Abstract
Progressively and projected integration of rare earth metals (REMs) in modern technologies, especially in the clean energy, consumer electronics, aerospace, automotive, and defense sectors, place REMs as critical raw materials in the supply chain and strategic metal from the fourth industrial revolution perspective. Current REM production from the primary mineral resources in the supply chain versus industrial demand is at a bottleneck. Alternatively, REM-bearing anthropogenic wastes are pertinent and potent to addressing the critical supply chain bottleneck. Although secondary REM resources are prudent to address the critical supply chain bottleneck, the absence of effective and efficient technologies to recover these REMs from anthropogenic waste imposes challenges and provides opportunities. Hence, this review analyses and discusses the significance of anthropogenic wastes for REM recovery, the status of recycling technologies for sustainable valorization of REMs, challenges, and opportunities. The current review covers the potential quantitative REM wealth locked in various anthropogenic waste like (i) spent rare earth permanent magnets, (ii) spent batteries, (iii) spent tri-band REM phosphors, (iv) bauxite industry residue red mud, (v) blast furnace slag and (v) coal mines, and coal byproducts and status of valorization technologies for circularizing the REMs. In industrial waste like red mud, steelmaking slag, blast furnace slag, and coal fly ash typically 109,000, 2000, 39,000, and 354,000 tons of REM get scrapped, respectively, in a conservative estimation. In the years 2020 and 2021, respectively, 240,000 and 280,000 tons of REM were produced by mine production in contrast to 504,000 tons of REM that were scrapped with REM-bearing industrial waste. This review revealed that total REM currently getting scrapped with anthropogenic waste versus projected REM demand for the years 2022, 2023, 2024, and 2025 could be standing at 2.66, 2.51, 2.37, and 2.23, respectively. Our investigation revealed that efficient recovery of REMs from anthropogenic waste is significant and promising but associated with challenges like lack of industrial-scale valorization process, lack of a clear strategy, road map, policy, effort, funding, and diversified research.
Similar content being viewed by others
1 Introduction
Rare earth metals (REMs) are progressively being integrated into modern technologies, especially in the clean energy, consumer electronics, sectors, aerospace, automotive, and defense. The REMs are not only getting integrated into various industrial technologies but also accelerating supply chain criticality driven by the fourth industrial revolution (4IR), clean energy demand, and environmental regulations. The 4IR is very much driven by data associated with connected devices, faster wireless internet, big data, and artificial intelligence which needs core technologies like connectivity, IT hardware, and software, enabling technologies for domains like consumer goods, services, vehicles, healthcare, industrial, home, infrastructure, and agriculture (https://www.epo.org/, 2020; David et al. 2022). Demand for REMs in each of these sectors is expanding and in some industries the growth is exponential, hence, the supply chain constantly getting bottlenecked. Current REM production from the primary mineral resources in the supply chain versus industrial demand is at a bottleneck and places REM as critical raw material in the supply chain and strategic metal from the fourth industrial revolution perspective.
China is monopolizing the global REM supply chain as the single most producer of these critical raw materials (CRMs) which accounts for 58% and 60% for the years 2020 and 2021, respectively (Gambogi 2021; Cordier 2022). China is not only a global leader in REM mine production but also REM reserve in China accounts for 37% of worldwide REM mine reserves (Gambogi 2021). Currently, the entire demand for REMs by various industries is mainly met by mine production as hardly less than 1% of REM is being recycled (Graedel et al. 2011). Progressive demands and lack of good distribution of resources around world-leading not only to supply chain discrepancies but also potentially put various industries and countries at a competitive disadvantage position. Considering all these factors the European Commission (EC) in its critical raw materials strategy continuously in the first (2011), second (2014), and third (2017) reports have identified REMs as critical raw materials (CRMs) with the highest risk of the supply chain (Commision 2017). Similarly, the US Department of Energy (DOE) (Energy 2011) and the American physical society (APS) (Jaffe et al. 2011) in the strategic metal report have emphasized REM as CRM for green energy and emerging technologies.
Challenges associated with REM-like strategic importance, industrial irreplaceability, sustainable alternative green energy, environmental carbon footprint control, geopolitical scenario, and REM supply chain monopoly; can be decisively addressed through circularizing REM from various anthropogenic waste. Hence, technological advancement for the sustainable valorization of REM from anthropogenic waste should be the panacea.
