Abstract
Context
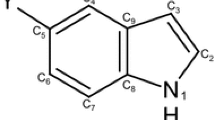
A comparative study of the change in different properties of electronic and structural of the free 1H-indole-2,3-dione molecule and its complexes has been obtained. HOMA analysis was performed to investigate the effects of lithium sodium and potassium cations on the aromaticity of lithium sodium and potassium complexes of 1H-indole-2,3-dione.
Methods
Several 1H-indole-2,3-dione complexes with lithium, sodium, and potassium cations were optimized at the B3LYP/6-311G(d,p) level. The cation and π interaction has been investigated from different aspects, including interaction energy calculations, charge transfer values, and changes in the aromaticity of the ring upon complexation. The charge transfer and natural population analysis for the complexes were performed with the natural bond orbital (NBO) analysis. The properties of bond critical points in complexes were studied by applying the quantum theory of atoms in molecules (QTAIM). Finally, the aromaticity change of phenyl induced upon complex formation was evaluated by applying the harmonic oscillator model of aromaticity (HOMA). [Li-INa]+ and [[Li-INb]+ were optimized with the wB97XD function using a version of Grimme’s D2 dispersion model, and the absorption energy was compared with the calculation made with the B3LYP functional.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Pandeya SN, Smitha S, Jyoti M, Sridhar SK (2005) Biological activities of isatin and its derivatives. Acta Pharm 55(1):27–46
Pakravan P, Kashanian S, Khodaie MM, Harding FJ (2013) Biochemical and pharmacological characterization of isatin and its derivatives: from structure to activity. Pharmacol Rep 65(2):313–335. https://doi.org/10.1016/S1734-1140(13)71007-7
Halket JM, Watkins PJ, Przyborowska A, Goodwin BL, Clow A, Glower V, Sandler MJ (1991) Isatin (indole-2,3-dione) in urine and tissues. Detection and determination by gas chromatography-mass spectrometry. Journal of Chromatograpy 562:279–287. https://doi.org/10.1016/0378-4347(91)80585-Z
Silva JFM, Garden SJ, Pinto AC (2001) The chemistry of isatins: a review from 1975 to 1999. J Braz Chem Soc 12(3):273–324. https://doi.org/10.1590/S0103-50532001000300002
Matos MAR, Miranda MS, Morais VMF, Liebman JF (2003) Are isatin and isatoic anhydride antiaromatic and aromatic respectively? A combined experimental and theoretical investigation. Org Biomol Chem 1(14):2566–2571. https://doi.org/10.1039/B304405J
Dehaghani MZ, Yousefi F, Seidi F, Bagheri B, Mashhadzadeh AH, Naderi G, Esmaeili A, Abida O, Habibzadeh S, Saeb MR, Rybachuk M (2021) Encapsulation of an anticancer drug Isatin inside a host nano-vehicle SWCNT: a molecular dynamics simulation. Sci Rep 11:18753. https://doi.org/10.1038/s41598-021-98222-2
Kashanian S, Khodaei MM, Pakravan P (2010) Spectroscopic studies on the ınteraction of ısatin with calf thymus DNA. DNA Cell Biol 29(10):639–646. https://doi.org/10.1089/dna.2010.1054
Bania KK, Guha AK, Bhattacharyya PK, Sinha S (2014) Effect of substituent and solvent on cation–π interactions in benzene and borazine: a computational study. Dalton Trans 43(4):1769–1784. https://doi.org/10.1039/C3DT52081A
Dinadayalane TC, Afanasiev D, Leszczynski J (2008) Structures and energetics of the cation−π ınteractions of Li+, Na+, and K+with cup-shaped molecules: effect of ring addition to benzene and cavity selectivity. J Phys Chem A 112(34):7916–7924. https://doi.org/10.1021/jp802236k
Ghenaatian HR (2017) Structural and electronic properties of alumaphosphinine complexes with metal ions: a theoretical study. Sci Iran Trans C: Chem Chem Eng 24(3):1181–1188. https://doi.org/10.24200/sci.2017.4099
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09. Gaussian Inc., Wallingford. https://doi.org/10.1017/CBO9781107415324.004
