Abstract
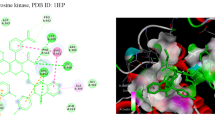
The present work describes the unrevealed potential of tetrahydropyridines as anticancer and anti-tubercular agents along with DFT, ADMET, drug-likeliness and molecular docking studies synthesized by using Bronsted acidic ionic liquid viz. 1-methyl-3-(4-sulfobutyl)-1H-imidazol-3-ium chloride [MSbIM-Cl]. The synthesized derivatives have been screened for their anticancer and anti-tubercular evaluation against MCF-7 cell lines and Mycobacterium tuberculosis respectively. The compound 4e showed the highest anticancer activity while the compound 4h showed the highest anti-tubercular activity. The in vitro evaluation has been supported by computational methods such as molecular docking, density functional study and in silico ADMET and drug-likeliness prediction.
Graphical abstract









Similar content being viewed by others
Data availability
The data have been included in the article and supplementary information file.
References
R. Kaur, S. Chaudhary, K. Kumar, M.K. Gupta, R.K. Rawal, Eur. J. Med. Chem. 132, 108 (2017)
L.E. Rodriguez, C.H. House, K.E. Smith, M.R. Roberts, M.P. Callahan, Sci. Rep. 9, 9281 (2019)
N. Kerru, L. Gummidi, S. Maddila, K.K. Gangu, S.B. Jonnalagadda, Molecules 25, 1909 (2020)
D. Zárate-Zárate, R. Aguilar, R.I. Hernández-Benitez, E.M. Labarrios, F. Delgado, J. Tamariz, Tetrahedron 71, 6961 (2015)
E.K. Davison, J. Sperry, J. Nat. Prod. 80, 3060 (2017)
W.Y. Fang, L. Ravindar, K.P. Rakesh, H.M. Manukumar, C.S. Shantharam, N.S. Alharbi, H.L. Qin, Eur. J. Med. Chem. 173, 117 (2019)
B. Eftekhari-Sis, M. Zirak, A. Akbari, Chem. Rev. 113, 2958 (2013)
Y. Ju, R.S. Varma, J. Org. Chem. 71, 135 (2006)
P.D. Leeson, B. Springthorpe, Nat. Rev. Drug Discov. 6, 881 (2007)
C. Lamberth, Pest Manag. Sci. 69, 1106 (2013)
B.R. Smith, C.M. Eastman, J.T. Njardarson, J. Med. Chem. 57, 9764 (2014)
J. Liu, J. Jiang, L. Zheng, Z.Q. Liu, Adv. Synth. Catal. 362, 4876 (2020)
K. Paruch, Ł Popiołek, A. Biernasiuk, A. Hordyjewska, A. Malm, M. Wujec, Molecules 25, 5844 (2020)
S.B. Bakare, Bull. Chem. Soc. Ethiop. 35, 449 (2021)
V. Alagarsamy, R. Giridhar, M.R. Yadav, J. Pharm. Pharmacol. 58, 1249 (2010)
S. Nabil, S.N. Abd El-Rahman, S.S. Al-Jameel, A.M. Elsharif, Biol. Pharm. Bull. 41, 1071 (2018)
M. Marinescu, Antibiotics 10, 1002 (2021)
R. Bouley, M. Kumarasiri, Z. Peng, L.H. Otero, W. Song, M.A. Suckow, V.A. Schroeder, W.R. Wolter, E. Lastochkin, N.T. Antunes, H. Pi, S. Vakulenko, J.A. Hermoso, M. Chang, S. Mobashery, J. Am. Chem. Soc. 137, 1738 (2015)
J. Jampilek, Molecules 24, 3839 (2019)
W. Wang, L. Lei, Z. Liu, H. Wang, Q. Meng, Molecules 24, 877 (2019)