Anthropogenic waste, in general, can be divided into two parts, i.e., (i) end-of-life (EOL) urban waste and (ii) industrial waste. Detailed classification of the REM bearing anthropogenic waste is presented in Fig. 1. As exhibited in Fig. 1, the context of REM bearing anthropogenic waste broadly can be divided into EOL urban waste and REM bearing industrial waste. REM bearing EOL products like electronic waste, waste catalysts, optical lenses, and rare earth permanent magnets (REPM) from various equipment can be classified as EOL urban waste. REM bearing industrial waste is also classified into two types, depending upon the generation process, i.e., (i) industrial process and end waste, and (ii) consumer product manufacturing waste. During the manufacturing of various consumer products for end uses, various process waste gets generated, which can be considered as consumer product manufacturing waste. The REM-bearing waste produced during rare earth permanent magnets (REPM) production, REM refining plant wastewater, and REM mines wastewater can also be classified as manufacturing waste. Similarly, in general, during the industrial metallurgical process, power generation process, and catalytic cracking process, REM bearing process waste gets generated as a by-product, or end waste products can be classified as industrial process and end waste. Spent fluid catalytic cracking catalysts (FCC) which are extremely important in the modern petroleum refining industry use REM mainly made from La and Ce zeolite because of its high activity, selectivity, thermal stability, hydrothermal stability, and toxicity resistance (Palos et al. 2018; Lu et al. 2020). The spent FCC contains about 3% REMs oxide (Lu et al. 2020). REM-bearing alumina industry waste red mud, iron, and steel industry waste blast furnace slag (BFS), coal mines wastewater, and coal byproducts can be classified as industrial process and end waste. Considering the similarity of EOL urban waste and consumer product manufacturing waste, in the current review sustainable valorization of rare earth metal from anthropogenic waste focusing on EOL urban waste and industrial process and end waste has been reviewed.
USGS reported, globally driven by demand REM oxide production from primary resources continuously increased year by year and during the last five years, respectively, 147, 190, 210, 240, and 280 thousand tons of REM oxide were produced in the year 2017, 2018, 2019, 2020, and 2021, from primary resources (Gambogi 2020; 2021; Cordier 2022). In the year 2020, worldwide REM consumption for various end uses was 38%, 23%, 13%, 8%, 9%, 5%, and 3% respectively for REPM, catalysts, glass polishing powder, and additives, metallurgy and alloy, battery alloy, ceramic and pigments, and phosphorus (https://www.nrcan.gc.ca/2020).
REPM is mainly used in information technology devices and battery alloy main used in electrical vehicles that are mainly driven by consumer demand and those consumer goods are also associated with rapid up-gradation and diversification. As majoring of REM are being used for consumer goods, considering the EOL of the respective product, all those REMs or REM bearing scraps enter the waste stream after a certain year(s) of time lag. The time lag depends upon which device/consumer good and how long it is being used. Hence, all these REM bearing urban scrap can be rich secondary resources. Similarly, Swain et. al has reported globally that 4 billion tons of red mud which contains 0.5–1.7 kg/ton of REMs have been stockpiled by 2020 (Swain et al. 2020b), and the same red mud being generated, annually between 120–150 million tons worldwide mainly goes for stockpiling. Kasina and Michalik have reported each ton of BFS could contain 0.12–14 kg/ton of REMs (Kasina and Michalik 2016). To put in perspective, quantitative estimation of REM gets slagged with BFS can be calculated from worldwide iron and steel production data. USGS reported in the year 2020, that worldwide 310–380 million tons of iron slag and 180–270 million tons of steel slag were generated (Ober 2021). USGS also reported in the year 2021 global iron slag and steel slag production was estimated to be 340–410 million tons and 190–280 million tons, respectively (Tuck 2022). The combination of the information above is indicative of plausible BFS as secondary resources for REM. Harris et al. have reported, that in the year 2017 worldwide 1.1 billion tons of coal combustion product that safely can consider coal fly ash was generated (Harris and Feuerborn 2020). Franus et al. have reported each ton of coal fly ash could contain 0.1–54 kg/ton of REMs (Franus et al. 2015). Ferella et al. have reported worldwide FCC supply is estimated at 840,000 tons/year (Ferella et al. 2016; Ye et al. 2017). Lu et al. have reported modern petroleum refining industries are crapping 210,000 tons of FCC annually (Ye et al. 2017; Lu et al. 2020). All the explained data suggest that efficient recovery of CRMs like REMs from anthropogenic waste is much more important and promising but associated with challenges like lack of industrial-scale valorization process, lack of a clear strategy, road map, policy, effort, funding, and diversified research. The significance, novelty, and objective of the review are described in the significance and novelty section below.