Boys SF, Bernardi R (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19(4):553–566. https://doi.org/10.1080/00268977000101561
DalgicSenturk S, Al-Sawaff ZH, Dalgic S, Kandemirli F (2023) A comparative DFT study on Al- and Si-doped single-wall carbon nanotubes (SWCNTs) for Ribavirin drug sensing and detection. Mater Sci Semicond Process 158:107360. https://doi.org/10.1016/j.mssp.2023.107360
Bader RFW (1985) Atoms in molecules. Acc Chem Res 18(1):9–15. https://doi.org/10.1021/ar00109a003
Lu T, Chen F (2012) Multiwfn: a multifunctional wave function analyzer. J Comput Chem 33(5):580–592. https://doi.org/10.1002/jcc.22885
Thomas W, Kelley C (2011) Gnuplot 4.5: an interactive plotting program. http://gnuplot.info. Accessed Oct 2023
Humphrey W, Dalke A, Schulten K (1996) VMD:visual molecular dynamics. J Mol Graphics 14(1):33–38. https://doi.org/10.1016/0263-7855(96)00018-5
O’Boyle NM, Tenderholt AL, Langner KM (2008) cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29(5):839–845. https://doi.org/10.1002/jcc.20823
Krygowski TM, Cyranski M (1996) Separation of the energetic and geometric contributions to the aromaticity. Part IV. A general model for the the (-electron systems. Tetrahedron 52(30):10255–10264. https://doi.org/10.1016/0040-4020(96)00560-1
Andrzejak M, Kubisiak P, Zborowski KK (2013) Avoiding pitfalls of a theoretical approach: the harmonicoscillator measure of aromaticity index from quantum chemistry calculations. Struct Chem 24:1171–1184. https://doi.org/10.1007/s11224-012-0148-2
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Phys 10:6615–6620. https://doi.org/10.1039/b810189b
de Miranda DB, Quintal S, Ferreira GB (2023) Theoretical studies of Zn2+ complexes with alkyl xanthate ligands: a thermochemical, electronic energy decomposition, and natural bond orbital analysis. J Mol Model 29:203. https://doi.org/10.1007/s00894-023-05604-
Bader RFW (1998) A bond path: A universal indicator of bonded interactions. J Phys Chem A 102:7314–7323. https://doi.org/10.1021/jp981794v
Matta CF, Boyd RJ (2007) In: the quantum theory of atoms in molecules: from solid state to DNA and drug design. An introduction to the quantum theory of atoms in molecules, 1st edn. https://doi.org/10.1002/9783527610709.ch1.
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132(18):6498–6506. https://doi.org/10.1021/ja100936w
Kruszewski J, Krygowski TM (1972) Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett 13(36):3839–3842. https://doi.org/10.1016/S0040-4039(01)94175-9
Krygowski TM (1993) Crystallographic studies of inter- and intramolecular interactions reflected in aromatic character of π-electron systems. J Chem Inf Comput Sci 33(1):70–78. https://doi.org/10.1021/ci00011a011
Krygowski TM, Cyrański M (1996) Separation of the energetic and geometric contributions to the aromaticity of π-electron carbocyclics. Tetrahedron 52(5):1713–1722. https://doi.org/10.1016/0040-4020(95)01007-6
Stanger A (2019) Reexamination of NICSπ, zz: height dependence, off-center values, and ıntegration. J Phys Chem A 123:3922–3927. https://doi.org/10.1021/acs.jpca.9b02083
Szymanski S, Majerz I (2019) Aromaticity and electron density of hypericin. J Nat Prod 82(8):2106–2115. https://doi.org/10.1021/acs.jnatprod.8b00872
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FK and SŞD. The first draft of the manuscript was written by FK, SŞD and FG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Genc, F., Kandemirli, F. & Senturk Dalgic, S. A theoretical study on 1H-indole-2,3-dione complexes with lithium, sodium, and potassium cations. J Mol Model 30, 100 (2024). https://doi.org/10.1007/s00894-024-05898-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05898-0