Abstract
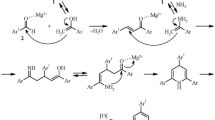
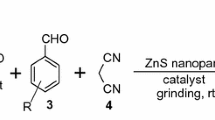
Design and development of a heterogeneous nanocatalyst for condensation reaction of acetophenone derivatives, aromatic aldehydes, and ammonium acetate to yield 2,4,6-triarylpyridines followed by microwave irradiation is described. Nanocrystalline MgAl2O4 as a novel heterogeneous recyclable catalyst shows high activity for the above reaction. In addition, the easily recoverable nanosized catalysts showed good reusability.

The design of a heterogeneous nanocatalyst for condensation reaction of acetophenone derivatives, aromatic aldehydes, and ammonium acetate to yield 2,4,6-triarylpyridines followed by microwave irradiation is described here. Nanocrystalline MgAl2O4 as a novel heterogeneous, recyclable catalyst shows high activity for the above reaction.


Similar content being viewed by others
References
De La Hoz A, Diaz-Ortiz A and Moreno A 2004 Curr. Org. Chem. 8 903
Clark D E, Folz D C and West J K 2000 Microstruct. Proc. 287 153
Bougrin K, Loupy A and Soufiaoui M 2005 J. Photochem. Photobiol. C6 139
Ju Y H and Varma R S 2005 Org. Lett. 7 2409
Kabalka G W, Wang L, Namboodiri V and Pagni R M 2000 Tetrahedron Lett. 41 5151
Kim B Y, Ahn J B, Lee H W, Kang S K, Lee J H, Shin J S, Ahn S K, Hong C I and Yoon S S 2004 Eur. J. Med. Chem. 39 433
Enyedy I J, Sakamuri S, Zaman W A, Johnson K M and Wang S 2003 Bioorg. Med. Chem. Lett. 13(3) 513
Pillai A D, Rathod P D, Franklin P X, Patel M, Nivsarkar M, Vasu K K, Padh H and Sudarsanam V 2003 Biochem. Biophys. Res. Commun. 301(1) 183
Klimešová V, Svoboda M, Waisser K, Pour M and Kaustová J 1999 Il Farmaco 54(10) 666
Constable E C, Housecroft C E, Neuburger M, Phillips D, Raithby P R, Schofield E, Sparr E, Tocher D A, Zehnder M and Zimmermann Y 2000 J. Chem. Soc. Dalton Trans. (13) 2219
(a) Krohnke F and Zecher W 1962 Angew. Chem. Int. Ed. 1(12) 626; (b) Krohnke F 1976 Synthesis 1 626
Zhao L X, Moon Y S, Basnet A, Kim E, Jahng Y, Park J G, Jeong T C, Cho W J, Choi S U, Lee C O, Lee S Y, Lee C S and Lee E S 2004 Bioorg. Med. Chem. Lett. 14 1333
Chubb F, Hay A S and Sandin R B 1953 J. Am. Chem. Soc. 75(23) 6042
Dilthey W 1921 J. Prakt. Chem. 102(8–10) 209
Lombard R and Stephen J P 1958 Bull. Soc. Chim. Fr. 1458
Zecher W and Krohnke F 1961 Chem. Ber. 94(3) 690
Frank R L and Seven R P 1949 J. Am. Chem. Soc. 71(8) 2629
(a) Potts K T, Cipullo M J, Ralli P and Theodoridis G 1981 J. Am. Chem. Soc. 103(12) 3584; (b) Potts K T, Cipullo M J, Ralli P and Theodoridis G 1981 J. Am. Chem. Soc. 103(12) 3585
Kobayashi T, Kakiuchi H and Kato H 1991 Bull. Chem. Soc. Jpn. 64(2) 392
Adib M, Tahermansouri H, Koloogani S A, Mohammadi B and Bi-janzadeh H R 2006 Tetrahedron Lett. 47 5957
Palacios F, de Retana A M O and Oyarzabal J 1996 Tetrahedron Lett. 37(26) 4577
