organic Ligands, compiled by Arthur E. Martell. (Second edition.) xviii + 754. Special Publication No. 17. The Chemical Society, London. 1964. E8 net. 25:1–754. https://doi.org/10.1016/0160-9327(66)90049-4
Högfeldt E (1982) (1979) IUPAC Chemical data series, no. 21. Pergamon, Oxford
Brugger J, McPhail DC, Black J, Spiccia L (2001) Complexation of metal ions in brines: application of electronic spectroscopy in the study of the cu(II)-LiCl-H2O system between 25 and 90 °C. Geochim Cosmochim Acta 65:2691–2708. https://doi.org/10.1016/S0016-7037(01)00614-7
Uchikoshi M (2017) Determination of the distribution of cupric chloro-complexes in hydrochloric acid solutions at 298 K. J Solut Chem 46:704–719. https://doi.org/10.1007/s10953-017-0597-8
Powell KJ, Brown PL, Byrne RH, Gajda T, Hefter G, Sjöberg S, Wanner H (2007) Chemical speciation of environmentally significant metals with inorganic ligands part 2: the Cu2+–OH–, Cl–, CO3 2–, SO4 2–, and PO4 3– systems (IUPAC Technical Report). Pure Appl Chem 79:895–950. https://doi.org/10.1351/pac200779050895
Uchikoshi M Determination of the distribution of cobalt-chloro complexes in hydrochloric acid solutions at 298 K. J Solut Chem, accepted on 17 September 2018. https://doi.org/10.1007/s10953-018-0831-z
Uchikoshi M, Shinoda K (2018) Determination of structures of cupric-chloro complexes in hydrochloric acid solutions by UV-Vis and X-ray absorption spectroscopy. Struct Chem, published online on 4 August 2018. https://doi.org/10.1007/s11224-018-1164-7
Tanimizu M, Takahashi Y, Nomura M (2007) Spectroscopic study on the anion exchange behavior of Cu chloro-complexes in HCl solutions and its implication to Cu isotopic fractionation. Geochem J 41:291–295. https://doi.org/10.2343/geochemj.41.291
Suzuki T, Ogata T, Tanaka M, Kobayashi T, Shiwaku H, Yaita T, Narita H (2018) Speciation of ruthenium(III) chloro complexes in hydrochloric acid solutions and their extraction characteristics with an amide-containing amine compound. Metals 8:558–510. https://doi.org/10.3390/met8070558
Hoogerstraete TV, Souza ER, Onghena B, Banerjee D, Binnemans K (2018) Mechanism for solvent extraction of lanthanides from chloride media by basic extractants. J Solut Chem 47:1351–1372. https://doi.org/10.1007/s10953-018-0782-4
Calvin S (2013) XAFS for everyone. CRC Press, Boca Raton
Ueno K (1976) Chelate titration methods. Nan-e dou, Tokyo
Liu W, Borg SJ, Testemale D, Etschmann B, Hazemann JL, Brugger J (2011) Speciation and thermodynamic properties for cobalt chloride complexes in hydrothermal fluids at 35–440 °C and 600 bar: an in-situ XAS study. Geochim Cosmochim Acta 75:1227–1248. https://doi.org/10.1016/j.gca.2010.12.002
Zabinsky SI, Rehr JJ, Ankudinov A, Albers RC, Eller MJ (1995) Multiple-scattering calculations of x-ray-absorption spectra. Phys Rev B Condens Matter Mater Phys 52:2995–3009. https://doi.org/10.1103/PhysRevB.52.2995
Ravel B, Newville M, IUCr (2005) ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12:537–541. https://doi.org/10.1107/S0909049505012719
Shulman GR, Yafet Y, Eisenberger P, Blumberg WE (1976) Observations and interpretation of x-ray absorption edges in iron compounds and proteins. Proc Natl Acad Sci U S A 73:1384–1388. https://doi.org/10.1073/pnas.73.5.1384
Gaur A, Klysubun W, Nair NN, Shrivastava BD, Prasad J, Srivastava K (2016) XAFS study of copper(II) complexes with square planar and square pyramidal coordination geometries. J Mol Struct 1118:212–218. https://doi.org/10.1016/j.molstruc.2016.04.008
