Abstract
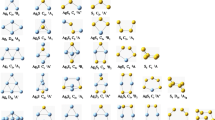
In the present study electronic structure and stabilities of cationic gold-doped germanium clusters, AuGen (n = 1 to 20), and their assemblies have been investigated by density functional theory (DFT) modeling. Computational results show a good relationship between the thermodynamic parameters, average binding energy, embedding energy, fragmentation energy, etc., with the percentage hybridization between different Ge 4s, Ge 4p, and Au 5d atomic orbitals, which plays a dominating role in the stabilization of anionic AuGe7, AuGe10, Au(Ge7)2, Au(Ge9)2, and Au(Ge10)2 clusters. Other thermodynamic and chemical parameters are also found consistent with the observed thermodynamic stabilities of the nanoclusters. In smaller size range (n < 11), Au atom always absorbs on the surface or vertex of pure Ge cluster. From n = 11, endohedral doping starts. In the assembled clusters, Au atom play the role as a bridging atom in Au(Ge7)2, Au(Ge9)2, and Au(Ge10)2 clusters. Stability of the AuGe7, AuGe10, Au(Ge7)2, Au(Ge9)2, and Au(Ge10)2 are explained using magic number in shell-filled model and mixed (π-σ) aromatic rule. As per the symmetry and structure of AuGe12 cluster, it is comparable to a nido-cluster, and hence, its stability is explained using Wade-Mingos rule. Calculated VDE, ADE, HOMO-LUMO gap, and VIP have very close agreement with the experimental results. IR and Raman frequencies show that the vibration nature of the clusters could produce electromagnetic radiation in the far infrared region which is useful for medical applications.






Similar content being viewed by others
References
Kumar V, Kawazoe Y (2001) Metal-encapsulated icosahedral superatoms of germanium and tin with large gaps: ZnGe12 and CdSn12. Appl Phys Lett 80:5
Zhang X, Wang Y, Wang H, Lim A, Gantefoer G, Bowen KH, Reveles JU, Khanna SN (2013) On the existence of designer magnetic Superatoms. J Am Chem Soc 135(12):4856
Reber AC, Khanna SN, Castleman Jr AW (2007) Superatom compounds, clusters, and assemblies: ultra-alkali motifs and architectures. J Am Chem Soc 129(33):10189
Bandyopadhyay D, Kaur P, Sen P (2010) New insights into applicability of Electron-counting rules in transition metal encapsulating Ge cage clusters. J Phys Chem A 114:12986
Kaxiras E, Jackson K (1993) Shape of small silicon clusters. Phys Rev Lett 71:727
Bandyopadhyay D, Sen P (2010) Density functional investigation of structure and stability of Gen and GenNi (n= 1− 20) clusters: validity of the electron counting rule. J Phys Chem A 114:1835
Bandyopadhyay D (2008) A density functional theory–based study of the electronic structures and properties of cage like metal doped silicon clusters. J Appl Phys 104:084308
Bandyopadhyay D (2009) The study of the electronic structures and properties of pure and transition metal-doped silicon nanoclusters: a density functional theory approach. Mol Simul 35:381
Bandyopadhyay D (2012) Architectures, electronic structures, and stabilities of cu-doped gen clusters: density functional modeling. J Mol Model 18:737
Bandyopadhyay D (2009) Density functional study of the electronic structure and properties of lithium intercalated graphite. Eur Phys J D 54:643
Bandyopadhyay D, Kumar M (2008) The electronic structures and properties of transition metal-doped silicon nanoclusters: a density functional investigation. Chem Phys 353:170
Gopakumar G, Wang X, Lin L, Haeck JD, Lievens P, Nguyen MT (2009) Lithium-doped germanium nanowire? Experimental and theoretical indication. J Phys Chem C 113:10858
Gopakumar G, Lievens P, Nguyen MT (2007) Stability and thermodynamics of ligand-free germanium-gold clusters. J Phys Chem A 111:4353
Kamata Y (2008) High-k/Ge MOSFETs for future nanoelectronics. Mater Today 11:30–38
Pillarisetty R (2011) Academic and industry research progress in germanium nanodevices. Nature 479:324–328