S.H. Sharma, J.L. Pablo, M.S. Montesinos, A. Greka, C.R. Hopkins, Bioorg. Med. Chem. Lett. 29, 155 (2019)
J. Liu, C. Wu, H. Jin, K. Du, H. Zheng, J. Heterocycl. Chem. 56, 3088 (2019)
P. Herdewijn, Antisense Nucleic Acid Drug Dev. 10, 297 (2000)
S.S. Gupta, S. Kumari, I. Kumar, U. Sharma, Chem. Heterocycl. Compd. (New York) 56, 433 (2020)
K.P. Srivastava, I. Singh, A. Kumari, Der Pharma Chem. 6, 119 (2014)
N.C. Desai, A.M. Dodiya, Arab. J. Chem. 9, S379 (2016)
M.S. Praceka, S. Megantara, R. Maharani, M. Muchtaridi, J. Adv. Pharm. Technol. Res. 12, 321 (2021)
R. Kakuchi, R. Ito, S. Nomura, H. Abroshan, K. Ninomiya, T. Ikai, K. Maeda, H.J. Kim, K. Takahashi, RSC Adv. 7, 9423 (2017)
J.D. Patil, S.N. Korade, D.M. Pore, RSC Adv. 4, 50449 (2014)
H.R. Safaei, M. Shekouhy, V. Shafiee, M. Davoodi, J. Mol. Liq. 180, 139 (2013)
H. Zang, Q. Su, Y. Mo, B.W. Cheng, S. Jun, Ultrason. Sonochem. 17, 749 (2010)
S.N. Korade, J.D. Patil, D.M. Pore, Monatsh. Chem. 147, 2143 (2016)
J.D. Patil, S.A. Patil, D.M. Pore, RSC Adv. 5, 21396 (2015)
V.I. Pârvulescu, C. Hardacre, Chem. Rev. 107, 2615 (2007)
G. Bhasin, R. Srivastava, R. Singh, Org. Prep. Proc. Int. 49, 370 (2017)
C. Maton, N. De Vos, C.V. Stevens, Chem. Soc. Rev. 42, 5963 (2013)
R. Sheldon, ChemComm 1, 2399 (2001)
B.J. Butler, J.B. Harper, New J. Chem. 39, 213 (2015)
R.L. Vekariya, J. Mol. Liq. 227, 44 (2017)
W.L. Wong, K.Y. Wong, Can. J. Chem. 90, 1 (2012)
R.C. Cioc, E. Ruijter, R.V.A. Orru, Green Chem. 16, 2958 (2014)
B.H. Rotstein, S. Zaretsky, V. Rai, A.K. Yudin, Chem. Rev. 114, 8323 (2014)
A. Rahmati, N. Pashmforoush, Iran. Chem. Soc. 12, 993 (2015)
J.D. Sunderhaus, S.F. Martin, Chem. Eur. J. 15, 1300 (2009)
J.R. Resting, I.L. Tolderlund, A.F. Pedersen, M. Witt, J.W. Jaroszewski, D. Staerk, J. Nat. Prod. 72, 312 (2009)
G.L. Lozano, H.B. Park, J.I. Bravo, E.A. Armstrong, J.M. Denu, E.V. Stabb, N.A. Broderick, J.M. Crawford, J. Handelsman, Appl. Environ. Microbiol. 85, e03058 (2019)
M. Misra, S.K. Pandey, V.P. Pandey, J. Pandey, R. Tripathi, R.P. Tripathi, Bioorg. Med. Chem. 17, 625 (2009)
M. Gangapuram, K. Redda, J. Heterocycl. Chem. 43, 709 (2006)
K. Rao, K. Redda, F. Onayemi, H. Melles, J. Choi, J. Heterocycl. Chem. 32, 307 (1995)
S. Petit, J. Nallet, M. Guillard, J. Dreux, R. Chermat, M. Poncelet, C. Bulach, P. Simon, C. Fontaine, M. Barthelmebs, J. Imbs, Eur. J. Med. Chem. 26, 19 (1991)
Y. Zhou, V.E. Gregor, B.K. Ayida, G.C. Winters, Z. Sun, D. Murphy, G. Haley, D. Bailey, J.M. Froelich, S. Fish, S.E. Webber, T. Hermann, D. Wall, Bioorg. Med. Chem. Lett. 15, 1206 (2007)
B. Ho, A.M. Crider, J.P. Stables, Eur. J. Med. Chem. 36, 265 (2001)