Verma A K, Koul S, Pannu A P S and Razdan T K 2007 Tetrahedron 63 8715
Kumar A, Koul S, Razdan T K and Kapoor K K 2006 Tetrahedron Lett. 47 837
Borthakur M, Dutta M, Gogoi S and Boruah R C 2008 Synlett (20) 3125
Cave G W V and Raston C L 2001 J. Chem. Soc., Perkin Trans. 1 3258
Zomordbakhsh S, Anaraki-Ardakani H, Zeeb M, Sadeghi M and Mazraeh-Seffid M 2012 J. Chem. Res. 36(3) 138
Cave G W V and Raston C L 2000 Chem. Commun. 2199
(a) Smith C B, Raston C L and Sobolev A N 2005 Green Chem. 7(9) 650; (b) Smith N M, Raston C L, Smith C B and Sobolev A N 2007 Green Chem. 9(11) 1185
Heravi M M, Bakhtiari Kh, Daroogheha Z and Bamoharram F F 2007 Catal. Commun. 8(12) 1991
Davoodnia A, Bakavoli M, Moloudi R, Tavakoli-Hoseini N and Khashi M 2010 Monatsh Chem. 141(8) 867
Nagarapu L, Peddiraju A R and Apuri S 2007 Catal. Commun. 8(12) 1973
Maleki B, Azarifar D, Veisi H, Hojati S F, Salehabadi H and Yami R N 2010 Chin. Chem. Lett. 21(11) 1346
Ren Y M and Cai C 2009 Monatsh. Chem. 140(1) 49
Montazeri N and Mahjoob S 2012 Chin. Chem. Lett. 23 419
Shinde P V, Labade V B, Gujar J B, Shingate B B and Shingare M S 2012 Tetrahedron Lett. 53 1523
Montazeri N, Ayoubi S F, Pourshamsian K and Bashtini F 2012 Oriental J. Chem. 28(1) 303
Reddy K S, Reddy R B, Mukkanti K, Thota G and Srinivasulu G 2011 Rasayan J. Chem. 4(2) 299
Davoodnia A, Razavi B and Tavakoli-Hoseini N 2012 E-J. Chem. 9(4) 2037
Maleki B, Salehabadi H, Sepehr Z and Kermanian M 2011 Collect. Czech. Chem. Commun. 76 1307
Mohammad Shafiee M R, Moloudi R and Ghashang M 2012 APCBEE Proc. 1 221
Mohammad Shafiee M R and Moloudi R 2011 J. Chem. Res. 35(5) 294
Banerjee S and Sereda G 2009 Tetrahedron Lett. 50 6959
Navaei Alvar E, Rezaei M, Navaei Alvar H, Feyzallahzadeh H and Yan Z F 2009 Chem. Eng. Commun. 196(11) 1417
Baudin C, Martinaz R and Pena P 1995 J. Am. Ceram. Soc. 78(7) 1857
Ganesh I, Bhattacharjee S, Saha B, Johnson R, Rajeshwari K, Sengupta R, Ramana Rao M and Mahajan Y 2002 Ceram. Int. 28(3) 245
Fairhurst C W 1992 Adv. Dent. Res. 6 78
Thomé L, Gentils A, Jagielski J, Garrido F and Thomé T 2007 Vacuum 81(10) s
Koroleva L 2004 Glass. Ceram. 61(9) 299
Huang X Q, Li H X, Wang J X and Jia X F 2005 Chin. Chem. Lett. 16(5) 607
Chiu C, Tang Z and Ellingboe J W 1999 J. Comb. Chem. 1(1) 73
Acknowledgement
The authors gratefully acknowledge the financial support from the Research Council of the University of Kashan (Grant No. 256722/17), Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
SAFARI, J., GANDOMI-RAVANDI, S. & BORUJENI, M.B. Green and solvent-free procedure for microwave-assisted synthesis of 2,4,6-triarylpyridines catalysed using MgAl2O4 nanocrystals. J Chem Sci 125, 1063–1070 (2013). https://doi.org/10.1007/s12039-013-0477-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0477-8