Zhao W-J, Wang Y-X (2008) Geometries, stabilities, and electronic properties of FeGen (n = 9–16) clusters: Density-functional theory investigations. Chem Phys 352:291–296
Wang J, Han J-G (2006) A Theoretical Study on Growth Patterns of Ni-Doped Germanium Clusters. J Phys Chem B 110:7820–7826
Jing Q, Tian FY, Wang YX (2008) No quenching of magnetic moment for the GenCo (n=1-13) clusters: first-principles calculations. J Chem Phys 128:124319
Wang J, Han J-G (2006) Geometries and electronic properties of the tungsten-doped germanium clusters: WGen (n = 1−17). J Phys Chem A (46):12670–12677
Zhao W-J, Wang Y-X (2009) Geometries, stabilities, and magnetic properties of MnGen (n = 2–16) clusters: density-functional theory investigations. Theochem 901:18–23
Deng X-J, Kong X-Y, Xu X-L, Xu H-G, Zheng W-J (2014) Structural and bonding properties of small TiGen − (n = 2–6) clusters: photoelectron spectroscopy and density functional calculations. RSC Adv 4:25963–25968
Deng XJ, Kong XY, Xu XL, Xu HG, Zheng WJ (2014) Structural and magnetic properties of CoGen − (n=2–11) clusters: photoelectron spectroscopy and density functional calculations. Chem Phys Chem 15:3987–3993
Furuse S, Koyasu K, Atobe J, Nakajima A (2008) Experimental and theoretical characterization of MSi16 −, MGe16 −, MSn16 − and MPb16 − (M= Ti, Zr, and Hf): the role of cage aromaticity. J Chem Phys 129:064311
Deng X-J, Kong X-Y, Xu H-G, Xu X-L, Feng G, Zheng W-J (2015) Photoelectron spectroscopy and density functional calculations of VGen − (n = 3–12) clusters. J Phys Chem C 119:11048–11055
Wang J, Han J-G (2007) The growth behaviors of the Zn-doped different sized germanium clusters: A density functional investigation. Chem Phys 342:253–259
Wang J, Han J-G (2008) Geometries, stabilities, and vibrational properties of bimetallic Mo2-doped Gen (n = 9−15) clusters: a density functional investigation. J Phys Chem A 112:3224–3230
Beck SM (1987) Studies of silicon cluster–metal atom compound formation in a supersonic molecular beam. J Chem Phys 87:4233
Beck SM (1989) Mixed metal–silicon clusters formed by chemical reaction in a supersonic molecular beam: implications for reactions at the metal/silicon interface. J Chem Phys 90:6306
Wang J, Han JG (2007) The growth behaviors of the Zn-doped different sized germanium clusters: a density functional investigation. Chem Phys 342:253
Abreu MB, Reber AC, Khanna SN (2014) Does the 18-Electron rule apply to CrSi12? J Phys Chem Lett 5:3492
Chauhan V, Abreu MB, Reber AC, Khanna SN (2015) Geometry controls the stability of FeSi14. Phys Chem Chem Phys 17:15718–15724
Abreu MB, Reber AC, Khanna SN (2015) Making sense of the conflicting magic numbers in WSin clusters. J Chem Phys 143:074310
Dhaka K, Bandyopadhyay D (2015) Study of the electronic structure, stability and magnetic quenching of CrGen (n=1–17) clusters: a density functional investigation. RSC Adv 5:83004–83012
Reveles JU, Khanna SN (2005) Nearly-free-electron gas in a silicon cage. Phys Rev B 72:16513
Koyasu K, Akutsu M, Mitsui M, Nakajima A (2005) Selective formation of MSi16 (M = Sc, Ti, and V). J Am Chem Soc 127:4998
Berlicki TM, Murawski E, Muszynski M, Osadnik SJ, Prociow EL (1995) Thin-film thermocouples of Ge doped with au and B. Sens Actuators, A 50:183–186
Wang XX, Getaneh M, Martoff CJ, Kaczanowicz E (1999) Hot electron effects and dynamic behavior of gold doped germanium thin films as cryogenic phonon sensors. J Appl Phys 85:8274–8280
Goicoechea JM, McGrady JE (2015) On the structural landscape in endohedral silicon and germanium clusters, M@Si12 and M@Ge12. Dalton Trans 44:6755–6766
McDermott D, Newman KE (2015) Wade’s rules and the stability of AunGem clusters. Eur Phys J D 69:90–102
Li X, Su K, Yang X, Song L, Yang L (2013) Effect of alkali metal atoms doping on structural and nonlinear optical properties of the gold-germanium bimetallic clusters. J Comput Chem 1010:32–37