S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, S.J. Shams-Najafi, Monatsh. Chem. 143, 939 (2012)
L. Wu, S. Yan, W. Wang, Y. Li, Res. Chem. Intermed. 46, 4311 (2020)
S. Mohammadi, M. Abbasi, J. Chem. Res. 39, 123 (2015)
M. Lashkari, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, S.S. Sajadikhah, R. Doostmohamadi, Synth. Commun. 43, 635 (2013)
J. Aboonajmi, M.T. Maghsoodlou, N. Hazeri, M. Lashkari, M. Kangani, Res. Chem. Intermed. 41, 8057 (2015)
D. Dogra, R. Bharti, R. Sharma, Orbital 12, 232 (2020)
S. Geetha, A. Thangamani, R. Valliappan, S. Vedanayaki, A. Ganapathi, Chem. Data Collect. 30, 100565 (2020)
A.T. Khan, M. Lal, M.M. Khan, Tetrahedron Lett. 51, 4419 (2010)
A. Mulik, P. Hegade, D. Patil, G. Mulik, S. Salunkhe, M. Deshmukh, Res. Chem. Intermed. 43, 729 (2016)
S. Sardar, C.D. Wilfred, L.J. Marc, AIP Conf. Proc. 1787, 030010 (2016)
S.A. Hattarki, C. Bogar, K. Bhat, Int. J. Pharm. Res. Health Sci. 8, 3220 (2020)
P. Mandroli, A. Prabhakar, K. Bhat, S. Krishnamurthy, C. Bogar, Ayu 40, 192 (2019)
S. Cho, H.S. Lee, S. Franzblau, Methods Mol. Biol. 1285, 281 (2015)
M.C.S. Lourenço, M.V.N. de Souza, A.C. Pinheiro, M.L. de Ferreira, R.S.B. Gonçalves, T.M. Cristina Nogueira, M.A. Peralta, ARKIVOC 15, 181 (2007)
E.J. Walsh, J. Chem. Educ. 74, 3 (1997)
Dassault Systèmes, (2020).
N.M. O’boyle, M. Banck, C.A. James, C. Morley, T. Vandermeersch, G.R. Hutchison, J. Cheminform. 3, 1 (2011)
V.K. Bagal, S.S. Rathod, M.M. Mulla, S.C. Pawar, P.B. Choudhari, V.T. Pawar, D.V. Mahuli, Nat. Prod. Res. 37, 1 (2023)
A. Daina, O. Michielin, V. Zoete, Sci. Rep. 7, 42717 (2017)
D.E.V. Pires, T.L. Blundell, D.B. Ascher, J. Med. Chem. 58, 4066 (2015)
S. Rochlani, M. Bhatia, S. Rathod, P. Choudhari, and R. Dhavale, Nat. Prod. Res. 1 (2023).
J. T. Kilbile, Y. Tamboli, S. A. Ansari, S. S. Rathod, P. B. Choudhari, H. Alkahtani, and S. B. Sapkal, Polycycl. Aromat. Compd. 1 (2023).
S. Rathod, S. Dey, S. Pawar, R. Dhavale, P. Choudhari, E. Rajakumara, D. Mahuli, D. Bhagwat, Y. Tamboli, P. Sankpal, S. Mali, and H. More, J. Biomol. Struct. Dyn. 1 (2023).
F. Neese, Wiley Interdiscip. Rev. Comput. Mol. Sci. 2, 73 (2012)
H.D. Snyder, T.G. Kucukkal, J. Chem. Educ. 98, 1335 (2021)
V. Puthanveedu, K. Muraleedharan, Struct. Chem. 33, 1423 (2022)
S. Choudhari, S. K. Patil, and S. Rathod, J Biomol Struct Dyn 1 (2023).
S. Rathod, D. Bhande, S. Pawar, K. Gumphalwad, P. Choudhari, and H. More, Chem. Afr. (2023).
H.M. Berman, J. Westbrook, Z. Feng, G. Gilliland, T.N. Bhat, H. Weissig, I.N. Shindyalov, P.E. Bourne, Nucleic Acids Res. 28, 235 (2000)
R. D. Bakale, P. S. Phatak, S. S. Rathod, P. B. Choudhari, E. M. Rekha, D. Sriram, R. S. Kulkarni, and K. P. Haval, J Biomol Struct Dyn 1 (2023).
