Abstract
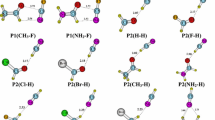
B3LYP/6-31++G(d,p) optimizations on models for the metal cyanin, Cy, complexes [MCy(H2O) n ]+, (M = Zn(II), Cu(II); n = 2, 3, 4) in aqueous solution indicate that 4 is the most favoured coordination number in both cases. SP -4 and T -4 geometries are nearly isoenergetic for the former, while SP -4 is the only one obtained for the latter. Anionic cyanin displays higher affinity for Cu(II) than for Zn(II) or Mg(II). The electron density reorganization of cyanin model accompanying the complexation process was analyzed by means of the quantum theory of atoms in molecules. This analysis reveals that: (1) the O4′–M bond is stronger than O3′–M; (2) anionic cyanin displays a dual character between 4′-keto-quinoidal and 3′,4′-dienolate resonance forms; (3) Cu(II) takes more electron density than Zn(II) from Cy− and water ligands; (4) when the coordination number increases, each ligand (Cy− or water) transfers less electron density; (5) complex formation modifies the electron density in all the atoms of the ligands, but the largest modifications are displayed within the AC bicycle of Cy−; and (6) a third part of density lost by the Cy− ligand is removed from hydrogens.




Similar content being viewed by others
References
Brouillard R (1982) In: Markakis P (ed) Anthocyanins as food colors. Academic Press, New York
Castañeda-Ovando A, Pacheco-Hernández L, Páez-Hernández E, Rodríguez JA, Galán-Vidal CA (2009) Chemical studies of anthocyanins: a review. Food Chem 113:859–871
Chou PH, Matsui S, Misaki K, Matsuda T (2007) Isolation and identification of xenobiotic aryl hydrocarbon receptor ligands in dyeing wastewater. Environ Sci Technol 41:652–657
Harborne J, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504
Galvano F, La Fauci L, Lazzarino G, Fogliano V, Ritieni A, Ciappellano S, Battistini NC, Tavazzi B, Galvano G (2004) Cyanidins: metabolism and biological properties. J Nutr Biochem 15:2–11
Strack D, Wray V (1989) In: Harbone JB (ed) Methods in plant biochemistry 1: plant phenolics. Academic Press, London
Haslam E (1998) Practical polyphenolics: from structure to molecular recognition and physiological action. Cambridge University Press, Cambridge
Rastelli G, Costantino L, Albasini A (1993) Physico-chemical properties of anthocyanidins. Part 1. Theoretical evaluation of the stability of the neutral and anionic tautomeric forms. J Mol Struct THEOCHEM 279:157–166
Leopoldini M, Marino T, Russo N, Toscano M (2004) Structure, conformation, and electronic properties of apigenin, luteolin, and taxifolin antioxidants. A first principle theoretical study. J Phys Chem A 108:4916–4922
Ferreira da Silva P, Lima JC, Freitas AA, Shimizu K, Macanita AL, Quina FH (2005) Charge-transfer complexation as a general phenomenon in the copigmentation of anthocyanins. J Phys Chem A 109:7329–7338
Woodford JN (2005) A DFT investigation of anthocyanidins. Chem Phys Lett 410:182–187
Borkowski T, Szymusiak H, Gliszczynska-Swiglo A, Rietjens IMCM, Tyrakowska B (2005) Radical scavenging capacity of wine anthocyanins is strongly pH-dependent. J Agric Food Chem 53:5526–5534
Sakata K, Saito N, Honda T (2006) Ab initio study of molecular structures and excited states in anthocyanidins. Tetrahedron 62:3721–3731
Leopoldini M, Russo N, Toscano M (2006) Gas and liquid phase acidity of natural antioxidants. J Agric Food Chem 54:3078–3085
Estévez L, Mosquera RA (2008) Where is the positive charge of flavylium cations. Chem Phys Lett 451:121–126
Guzmán R, Santiago C, Sánchez M (2009) A density functional study of antioxidant properties on anthocyanidins. J Mol Struct 935:110–114
Estévez L, Mosquera RA (2009) Conformational and substitution effects on the electron distribution in a series of anthocyanidins. J Phys Chem A 113:9908–9919
Estévez L, Otero N, Mosquera RA (2010) A computational study on the acidity dependence of radical-scavenging mechanisms of anthocyanidins. J Phys Chem B 114:9706–9712
Monica L, Russo N, Toscano M (2011) The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem 125:288–306
Calzolari A, Monti S, Ruini A, Catellani A (2010) Hydration of cyanin dyes. J Chem Phys 132:114304-1–114304-9
Anderson ØM, Jordheim M (2006) In: Andersen ØM, Markham KR (eds) Flavonoids: chemistry, biochemistry and applications. CRC Press, Boca Raton
Kondo T, Ueda M, Isobe M, Goto T (1998) A new molecular mechanism of blue color development with protocyanin a supramolecular pigment from cornflower, Centaurea cyanus. Tetrahedron Lett 39:8307–8310
Shiono M, Matsugaki N, Takeda K (2005) Structure of the blue cornflower pigment. Nature 436:791
Marković JMD, Veselinović DS, Baranac JM, Brdarić TP (2008) Spectroscopic and theoretical study of cyanidin–aluminum (III) complexes. Spectrosc Lett 41:104–115
Estévez L, Otero N, Mosquera RA (2011) Molecular structure of cyanidin metal complexes: Al(III) versus Mg(II). Theor Chem Acc 128:485–495
Kirchgessner M, Weigand E (1979) In: Sigel H (ed) Metal ions in biological systems 15: zinc and its role in biology and nutrition. Marcel Dekker, New York
Martin RB (1986) In: Sigel H (ed) Metal ions in biological systems 20: concepts and metal ion toxicity. Marcel Dekker, New York