2 Significance and novelty of review over REM valorization from anthropogenic waste
The EOL waste material contains a higher REM concentration compared to primary resources whence a significant volume of industrial waste is generated with a moderate or small amount of REM content. The volume of industrial waste and concentrated resources from urban mining provides significant opportunities for addressing the REM supply chain bottleneck. Simultaneously, REM valorization through urban mining and REM recovery from industrial waste also imposes significant challenges. For techno-economic industrial recovery of REM from EOL scrap, the volume of raw material is very important for the efficient circular economy, hence, efficient waste management and collection play a vital role. Although waste management policies and resource recycling policies for EOL scrap are available in some developed countries, the same is not true for the majority of the countries around the world (Domenech and Bahn-Walkowiak 2019; Ferronato and Torretta 2019). Every other nation consumes but the nonexistence of REM waste management processes and policies in most parts of the world also imposes substantial challenges despite the rich resources of REM. Hence, from waste to wealth vision and to achieve sustainable development goals, starting from policy development to process development, understanding the current state of REM bearing anthropogenic waste generation is essential and the current review addresses those fundamental aspects. The current review revealed that industrial-scale, efficient recovery economically feasible technology hardly available for EOL scraps hinders the valorization of REM for the circular economy. Also. the review indicated that laboratory-scale technology development may not be completely neglected and still not in a significantly advanced state that can fill the gap between lab-scale investigation and industrial-scale REM recovery from EOL waste (Ilankoon et al. 2022). As the volume of EOL waste as raw material is limited and technology for economic feasibility is rarely available, hardly grabs the attention of the metallurgical industry, unlike primary resources. Hence, sustainable REM waste management and recycling process technology for EOL scrap need of the hour and need to be developed.
Unlike EOL scrap, industrial waste like red mud, blast furnace slag, coal mines, and coal byproducts volume or input raw material are not scarce but the efficient industrial-grade technology for REM recovery (Deng et al. 2022). Not only technology but also the scarcity of precise data regarding REM content in the above industrial waste product, the exact volume of waste generation, and the lack of country-wide data accelerated the concern. The current review also highlights the abundance of REM wealth in the industrial waste whence, relevant data like REM content on individual industrial waste, the volume of waste being generated, and the fate of generated waste are hardly available which is the rudimentary stage for the policy or process development. Though some of the researchers report plausible economic feasibility, an equally opposite conclusion also hinders industrial recovery interest (Innocenzi et al. 2016b; Joseph et al. 2019; Deng et al. 2020; Ilankoon et al. 2022). Through, dedicated research and precise data on the context of industrial-scale REM recovery and economic impact can address the concern. The current investigation also reviewed the reported review literature, and the details are summarized in Table 1. Investigation of the literature and reported review (Table 1) indicated that extensive research on the integrated sustainable valorization of REM from anthropogenic waste lacks the following aspect, i.e., (i) quantitative estimation of volume waste generated, (ii) discussion on the prudent potential of anthropogenic waste for addressing CRMs issue, (iii) possible recommendation for bridging the gap among industry requirement and policy deficit, and (iv) lack of recommendation addressing research development and real-life REM criticality issue. The significance and objective of the current review are highlighted below.
-
(i)
Reviews published elsewhere (Binnemans et al. 2013, 2018) in the field are almost a decade old, considering rapid waste generation and sustainable development an update in the information is essential, hence, reviewed over recent and if any advances developments.
-
(ii)
Fundamental difference and classification of waste in term of REM-bearing EOL and industrial waste has been introduced, and the broader characteristics of waste from REM content and the amount of waste generation which has been lacking in the literature has been discussed.
-
(iii)
Essentially potential of each waste in the ideal circular economy scenario has been highlighted and their possible contribution to resolving the supply chain criticality of REM has been analyzed.
-
(iv)
Status of technology and drawback associated with efficient management of REM bearing waste and constraint for REM circular economy has been discussed.
3 Sustainable valorization of REM from anthropogenic waste: challenges
To learn well challenges and opportunities associated with sustainable valorization of REM from anthropogenic waste, three important aspects of REM, i.e., (i) production of REM from primary resources, mineral reserve, and its distribution, (ii) current demand, estimated future demand for REM and its distribution according to end-use and (iii) possible substitute from a technology efficiency perspective and status of recycling of each category need to be well understood. All the explained aspects are discussed below individually.