J. Eberhardt, D. Santos-Martins, A.F. Tillack, S. Forli, J. Chem. Inf. Model. 61, 3891 (2021)
S. Rathod, P. Chavan, D. Mahuli, S. Rochlani, S. Shinde, S. Pawar, P. Choudhari, R. Dhavale, P. Mudalkar, F. Tamboli, J. Mol. Model. 29, 1 (2023)
S.Y. Salunkhe, R.P. Gurav, S.S. Rathod, P.B. Choudhari, T.P. Yadav, S.B. Wakshe, P.V. Anbhule, G.B. Kolekar, J. Mol. Struct. 1301, 137288 (2024)
S. Rathod, K. Shinde, J. Porlekar, P. Choudhari, R. Dhavale, D. Mahuli, Y. Tamboli, M. Bhatia, K.P. Haval, A.G. Al-Sehemi, M. Pannipara, ACS Omega 8, 391 (2022)
P. Swami, S. Rathod, P. Choudhari, D. Patil, A. Patravale, Y. Nalwar, S. Sankpal, S. Hangirgekar, J. Mol. Struct. 1292, 136079 (2023)
S. Dey, S. Rathod, K. Gumphalwad, N. Yadav, P. Choudhari, E. Rajakumara, R. Dhavale, and D. Mahuli, J Biomol Struct Dyn 1 (2023).
H. Eshghi, A. Khojastehnezhad, F. Moeinpour, M. Bakavoli, S.M. Seyedi, M. Abbasi, RSC Adv. 4, 39782 (2014)
M. Abbasi, RSC Adv. 5, 67405 (2015)
R. Jahanshahi, B. Akhlaghinia, New J. Chem. 41, 7203 (2017)
N.R. Agrawal, S.P. Bahekar, P.B. Sarode, S.S. Zade, H.S. Chandak, RSC Adv. 5, 47053 (2015)
R. Ramachandran, S. Jayanthi, Y.T. Jeong, Tetrahedron 68, 363 (2012)
G.V. Ramin, S. Hajar, C. R. Chim. 16, 1047 (2013)
S.S. Sajadikhah, N. Hazeri, M. Taher Maghsoodlou, M. Habibi-Khorassani, A. Beigbabaei, M. Lashkari, J. Chem. Res. 36, 463 (2012)
M. Zarei, S.S. Sajadikhah, Res. Chem. Intermed. 42, 7005 (2016)
D.D. Gadge, P.S. Kulkarni, J. Heterocycl. Chem. 59, 1320 (2022)
C.A. Lipinski, Drug Discov. Today Technol. 1, 337 (2004)
C.A. Lipinski, B.W. Dominy, P.J. Feeney, Adv. Drug Deliv. Rev. 23, 3 (1997)
C.A. Lipinski, F. Lombardo, B.W. Dominy, P.J. Feeney, Adv. Drug Deliv. Rev. 23, (2001)
D.F. Veber, S.R. Johnson, H.Y. Cheng, B.R. Smith, K.W. Ward, K.D. Kopple, J. Med. Chem. 45, 2615 (2002)
J.X. Mu, Y.X. Shi, M.Y. Yang, Z.H. Sun, X.H. Liu, B.J. Li, N.B. Sun, Molecules 21, 68 (2016)
P. Patil, P. Patil, P. Dandge, P. Bansode, B. Kumbhar, W. Chandane, S. Rathod, P. Choudhari, S. Khot, N. Valekar, D. Pore, G. Rashinkar, J. Mol. Struct. 1301, 137202 (2024)
E.B. Elkaeed, R.G. Yousef, H. Elkady, I.M.M. Gobaara, B.A. Alsfouk, D.Z. Husein, I.M. Ibrahim, A.M. Metwaly, I.H. Eissa, Molecules 27, 4606 (2022)
P.K. Patial, D.S. Cannoo, Nat. Prod. Res. 35, 4611 (2021)
M.A. Mumit, T.K. Pal, M.A. Alam, M.A.A.A.A. Islam, S. Paul, M.C. Sheikh, J. Mol. Struct. 1220, 128715 (2020)
R.D. Bakale, S.M. Sulakhe, S.L. Kasare, B.P. Sathe, S.S. Rathod, P.B. Choudhari, E. Madhu Rekha, D. Sriram, K.P. Haval, Bioorg. Med. Chem. Lett. 97, 129551 (2023)