Esparza I, Santamaría C, García-Mina JM, Fernández JM (2007) Complexing capacity profiles of naturally occurring ligands in Tempranillo wines for Cu and Zn. An electroanalytical approach for cupric casse. Anal Chim Acta 599:67–75
Bodini ME, del Valle MA, Tapia R, Leighton F, Berrios P (2001) Zinc catechin complexes in aprotic medium. Redox chemistry and interaction with superoxide radical anion. Polyhedron 20:1005–1009
Le Nest G, Caille O, Woudstra M, Roche S, Burlat B, Belle V, Guigliarelli B, Lexa D (2004) Zn–polyphenol chelation: complexes with quercetin, (+)-catechin, and derivatives: II electrochemical and EPR studies. Inorg Chim Acta 357:2027–2037
Vestergaard M, Kerman K, Tamiya E (2005) An electrochemical approach for detecting copper-chelating properties of flavonoids using disposable pencil graphite electrodes: possible implications in copper-mediated illnesses. Anal Chim Acta 538:273–281
Esparza I, Salinas I, Santamaría C, García-Mina JM, Fernández JM (2005) Electrochemical and theoretical complexation studies for Zn and Cu with individual polyphenols. Anal Chim Acta 543:267–274
Esparza I, Salinas I, Caballero I, Santamaría C, Calvo I, García-Mina JM, Fernández JM (2004) Evolution of metal and polyphenol content over a 1-year period of vinification: sample fractionation and correlation between metals and anthocyanins. Anal Chim Acta 524:215–224
Bader RFW (1990) Atoms in molecules—a quantum theory. Oxford University Press, Oxford
Tomasi J, Mennuci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham, MA Peng, CY Nanayakkara, A Challacombe, M Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision E.01. Gaussian, Inc., Wallingford
Rezabal E, Mercero JM, Lopez X, Ugalde JM (2007) A theoretical study of the principles regulating the specificity for Al(III) against Mg(II) in protein cavities. J Inorg Biochem 101:1192–1200
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928
Biegler-König FW, Schönbohm J, Bayles D (2001) AIM2000. J Comp Chem 22:545–559
Bader RFW. AIMPAC: a suite of programs for the theory of atoms in molecules; McMaster University, Hamilton. http://www.chemistry.mcmaster.ca/aimpac/download/download.htm
Estévez L, Mosquera RA (2007) A density functional theory study on pelargonidin. J Phys Chem A 111:11100–11109
Estévez L, Mosquera RA (2008) Molecular structure and antioxidant properties of delphinidin. J Phys Chem A 112:10614–10623
González Moa MJ, Mosquera RA (2003) Applicability of resonance forms in pyrimidinic bases. An AIM study. J Phys Chem A 107:5361–5367
Glaser R, Horan CJ, Lewis M, Zollinger H (1999) σ-Dative and π-backdative phenyl cation–dinitrogen interactions and opposing sign reaction constants in dual substituent parameter relations. J Org Chem 64:902–913
Persson I (2010) Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl Chem 82:1901–1917
Deeth RJ, Randell K (2008) Ligand field stabilization and activation energies revisited: molecular modeling of the thermodynamic and kinetic properties of divalent, first-row aqua complexes. Inorg Chem 47:7377–7388
Johnson DA, Nelson PG (1995) Factors determining the ligand field stabilization energies of the hexaaqua 2+ complexes of the first transition series and the Irving–Williams order. Inorg Chem 34:5666–5671
Bryantsev VS, Diallo MS, van Duin ACT, Goddard WA III (2008) Hydration of copper(II): new insights from density functional theory and the COSMO solvation model. J Phys Chem A 112:9104–9112
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–556
Langmuir I (1921) Types of valence. Science 54:59–67
Cirera J, Alemany P, Alvarez S (2004) Mapping the stereochemistry and symmetry of tetracoordinate transition-metal complexes. Chem Eur J 10:190–207
Ribas GJ (2008) Coordination chemistry. Wiley-VCH, Weinheim
Alvarez S, Alemany P, Casanova D, Cirera J, Llunell M, Avnir D (2005) Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord Chem Rev 249:1693–1708
Cremer D, Kraka E (1984) A description of the chemical bond in terms of local properties of electron density and energy. Croat Chem Acta 57:1259–1282
Cortés-Guzmán F, Bader RFW (2005) Complementarity of QTAIM and MO theory in the study of bonding in donor–acceptor complexes. Coord Chem Rev 249:633–662
González Moa MJ, Mandado M, Mosquera RA (2006) Explaining the sequence of protonation affinities of cytosine with QTAIM. Chem Phys Lett 428:255–261
Ferro-Costas D, Mosquera RA (2013) Influence of the O-protonation in the O=C–O–Me Z preference. A QTAIM study. J Phys Chem A 117:257–265
Holleman AF, Wiberg E (2001) Inorganic chemistry. Academic Press, Berlin
Acknowledgments
The authors thank “Centro de Supercomputación de Galicia” (CESGA) for free access to its computational facilities, and financial support from Spanish Ministry of Economy through research project CTQ2010-21500.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11224_2014_445_MOESM1_ESM.doc
Supplementary material 1 (DOC 388 kb): Absolute values for atomic electron populations in the model of anionic cyanin and their variations upon complexation in the diverse cases here considered are available as Supporting Information
Rights and permissions
About this article
Cite this article
García Bugarín, M., Mosquera, R.A. On the structure of Zn(II) and Cu(II) cyanin complexes in aqueous solution. Struct Chem 25, 1647–1657 (2014). https://doi.org/10.1007/s11224-014-0445-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0445-z