Abstract
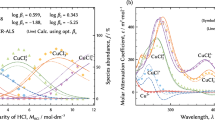
The distribution of metal-chloro complexes in hydrochloric acid solutions is a fundamental aspect of anion exchange reactions that allows ultrahigh purification during hydrometallurgical processes. However, these exchange reactions are not yet understood in detail. To clarify and improve anion exchange separation so as to obtain a more sophisticated purification process, it is necessary to accurately determine the distribution of metal-chloro complexes. In the present work, cupric-chloro complexes were investigated because copper is one of the most important base metals in modern society. The absorption spectra of solutions of these complexes were acquired at 298 K and analyzed by multivariate curve resolution–alternating least squares method (MCR–ALS), a factor analysis technique widely used in chemometrics. The resulting cupric-chloro complex distributions were fitted with a thermodynamic model using appropriate activity coefficients extended to the concentrated solutions. These calculations employed a modified Debye–Hückel model because the distributions acquired through the model-free MCR–ALS analysis were less meaningful, both physically and chemically. It was concluded that five [CuIICl n ]2−n species, where n = 0–4, are present in the hydrochloric acid solutions. In addition, cumulative formation constants and pure molar attenuation coefficients were obtained.
Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.






Similar content being viewed by others

References
Arakawa, K., Ono, K., Isshiki, M., Mimura, K., Uchikoshi, M., Mori, H.: Observation of the one-dimensional diffusion of nanometer-sized dislocation loops. Science 318, 956–959 (2007)
Fukuyama, H., Morohoshi, K., Uchikoshi, M., Isshiki, M.: Dynamic surface tension behavior of liquid iron during carburization and decarburization processes. ISIJ Int. 54, 2109–2114 (2014)
Kitahara, T., Tanada, K., Ueno, S., Sugioka, K., Kubo, M., Tsukada, T., Uchikoshi, M., Fukuyama, H.: Effect of static magnetic field on recalescence and surface velocity field in electromagnetically levitated molten CuCo droplet in undercooled state. Metall. Mater. Trans. B 46B, 2706–2712 (2015)
Bost, M.C., Mahan, J.E.: Optical properties of semiconducting iron disilicide thin films. J. Appl. Phys. 58, 2696–2703 (1985)
Gotoh, K., Suzuki, H., Udono, H., Kikuma, I., Esaka, F., Uchikoshi, M., Isshiki, M.: Single crystalline β-FeSi2 grown using high-purity FeSi2 source. Thin Solid Films 515, 8263–8267 (2007)
Kekesi, T., Uchikoshi, M., Mimura, K., Isshiki, M.: Anion-exchange separation in hydrochloric acid solutions for the ultrahigh purification of cobalt. Metall. Mater. Trans. B 32B, 573–582 (2001)
Uchikoshi, M., Shibuya, H., Kékesi, T., Mimura, K., Isshiki, M.: Mass production of high-purity iron using anion-exchange separation and plasma arc melting. Metall. Mater. Trans. B 40B, 615–618 (2009)
Uchikoshi, M., Imai, K., Mimura, K., Isshiki, M.: Oxidation refining of iron using plasma-arc melting. J. Mater. Sci. 43, 5430–5435 (2008)
Uchikoshi, M., Nagahara, T., Lim, J.W., Kim, S.B., Mimura, K., Isshiki, M.: Anion-exchange behavior of Mo(V) and W(VI) in HCl solutions. High Temp. Mater. Process 30, 345–351 (2011)
Kraus, K.A., Nelson, F.: Anion exchange studies of the fission products. In: Proceedings international conference on peaceful uses of atomic energy, pp. 113–125. Geneva (1955)