A. Das, A. Das, B.K. Banik, J. Indian Chem. Soc. 98, 100005 (2021)
A. Das, B.K. Banik, Green Approaches in Medicinal Chemistry for Sustainable Drug Design (Elsevier, Amsterdam, 2020), pp.921–964
D.M. Kara, I.J. Smallman, J.J. Hudson, B.E. Sauer, M.R. Tarbutt, E.A. Hinds, New J. Phys. 14, 103051 (2012)
M.N. Tahir, M. Khalid, A. Islam, S.M. Ali Mashhadi, A.A.C. Braga, J. Mol. Struct. 1127, 766 (2017)
A.R. Pasha, M. Khalid, Z. Shafiq, M.U. Khan, M.M. Naseer, M.N. Tahir, R. Hussain, A.A.C. Braga, R. Jawaria, J. Mol. Struct. 1230, 129852 (2021)
G. Mustafa, I. Shafiq, Q.U.A. Shaikh, A. Mustafa, R. Zahid, F. Rasool, M.A. Asghar, R. Baby, S.M. Alshehri, M. Haroon, ACS Omega 8, 22673 (2023)
A. Ali, M. Khalid, K.P. Marrugo, G.M. Kamal, M. Saleem, M.U. Khan, O. Concepción, A.F. de la Torre, J. Mol. Struct. 1207, 127838 (2020)
M. Khalid, H.M. Lodhi, M.U. Khan, M. Imran, RSC Adv. 11, 14237 (2021)
M. Riaz, F. Jan, A. Khan, A. Ullah, H. Haq, A.F. AlAsmari, M. Alharbi, F. Alasmari, MominKhan, Arab. J. Chem. 16, 105259 (2023)
C. Weetman, S. Inoue, ChemCatChem 10, 4213 (2018)
P. Wu, P. Du, H. Zhang, C. Cai, J. Phys. Chem. C 116, 20472 (2012)
S.K. Seth, G.C. Maity, T. Kar, J. Mol. Struct. 1000, 120 (2011)
S.K. Seth, N.C. Saha, S. Ghosh, T. Kar, Chem. Phys. Lett. 506, 309 (2011)
S.K. Seth, S. Banerjee, T. Kar, J. Mol. Struct. 965, 45 (2010)
Acknowledgements
We are thankful to Instrumentation Centre, Punyashlok Ahilyadevi Holkar Solapur University, Solapur, Council for Scientific and Industrial Research-Indian Institute of Chemical Technology (CSIR-IICT), Hyderabad and Sophisticated Analytical Instrumental Facility-Common Facility Center (SAIF-CFC), Shivaji University, Kolhapur for spectral analysis. The authors are thankful to Maratha Mandal’s Central Research Laboratory, Belgaum for providing biological activities.
Funding
This research work has not received any funds.
Author information
Authors and Affiliations
Contributions
PRK and SSR analyzed the data and wrote the main manuscript, SSR worked on software, SSD supervised the work, All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kharade, P.R., Chougale, U.B., Gaikwad, D.S. et al. Synthesis and in vitro evaluation of tetrahydropyridines as potential CDK2 and DprE1 inhibitors. Res Chem Intermed 50, 1777–1808 (2024). https://doi.org/10.1007/s11164-024-05228-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-024-05228-2