3.1 Global mine production, reserve, and distribution
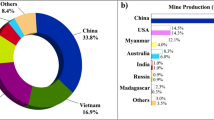
Detailed analysis of REM oxide worldwide mine production during 2010–2020, country-wise production for the year 2020, and country-wise reserve data known by the year 2020 is presented in Fig. 2. From the U.S. Geological Survey (USGS) data on the global mine production of REM oxide for the year 2010–2020 is summarized in Fig. 2a and indicates 130, 130, 110, 110, 123, 124, 129, 130, 190, 220, and 240 thousand tons respectively (Gambogi 2020; 2021). The same USGS also reported global REM oxide mines production data for the years 2015–1920 were 124, 129, 130, 190, 220, and 240 thousand tons, respectively (Gambogi 2018). The mass of countrywide REM oxide mine production depicted in Fig. 2b for the year 2020, indicates China is the largest producer of REM oxide production accounting for 58% of world mine production amounting to 140 thousand tons.
The same Fig. 2b indicates from a supply chain perspective worldwide 58, 15.5, 12.3, and 7% of REM were produced by China, the USA, Myanmar, and Australia, respectively (https://www.nrcan.gc.ca/2021), accounting for 140, 38, 30, and 17 thousand tons. The data presented in Fig. 2b indicates not only does China monopolize the REM oxide production but also approximately 93% of worldwide REM oxide production is concentrated in those four countries only. Global REM oxide reserve data known by the year 2020 presented in Fig. 2c indicates Chinese primary resources REM oxide reserve is 44 million tons accounting for 38% of worldwide REM oxide reserve. The next four major REM oxide reserve-holding countries are Brazil, Vietnam, Russia, and India accounting for 19%, 18%, 10%, and 6% respectively. The major reserve of REM oxide accosting 91% of the worldwide reserve is concentrated in the above five countries only. The rate of global REM oxide production for the 2011–2020 decades and projected demand for the near-term future between the year 2021–25 indicates REMs are at a critical metal bottleneck in the global supply chain. The dominance and monopoly of China on reserve, production, import, and utilization of value-added products put global competitiveness and better business incentives in jeopardy (Wübbeke 2013; Golev et al. 2014).
3.2 REM current, future demand, and its distribution according to end-use
Detailed analysis of projected REM oxide demands for the years 2021–2025, distribution of REM oxide consumption by the end uses of the year 2019, and distribution of projected REM oxide consumption by the end uses by the year 2018 has been analyzed and depicted in Fig. 3. REM oxide consumption and distribution of projected REM oxide data were collected from web references of https://www.statista.com/ (Garside 2020; 2021), and https://www.nrcan.gc.ca/ (https://www.nrcan.gc.ca/2020). Although when the manuscript was revised the REM oxide consumption data has been updated but reported values are different and observed discrepancies mainly because of Coronavirus disease (COVID-19) related interruption during the year 2020. Because the COVID 19 is a one-of-its-kind disruption it may not reflect the usual trend hence, the updated data has not been considered for the current analysis. Projected REM oxide demand presented in Fig. 3a for the years 2021–25 are 242, 256, 271, 287, and 305 thousand tons, respectively (Garside 2020). REM oxide demand data indicates the exponential rate of growth between the year 2016–2025. Figure 3b depicts the distribution of REM oxide consumption by end uses for the year 2019. The figure indicated that in the year 2019, 38% of global REM production was used for manufacturing rare earth permanent magnets (REPM) signifies the single largest consumption (Garside 2020; 2021). Followed by REPM, catalyst, glass polishing industry, metallurgical industry, battery manufacturing, and phosphors producing industry consumed 23, 13, 8, 9, and 4% REM oxide of global production. The same was 5% for ceramic, pigment, and glaze manufacturing.
a Projected global REM oxide demand for the year 2022–2025, b Distribution of REM consumption in the year 2019, and c projected sector-wise REM oxide distribution by the year 2028 (Garside 2021; https://www.nrcan.gc.ca/, 2021, 2022; https://www.statista.com/)
Figure 3c depicts the forecasted distribution of REM oxide consumption by end uses for the year 2028. The forecasted consumption indicates the market share of REM oxide for REPM could reach 68% of global REM oxide production, which is 79% growth only for REPM just in 8–9 years (https://www.statista.com/2022). All other applications like the catalyst, glass polishing industry, metallurgical industry, battery manufacturer, and phosphors producing industry consumption could be only 21%. On the contrary, the replaceability of REM oxide with other materials either compromises desired functional characteristics or is expensive, hence, industrial substitution in the near-term future is hardly an option. Hence, the valorization of REM oxide-bearing secondary resources like anthropogenic waste could be a sustainable and much-needed alternative. The forecasted exponential growth of REM oxide consumption is mainly driven by rare earth permanent magnets (REPM). Among all (end users) EOL REM bearing products such as permanent magnets, fuel cracking catalysts (FCC), battery alloy, metallurgical alloy, and phosphorous, are fundamentally feasible for valorization. Other (end users) EOL REM bearing products like glass polishing and ceramics may be hard for valorization. Permanent magnet production not only consumes the largest share of global REM oxide produced but also progressively going to consume a higher % and the largest share of global REM oxide to be produced.