Dorfner, K. (ed.): Ion Exchangers. Walterde Guyter, Boston (1991)
Crerar, D.A.: A method for computing multicomponent chemical equilibria based on equilibrium constants. Geochim. Cosmochim. Acta 39, 1375–1384 (1975)
Seward, T.M.: The formation of lead(II) chloride complexes to 300 °C: a spectrophotometric study. Geochim. Cosmochim. Acta 48, 121–134 (1984)
Gammons, C.H., Seward, T.M.: Stability of manganese(II) chloride complexes from 25 to 300 °C. Geochim. Cosmochim. Acta 60, 4295–4311 (1996)
Brugger, J., McPhail, D.C., Black, J., Spiccia, L.: Complexation of metal ions in brines: application of electronic spectroscopy in the study of the Cu(II)–LiCl–H2O system between 25 and 90 °C. Geochim. Cosmochim. Acta 65, 2691–2708 (2001)
Brugger, J.: BeerOz, a set of Matlab routines for the quantitative interpretation of spectrophotometric measurements of metal speciation in solution. Comput. Geosci. 33, 248–261 (2007)
Anderson, G.M., Crerar, D.A.: Thermodynamics in Geochemistry. The Equilibrium Model. Oxford University Press, Oxford (1993)
Bjerrum, J.: Determination of small stability constants. A spectrophotometric study of copper(II) chloride complexes in hydrochloric acid. Acta. Chem. Scand. A 41A, 328–334 (1987)
Sverjensky, D.A., Shock, E.L., Helgeson, H.C.: Prediction of the thermodynamic properties of aqueous metal complexes to 1000 °C and 5 kb. Geochim. Cosmochim. Acta 61, 1359–1412 (1997)
Uekawa, E., Murase, K., Matsubara, E., Hirato, T., Awakura, Y.: Determination of chemical species and their composition in Ni–Mo alloy plating baths by factor analysis of visible absorption spectra. J. Electrochem. Soc. 145, 523–528 (1998)
Ikeda, A., Hennig, C., Rossberg, A., Tsushima, S., Scheinost, A.C., Bernhard, G.: Structural determination of individual chemical species in a mixed system by iterative transformation factor analysis-based X-ray absorption spectroscopy combined with UV–Visible absorption and quantum chemical calculation. Anal. Chem. 80, 1102–1110 (2008)
Murase, K., Ando, H., Matsubara, E., Hirato, T., Awakura, Y.: Determination of Mo(VI) species and composition in Ni–Mo alloy plating baths by Raman spectra factor analysis. J. Electrochem. Soc. 147, 2210–2217 (2000)
Furlani, C., Morpurgo, G.: Properties and electronic structure of tetra halogeno cuprate (II)-complexes. Theor. Chim. Acta 1, 102–115 (1963)
Malinowski, E.R., Howery, D.G.: Factor Analysis in Chemistry, 3rd edn. Wiley, New York (1980)
Gemperline, P.J: Pratical Guide to Chemomertrics, 2nd edn. Taylor & Francis Group, Boca Raton (2006)
Jaumot, J., de Juan, A., Tauler, R.: MCR-ALS GUI 2.0: new features and applications. Chemom. Intell. Lab. Syst. 140, 1–12 (2015)
Maeder, M.: Evolving factor analysis for the resolution of overlapping chromatographic peaks. Anal. Chem. 59, 527–530 (1987)
Filipponi, A., D’Angelo, P., Pavel, N.V., Dicicco, A.: Triplet correlations in the hydration shell of aquaions. Chem. Phys. Lett. 225, 150–155 (1994)
D’Angelo, P., Bottari, E., Festa, M.R., Nolting, H.F., Pavel, N.V.: Structural investigation of copper(II) chloride solutions using x-ray absorption spectroscopy. J. Chem. Phys. 107, 2807–2812 (1997)
Marsh, A.R.W., Mcelroy, W.J.: The dissociation constant and Henry law constant of HCl in aqueous solution. Atmos. Environ. 19, 1075–1080 (1985)