Truong BT, Nguyen MT (2011) Enhanced stability by three-dimensional aromaticity of Endohedrally doped clusters X10M0/− with X = Ge, Sn, Pb and M = cu, ag, au. J Phys Chem A 115:9993–9999
Li X-J, Ren H-J, Yang L-M (2012). J Nanomater 2012:1–8
Mahtout S, Siouani C, Rabilloud F (2018) Growth behavior and electronic structure of Noble metal-doped germanium clusters. J Phys Chem A 122:662–677
Lu S-J, Hu L-R, Xu X-L, Xu H-G, Chen H, Zheng W-J (2016) Transition from exohedral to endohedral structures of AuGen (n = 2–12) clusters: photoelectron spectroscopy and ab initio calculations. Phys Chem Chem Phys 18:20321
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868
Perdew JP, Burke K, Ernzerhof M (1997) Generalized gradient approximation made simple [ERRATA: Phys. Rev. Lett. 77, 3865 (1996)]. Phys Rev Lett 78(7):1396
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1. Gaussian, Inc., Wallingford CT
Dolg M, Wedig U, Stoll H, Pereuss H (1987) Energy-adjusted ab initio pseudopotentials for the first row transition elements. J Chem Phys 86:866
Glass CW, Oganov AR, Hansen N (2006) USPEX – evolutionary crystal structure prediction. Comput Phys Commun 175:713
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758
Bandyopadhyay D (2009) Study of pure and doped hydrogenated germanium cages: a density functional investigation. Nonotechnology 20:275202
Kumar M, Singh BJ, Kajjam S, Bandyopadhyay D (2010) Effect of transition metal doping on hydrogenated germanium Nano-cages: a density functional investigation. J Comp Theo Nanoscience 7(1):296
Trivedi R, Dhaka K, Bandyopadhyay D (2014) Study of electronic properties, stabilities and magnetic quenching of molybdenum-doped germanium clusters: a density functional investigation. RSC Adv 4:64825–64834
Yoshida S, Fuke K (1999) Photoionization studies of germanium and tin clusters in the energy region of 5.0–8.8 eV: ionization potentials for Gen (n=2–57) and Snn (n=2–41). J Chem Phys 111:3880
Zhou B, Kramer T, Thompson AL, McGrady JE, Goicoechea JM (2011) A highly distorted open-Shell endohedral Zintl cluster: [Mn@Pb12]3−. Inorg Chem 50:8028
Tai TB, Nguyen HMT, Nguyen MT (2011) The group 14 cationic clusters by encapsulation of coinage metals X10M+, with X = Ge, Sn, Pb and M = cu, ag, au: enhanced stability of 40 valence electron systems. Chem Phys Lett 502:187
Wade K (1971) The structural significance of the number of skeletal bonding electron-pairs in carboranes, the higher boranes and borane anions, and various transition-metal carbonyl cluster compounds. J Chem Soc D:792
Wade K (1976) Structural and Bonding Patterns in Cluster Chemistry. Adv Inorg Chem Radiochem 18:1
Mingos DMP (1972) A general theory for cluster and ring compounds of the Main group and transition elements. Nat Phys Sci 236:99
Huang X, Xu H-G, Lu S, Su Y, King RB, Zhao J, Zheng W (2014) Discovery of a silicon-based ferrimagnetic wheel structure in VxSi12 − (x = 1–3) clusters: photoelectron spectroscopy and density functional theory investigation. Nanoscale 6:14617
Esenturk EN, Fettinger J, Eichhorn B (2006) The Pb12 2− and Pb10 2− Zintl Ions and the M@Pb12 2- and M@Pb10 2- Cluster Series Where M = Ni, Pd, Pt. J Am Chem Soc 128:9178
Uta MM, Cioloboc D, King RB (2012) Cobalt-centered ten-vertex germanium clusters: the pentagonal prism as an alternative to polyhedra predicted by the Wade-Mingos rules. Inorg Chem 51:3498–3504
Hirsch A, Chen Z, Jiao H (2000) Spherical aromaticity in Ih symmetrical fullerenes: the 2(N+1)2 rule. Angew Chem Int Ed 39:3915
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 4385 kb)
Rights and permissions
About this article
Cite this article
Bandyopadhyay, D. Electronic structure and stability of anionic AuGen (n = 1–20) clusters and assemblies: a density functional modeling. Struct Chem 30, 955–963 (2019). https://doi.org/10.1007/s11224-018-1239-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1239-5