3.3 REM bearing anthological waste and status of recycling
A detailed list of REMs bearing EOL urban waste has been summarized in Table 2. Table 2 shows in a vast range of products REMs are being used for their superior functionality. The table also indicated most of the REM bearing EOL urban waste is mainly associated with electronics waste (e-waste). The recent report on the global e-waste monitor 2020 reported in the year 2019 is mainly 17.4 Mt of small equipment, 13.1 Mt of large equipment, 6.7 Mt of screens and monitors, 4.7 Mt of small IT and telecommunication equipment, and 0.9 Mt of lamps were scrapped (Forti et al. 2020). The same report also indicated that from 2014 to 2019, e-waste comprising large equipment, and lamps and small equipment increased by 5%, and 4%, respectively (Forti et al. 2020). Global distribution for that e-waste generated per capita for the year 2019 across the content is as follows; 13.3 kg in the Americas, 16.3 kg in Europe, 16.1 kg in Oceania, 5.6 kg in Asia, and 2.5 kg in Africa (Forti et al. 2020). In the absence of REM bearing EOL waste data in particular from the above four sets of information, i.e., Table 2, amount of e-waste generated, rate of increasing e-waste generation, and per capita e-waste generation around the globe, massive EOL waste generation is indicated. All the information on e-waste together suggests that the EOL life waste is not only an environmental problem but also a prudent secondary resource for the various metal in general and REM in particular. From the listed urban waste in Table 2, it can be reasonably concluded that through proper waste management like collection strategy, segregation, and transportation strategy followed by valorization, the REMs being scrapped along with EOL life products can be brought back to the material flow stream. From an economy and process development perspective, a quantitative understanding of REM content on the listed EOL product is essential.
Table 3 lists the content of REMs in the various anthropogenic waste. Table 3 exhibits, individual REM in industrial waste varies significantly, which is purely contributed by the geological origin of the minerals and mining products for either metal production purposes or energy production purposes. The same table also indicates that the red mud, iron, and steel industry slag, coal mines, and coal ash scraps reasonably fair amount of REM in the context of the critical nature of REMs in the supply chain. REM content is summarized in Table 3 from various resources reasonably concluded that red mud, steel making slag (SMS), BFS, electric arc furnace dust (EAF), ladle furnace slag (LFS), coal mines, and coal fly ash scraps 0.5–1.7 kg/ton, 12.14–11.94 kg/ton, 127–145 kg/ton, 41.56 kg/ton, 62.31 kg/ton, 0.29–1134 kg/ton of REM respectively. Literature investigation indicated that globally that 4 billion tons of red mud have been stockpiled by 2020 and the same is being generated, annually between 120 and 150 million tons worldwide that mainly get stockpiled (Swain et al. 2020b). USGS has reported in the year 2020, globally 310–380 million tons of iron slag and 180–270 million tons of steel slag were generated (Ober 2021). USGS also reported in the year 2021 global iron slag and steel slag production was estimated to be 340–410 million tons and 190–280 million tons, respectively (Tuck 2022). Similarly, Harris et al. have reported, that in the year 2017 worldwide 1.1 billion tons of coal combustion products that are reasonable can be considered as coal fly ash was generated (Harris and Feuerborn 2020). Table 3 indicates from a quantitative REM content perspective the REM bearing EOL urban waste and industrial waste poses two different sets of challenges for REM value recovery. In the EOL urban waste usually, REM content is at a higher concentration side, and only selective REM elements are present. Selective REM present could be relatively easier for separation and purification and efficient valorization. Whence, in industrial waste usually REM content is at the lower concentration side and contains the whole range of REM at varying concentrations. Because of similarities in chemical properties of REM, when present all together in the industrial waste, the recycling process needs significant technological effort for the separation and purification of individual REM in the valorization process. Table 3 indicates both kinds of waste pose two distinct sets of challenges for the valorization of REM bearing anthropogenic waste.