Newville M, LīviņŠ P, Yacoby Y, Rehr JJ, Stern EA (1993) Near-edge x-ray-absorption fine structure of Pb: a comparison of theory and experiment. Phys Rev B Condens Matter Mater Phys 47:14126–14131. https://doi.org/10.1103/PhysRevB.47.14126
Bunker G (2010) Introduction to XAFS. Cambridge University Press, Cambridge
Abragam A, Pryce MHL (1951) The theory of paramagnetic resonance in hydrated cobalt salts. Proc R Soc A 206:173–191. https://doi.org/10.1098/rspa.1951.0063
Holmes OG, McClure DS (1957) Optical spectra of hydrated ions of the transition metals. J Chem Phys 26:1686–1694. https://doi.org/10.1063/1.1743606
Liehr AD, Ballhausen CJ (1958) Inherent configurational instability of octahedral inorganic complexes in Eg electronic states. Ann Phys 3:304–319. https://doi.org/10.1016/0003-4916(58)90022-8
Cotton FA, Goodgame DML, Goodgame M (1961) The electronic structures of tetrahedral cobalt(II) complexes. J Am Chem Soc 83:4690–4699. https://doi.org/10.1021/ja01484a002
Wiesner JR, Srivastava RC, Kennard CHL, DiVaira M, Lingafelter EC (1967) The crystal structures of tetramethylammonium tetrachloro-cobaltate(II), −nickelate(II), and -zincate(II). Acta Cryst 23:565–574. https://doi.org/10.1107/S0365110X67003214
Susak NJ, Crerar DA (1985) Spectra and coordination changes of transition-metals in hydrothermal solutions—implications for ore genesis. Geochim Cosmochim Acta 49:555–564. https://doi.org/10.1016/0016-7037(85)90047-X
Waizumi K, Masuda H, Ohtaki H, Tsukamoto K, Sunagawa I (1990) In situ observations of the phase transition among cobalt(II) dichloride hydrates and crystal structures of the tetra- and hexahydrates. Bull Chem Soc Jpn 63:3426–3433. https://doi.org/10.1246/bcsj.63.3426
Okumura T, Yamaguchi Y, Shikano M, Kobayashi H (2012) Correlation of lithium ion distribution and X-ray absorption near-edge structure in O3 – and O2 – lithium cobalt oxides from first-principle calculation. J Mater Chem 22:17340–17349. https://doi.org/10.1039/c2jm32024j
Bunau O, Joly Y (2009) Self-consistent aspects of x-ray absorption calculations. J Phys Condens Matter 21:345501. https://doi.org/10.1088/0953-8984/21/34/345501
Acknowledgments
The authors wish to thank Mr. Yuji Baba and Mr. Kouji Nagahashi for their great devotion to this experimental work. The synchrotron radiation experiments of X-ray absorption spectroscopy were performed at BL14B2 of SPring-8 with the approval of the the Japan Synchrotron Radiation Research Institute (Proposal No. 2008A1941). This work was performed under the Research Program of “The Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” in “The Network Joint Research Center for Materials and Devices” and was one of the projects conducted in the MSTeC Research Center at the Institute of Multidisciplinary Research for Advanced Materials, Tohoku University.
Author information
Authors and Affiliations
Contributions
The manuscript was composed by contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uchikoshi, M., Shinoda, K. Determination of structures of cobalt(II)-chloro complexes in hydrochloric acid solutions by X-ray absorption spectroscopy at 298 K. Struct Chem 30, 945–954 (2019). https://doi.org/10.1007/s11224-018-1245-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1245-7