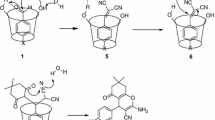
Abstract
Simple and green procedure was developed for the synthesis of various xanthene and coumarin derivatives using beta-cyclodextrin (β-CD) as reusable catalyst at 70 °C in water. Condensation of salicylaldehyde (1 mmol) and dimedone (2 mmol) or 1,3 cyclohexadione (2 mmol) gave corresponding xanthene derivatives, while condensation of salicylaldehyde (1 mmol) with Meldrum’s acid (1 mmol) or 4-Hydroxy-6-methyl-2H-pyran-2-one (1 mmol) gave respective coumarin derivatives in impressive yields. Involvement of β-CD as catalyst was ascertained by inclusion complex evaluation of β-CD-salicylaldehyde with 1H NMR analysis at 70 °C.
Graphic abstract







Similar content being viewed by others
References
A. Chaudhary, J.M. Khurana, Curr. Org. Synth. 15, 341 (2018)
P. Mane, B. Shinde, P. Mundada, V. Gawade, B. Karale, A. Burungale, Res. Chem. Intermed. 46, 231 (2020)
K.M. Khan, I. Khan, S. Perveen, M.I. Malik, J. Fluorine Chem. 158, 1 (2014)
D. Prasad, A. Preetam, M. Nath, C. R. Chimie. 16, 1153 (2013)
L. Nagarapu, S. Karnakanti, R. Bantu, R. Bantu, B. Sridhar, Synth. Commun. 42, 967 (2012)
J.M. Khurana, K. Vij, J. Chem. Sci. 124, 907 (2012)
H.F. Niya, N. Hazeri, M. Fatahpour, M.T. Maghsoodlou, Res. Chem. Intermed. 46, 3651 (2020)
D. Thirumalai, S. Gajalakshmi, Res. Chem. Intermed. 46, 2657 (2020)
M.M. Heravi, P. Ansari, M. Saeed, N. Karimi, N. Tavakoli-Hosseini, Bull. Chem. Soc. Ethiop. 25, 315 (2011)
M. Salami, A. Ezabadi, Res. Chem. Intermed. 46, 4611 (2020)
S.E.S.M.M.A. SorkhiHashemiEzabadi, Res. Chem. Intermed. 46, 2229 (2020)
E. Yoshioka, S. Kohtani, H. Miyabe, Angew. Chem. Int. Ed. 50, 6638 (2011)
B.S. Kuarm, J.V. Madhav, S.V. Laxmi, B. Rajitha, Y.T. Reddy, P.N. Reddy, P.A. Crooks, Synth. Commun. 41(12), 1719–1724 (2011)
G. Sabitha, K. Arundhathi, K. Sudhakar, B.S. Sastry, J.S. Yadav, Synth. Commun. 38, 3439 (2008)
P. Zhang, Y. Yu, Z. Zhang, Synth. Commun. 38, 4474 (2008)
X. Wang, D. Shi, Y. Li, H. Chen, X. Wei, Z. Zong, Synth. Commun. 97, 35 (2005)
D.M. Pore, T.S. Shaikh, K.A. Undale, D.S. Gaikwad, C. R. Chimie. 13, 1429 (2010)
D. Shi, Y. Wang, Z. Lu, G. Dai, Synth. Commun. 30, 713 (2000)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517 (1993)
K.A. Ohemeng, C.F. Schwender, K.P. Fu, J.F. Barrett, Bioorg. Med. Chem. Lett. 3, 225 (1993)
A. Mitra, S.K. Misra, A. Patra, Synth. Commun. 10, 915 (1980)
S. Kumar, R. Girl, S.C. Mishra, M.K. Machwe, Spectrochim. Acta, Part A 51, 1459 (1995)
W.C. Sun, K.R. Gee, R.P. Haugland, Bioorg. Med. Chem. Lett. 8, 3107 (1998)
H.V. Chavan, B.P. Bandgar, ACS Sustainable Chem. Eng. 1, 929 (2013)
N.N. Karade, S.V. Gampawar, S.V. Shinde, W.N. Jadhav, Chin. J. Chem 25, 1686 (2007)
G. Sabitha, N. Fatima, E.V. Reddy, J.S. Yadav, Adv. Synth. Catal 347(10), 1353–1355 (2005)
A. Shaabani, R. Ghadari, A. Rahmati, A.H. Rezayan, J. Iran. Chem. Soc. 6, 710 (2009)
D.-Q. Shi, Y. Zhou, S.-F. Rong, Synth. Commun. 39, 3500 (2009)
K. A. Undale, D. S. Gaikwad, T. S. Shiakh, U. V. Desai, D. M. Pore, Indian J. Chem., Sect B 51B, 1039 (2012)
H. Xinwei, S. Yongjia, Z. Yao, Y. Zhiyu, H. Guang, J. Wenjing, C. Jiaojiao, Tetrahedron 71, 863 (2015)
J. Safaei-Ghomi, Z. Akbarzadeh, R. Teymuri, Res. Chem. Intermed. 45, 3425 (2019)
M.H. Sayahi, S. Bahadorikhalili, S.J. Saghanezhad, M.A. Miller, M. Mahdavi, Res. Chem. Intermed. 46(1), 491–507 (2020)
M. Torabi, M. Yarie, F. Karimi, M.A. Zolfigol, Res. Chem. Intermed. 291–16(2020).
G. Crini, Chem. Rev. 114, 10940 (2014)
W. Zhao, Q. Zhong, J. Incl. Phenom. Macrocycl. Chem. 72, 1 (2012)
K. Takahashi, Chem. Rev. 98, 2013 (1998)
Y. Chen, L. Yu, Chem. Soc. Rev. 39, 495 (2010)
A. Patil, S. Gajare, G. Rashinkar, R. Salunkhe, Catal. Lett. 150, 127 (2020)
S. Sadjadi, M.M. Heravi, M. Daraie, Res. Chem. Intermed. 43, 843 (2017)
M.J. Nasab, A.R. Kiasat, Res. Chem. Intermed. 44, 2719 (2018)
N. Mohammadian, B. Akhlaghinia, Res. Chem. Intermed. 45, 4737 (2019)
M.R. Bhosle, S.A. Joshi, G.M. Bondle, J.N. Sangshetti, Res. Chem. Intermed. 46, 737 (2020)
R. Kardooni, A.R. Kiasat, N.E. Sabzi, Res. Chem. Intermed. 46, 1857 (2020)
Acknowledgement
We gratefully acknowledge the financial support from the University Grants Commission (UGC), New Delhi, India for BSR-SAP fellowship No.F.7-183/2007(BSR), Dated 27th Jan. 2014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kamat, S.R., Mane, A.H., Patil, A.D. et al. Synthesis of xanthene and coumarin derivatives in water by using β-Cyclodextrin. Res Chem Intermed 47, 911–924 (2021). https://doi.org/10.1007/s11164-020-04308-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04308-3