3.4 Valorization of REM bearing anthological waste: Challenges
Despite explained importance and significance, the current global REM recovery status, resources recycling policy, and REM bearing anthropogenic waste exploitation scenario are purely miserable and neglected. Graedel et al. have reported that < 1% of REM-bearing waste is recycled, and even after decades, the rate of recycling for REM or REM valorization from secondary resources stands at no charge (Graedel et al. 2011). A recent report by U.S. Geological survey on Mineral commodity summarizes the recycling rate for REMs are limited (Cordier 2023). Chinwego et al. have reported a maximum REM recycling rate of 20% could be accomplished after a decade provided efficient recovery and a continuous growth trend could be achieved (Chinwego et al. 2022). Though the thousand tons of REM oxide flows to EOL and apparently to the waste stream, in the absence of technology, policy, and process, the critical REM gets scrapped. The amount of REM in EOL scraps could provide the opportunity to overcome the REM critical supply chain bottlenecks through proper valorization. Currently, valorization REM bearing EOL scrap is associated with several challenges like, (i) technology for recovery from EOL scrap is not well developed, (ii) Collection of urban waste and its management is not well organized and streamlined, (iii) several nations does not have a policy in place for recycling, and (iv) scarcity of data for the material flow of REM bearing EOL scrap. From the report of Graedel et al., it can reasonably be concluded that most of the REM bearing waste mainly follows the cradle (mines) to grave approach, rather than the cradle-to-cradle waste management and valorization approach (Graedel et al. 2011). Challenges for REM bearing EOL urban waste and industrial waste are not the same or similar, rather district and different. Challenges associated with valorization and the circular economy of REM from anthropogenic waste are summarized below.
-
(i)
Lack of precise data regarding the material flow of REM bearing EOL urban waste, collection strategy, segregation, and transportation strategy as a raw material for the recycling industry, and policy direction to bring back a certain amount or % of REM from EOL scrap. On the contrary industrial waste, collection, segregation, and transportation do not as issues as those that are readily available at the production site abundantly. Industrial waste faces precise assessment and data mining challenges for REM content in waste, the volume of REM bearing waste generation, and policy issues.
-
(ii)
The lack of industrial-scale process for selective recovery of REM or REM valorization, Lack of strategy for bringing back the REM from either waste to raw material stream or circular economy is equally challenging for both the waste.
-
(iii)
Available information, regarding waste generation, stockpiled, and REM content in respective waste are haphazard and literature reports are quite speculative and rather precise. The absence of waste-wide, country-wise, and industry-wide accurate and precise quantitative data related to REM bearing waste generation makes it more tedious to understand the REM worth in the anthropogenic waste.
-
(iv)
Serious efforts for REM value recovery on an industrial scale from anthropogenic waste. The literature on REM recovery from anthropogenic waste is limited which is not only counterproductive for REM but also a huge challenge for massive solid waste management challenges posed by this anthropogenic waste.
-
(v)
Bench-scale to industrial-scale REM recovery from anthropogenic waste is the biggest challenge needed not only to be addressed to CRMs supply chain issue but also challenges posed by these solid waste management issues.
4 Review of REM valorization technologies from anthropogenic waste
Several research reports have indicated that anthropogenic waste like spent REPM, spent batteries, phosphor powders, red mud, BFS, coal mines, and coal byproducts can be a sustainable alternative to primary resources. Hence, the current investigation reviews over challenges and opportunities for the sustainable valorization of REM from anthropogenic waste like (i) spent REPM, (ii) spent batteries, (iii) spent tri-band REM phosphors, (iv) alumina industry residue red mud, (v) BFS and (v) coal mines, and coal byproducts. Various aspect of sustainable valorization of REM from anthropogenic waste and associated challenges and opportunities has been discussed thoroughly in the below sections.