Helgeson, H.C., Kirkham, D.H.: Theoretical prediction of thermodynamic behavior of aqueous electrolytes at high pressures and temperatures. Am. J. Sci. 274, 1199–1261 (1974)
Partanen, J.I., Juusola, P.M., Vahteristo, K.P., de Mendonca, A.J.G.: Re-evaluation of the activity coefficients of aqueous hydrochloric acid solutions up to a molality of 16.0 mol kg−1 using the Hückel and Pitzer equations at temperatures from 0 to 50 °C. J. Solution Chem. 36, 39–59 (2007)
Kielland, J.: Individual activity coefficients of ions in aqueous solutions. J. Am. Chem. Soc. 59, 1675–1678 (1937)
Helgeson, H.C., Kirkham, D.H.: Theoretical prediction of thermodynamic behavior of aqueous electrolytes at high pressures and temperatures. Am. J. Sci. 274, 1089–1198 (1974)
Setchénow, M.: Über die constitution der salzlösungen auf grund ihres verhaltens zu kohlensäure. Z. Phys. Chemie-Int. J. Res. Phys. Chem. Chem. Phys. 4, 117–126 (1889)
Hershey, J.P., Damesceno, R., Millero, F.J.: Densities and compressibilities of aqueous HCl and NaOH From 0 to 45 °C. The effect of pressure on the ionization of water. J. Solution Chem. 13, 825–848 (1984)
Kell, G.S.: Density, Thermal expansivity, and compressibility of liquid water from 0 to 150 °C: correlations and tables for atmospheric pressure and saturation reviewed and expressed on 1968 temperature scale. J. Chem. Eng. Data 20, 97–105 (1975)
Lagarias, J.C., Reeds, J.A., Wright, M.H., Wright, P.E.: Convergence properties of the Nelder-Mead simplex method in low dimensions. SIAM J. Optim. 9, 112–147 (1998)
Xia, F.F., Yi, H.B., Zeng, D.: Hydrates of copper dichloride in aqueous solution: a density functional theory and polarized continuum model investigation. J. Phys. Chem. A 113, 14029–14038 (2009)
Xia, F.F., Yi, H.B., Zeng, D.: Hydrates of Cu2+ and CuCl+ in dilute aqueous solution: a density functional theory and polarized continuum model investigation. J. Phys. Chem. A 114, 8406–8416 (2010)
Yi, H.B., Xia, F.F., Zhou, Q., Zeng, D.: [CuCl3]− and [CuCl4]2− hydrates in concentrated aqueous solution: a density functional theory and ab Initio study. J. Phys. Chem. A 115, 4416–4426 (2011)
Allen, P.G., Bucher, J.J., Shuh, D.K., Edelstein, N.M., Reich, T.: Investigation of aquo and chloro complexes of UO2 2+, NpO2 +, Np4+, and Pu3+ by X-ray absorption fine structure spectroscopy. Inorg. Chem. 36, 4676–4683 (1997)
Ikeda, A., Yaita, T., Okamoto, Y., Shiwaku, H., Suzuki, S., Suzuki, T., Fujii, Y.: Extended X-ray absorption fine structure investigation of adsorption and separation phenomena of metal ions in organic resin. Anal. Chem. 79, 8016–8023 (2007)
Kékesi, T., Mimura, K., Isshiki, M.: Ultra-high purification of iron by anion exchange in hydrochloric acid solutions. Hydrometallurgy 63, 1–13 (2002)
Kékesi, T., Isshiki, M.: Anion-exchange behavior of copper and some metallic impurities in HCl solutions. Mater. T. Jim 35, 406–413 (1994)
Acknowledgements
The author wishes to thank Mr. Yuji Baba for his great devotion to this experimental work. This research was performed at the MSTeC Research Center at Institute of Multidisciplinary Research for Advanced Materials, Tohoku University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uchikoshi, M. Determination of the Distribution of Cupric Chloro-Complexes in Hydrochloric Acid Solutions at 298 K. J Solution Chem 46, 704–719 (2017). https://doi.org/10.1007/s10953-017-0597-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-017-0597-8