4.1 REM valorization through urban mining
4.1.1 REM recovery from EOL permanent magnets
Rare earth permanent magnet (REPM) materials can generally categorize into two types, i.e., high-performance permanent magnets mainly made from magnetic material based on Sm2(Co, Cu, Fe, Zr)17 otherwise called SmCo magnet and NdFeB permanent magnets primarily made from (Nd, Pr)2Fe14B (Goll and Kronmüller 2000; Wang et al. 2020). The NdFeB permanent magnet contains 30–40% REMs, of which 15–30% is Nd along with 2–5% Dy, Tb, and Gd, and the rest are Fe and B. In the NdFeB permanent magnet, Fe and B constitute up to 60–70 where B hardly up to 1% (Önal et al. 2015; Yang et al. 2016). Similarly, high-performance permanent magnets contain mainly Sm and with an atomic ratio of 1:5 or 2:17 depending on the requirement of magnetic property and operating temperature (Zhang et al. 2018; Palasyuk et al. 2021). Worldwide demand for NdFeB permanent magnet from 2018 to 2022 was 6.7 kt, 6.8 kt, 8.35 kt, 11.73 kt, and 16.14 kt, respectively (https://www.statista.com/2023). Worldwide production of NdFeB permanent magnet production in the year 2017 was 170 kt which includes high-performance permanent magnets. In the same year, the high-performance permanent magnet worldwide production was 60 kt. Out of worldwide 170 kt production, ~ 90% of NdFeB permanent magnet production had come from China alone which is 150 kt. Globally, TDK, Hitachi Metals, Shin-Etsu Chemical, and VAC are the leading producer of high-performance NdFeB permanent magnet accounts, with 50% of the market share (Markets June 2018). Although mid or low-performance permanent magnets dominate the consumer market emerging applications of permanent magnets for various applications in new energy vehicles, robots, and energy-saving household appliances projected demand more for high-performance NdFeB permanent magnets (Matizamhuka 2018). Whereas globally, China is the largest REPM producer owing to its availability of primary resources and as well as superiority production facilities. In the year 2017, China had produced 150kt of NdFeB permanent magnets mainly in the mid and low-end products segment, which account for 90% of (Markets June 2018) global productions (https://www.stanfordmagnets.com/).
Considering resource scarcity, and Chinese monopoly over REM, to develop the competitive advantage of the REM consuming industry, REM recovery from the end of life (EOL) REPM can be a lucrative option that can address the above issues simultaneously. This EOL REPM is a rich source of REMs such as Nd, Sm, and other REM although comparatively smaller content than Nd, and Sm. Besides, in the REPM manufacturing process, about 20–30% of process waste is generated and rejected as industrial sludge (Bian et al. 2015; Li et al. 2021). In the REPM manufacturing process, the aforesaid 20–30% get rejected during sequential vacuum melting, atomization, and quenching process which are difficult to reutilize under the given process design. These REPM manufacturing industrial sludge can also be rich secondary resources for REM recovery. Considering the criticality of supply chain scarcity, various REM valorization technique from REPM has been reported. The valorization REPM process commonly follows dismantling, physical separation for accessing the REPM from various discarded equipment, and a pretreatment process followed by the valorization process.
Physical separation of REPM is the primary difficulty that has been successfully addressed by Hitachi, Ltd., Tokyo, Japan. Hitachi, Ltd., has developed a complete integrated physical separation process for the separation of HDDs and compressors of air conditioners from discarded equipment. In the reported process by Hitachi, Ltd., before the pretreatment, for physical separation of REPM, the automated mechanical dismantling of the components from hard disks and compressors motors/ generators has been employed. A similar, valorization approach for recovery REM from MRI (magnetic resonance imaging equipment) has also been outlined (Haque et al. 2014). Hitachi has also developed a process to recover the NdFeB-based magnets from HDDs and compressors of air conditioners using a combination of vibration, demagnetization, and manual collection. Satio et al. have reported valorization of Nd from EOL NdFeB magnets through glass slag formation where the EOL NdFeB magnets melted with BO3 in an induction furnace where Nd gets completely slagged. From the Nd bearing slag, recovery of Nd through sequential sulfuric acid leaching followed by precipitation using sodium sulfate has been proposed (Saito et al. 2003, 2006). The same author also reported Sm recovery from Sm–Co magnets through glass slag formation with 99% efficacy (Tetsuji Saito et al. 2003; Saito et al. 2005). Takeda et al. have reported a scrap-to-scrap integration recovery process where Nd was recovered from NdFeB magnet through alloying with Mg at high temperature followed by hydrothermal treatment (Takeda et al. 2006). Effectively, Nd from the NdFeB magnet was alloyed with Mg as Nd-Mg followed by hydrothermal treatment using NaOH, H3PO4, HCl, and H2SO4 using both high temperature and pressure (Itakura et al. 2006). As reported above though pyro-metallurgical processes have been used dominatingly, still, pyro-metallurgical processes use subsequent processes like hydrogenation, oxidation, chlorination, liquid molten extraction, and electrolysis downstream. Though various pyro-metallurgical processes have been reported, none is fully developed for commercial exploitation. Alternatively, hydrometallurgical processes for the valorization of REPM have been reported in the literature (Binnemans et al. 2013; Sinha et al. 2017). Sinha et al. have reported the recycling of Sm–Co magnet by hydrometallurgical route, where a sequentially demagnetization-mineral acid leaching-purification process was followed (Sinha et al. 2017). In the reported process by Sinha et al., the magnet was demagnetized by roasting at 1123 K followed by leaching at the optimized conditions using 4 M HCl, and 100 g/L pulp density. Quantitative leaching of Sm and Co has been achieved (Sinha et al. 2017). Sn/Co from the leach liquor was separated by solvent extraction with Cyanex 572. Despite several developments, direct re-use of the magnets or alloys seems impossible, and substantial technological challenges exist for REMs recovery (Binnemans et al. 2013). Kaya et al. have reported NdFeB permanent magnet recycling by an alternate route sequential griding, sieving, acid baking, calcination, water leaching to recover Fe2O3 and purify REM and finally REM recovery by ultrasonic spray pyrolysis (Kaya et al. 2021). Chowdhury et al. analyzed the sustainable recycling aspect of NdFeB permanent magnet from a techno-economical-environment perspective (Chowdhury et al. 2021). Chowdhury et al. have investigated the techno-economical aspect of REM recovery by sequential acid-free Cu(NO3)2 leaching followed by REM recovery as REM oxalate and finally REM oxide valorization by calcination. Apart from process development studies for REM recovery from EOL permanent magnets, several authors also reported fundamental and lab-scale investigation by various techniques has been summarized in Table 4 below. Our literature investigation indicated recycling of NdFeB permanent magnets by hydrometallurgical route relatively extensive though pyrometallurgical and chemical metallurgy route is extensive and diversified. Table 4 represents comprehensive discussion only as it has limited implication on current review rather fundamental investigation. As extensive discussion over fundamental studies for REPM recycling is not scope of the study, it has been kept comprehensive rather extensive and focused on hydrometallurgical route.
From a review of the literature and discussion in the current inferred that recycling of permanent magnets usually follows the following stages, i.e., (i) demagnetization of REPM, (ii) size reduction by crushing, (iii) pulverization for getting a superior surface area that accelerates subsequent leaching, (iv) roasting or oxidative roasting, (v) mineral acid or organic acid leaching, (vi) solvent extraction by conventional solvent extraction using commercial extractant or solvent extraction by ionic liquids, (vii) REM recovery either by direct precipitation stripping using oxalic acid or mineral acid stripping followed by precipitation by oxalic acid and (viii) Finally, REM materialization as REM oxide (RE2O3) from REM oxalate. In some cases, further value addition of recovered REM oxalate or REM oxide by subsequent investigations has also been reported.
The leaching chemistry for NdFeB permanent magnet by oxidation roasting, mineral acids, and organic acid can be explained using the Equations given below. The chemistry associated with oxidative roasting NdFeB permanent magnet followed by commonly used mineral acids (H2SO4, HCl, and HNO3) leaching can be explained using Eqs. 1 to 4 given bellow (Emil-Kaya et al. 2023). Leaching chemistry for NdFeB permanent magnet with commonly used mineral acids (H2SO4, HCl, and HNO3) without roasting can be explained using Eqs. 5 to 7 given bellow. Similarly, leaching chemistry for NdFeB permanent magnet using Organic acids like, Acetic acid, Ascorbic acids, Glycolic acids, and Maleic acid can be explained using chemical Eqs. 8–9 given below (Liu et al. 2019).
In the above Eqs. 8–9, the OrgH and Org− are the organic component and cationic organic component, respectively.
The generalized process flowsheet represented in Fig. 4 implies that different mineral acids (H2SO4, HCl, and HNO3) have been employed for REPM leaching and followed by mineral acids (H2SO4, HCl and HNO3) leach liquor, the REM has been recovered by different commercial extractants like D2EPHA, PC 88A, and Cyanex 272. Hence, more generalized solvent extraction chemistry for REM recovery from sulfate, chloride, and nitrate media, respectively, using commonly used commercial extractant D2EPHA, PC 88A, and Cyanex 272 can be explained using the Eqs. 10–12 given below (Swain and Otu 2011).
In Eqs. 10–12, the double over bar on (HL)2 and single over bar on L− species represent dimeric HL and monomeric L− species, respectively. The HL represents cation exchange solvents such as D2EPHA, PC 88A, and Cyanex 272.
To make it straightforward and for easier understanding, more simplified solvent extraction chemistry for REM from sulfate, chloride, and nitrate media, respectively, using commonly used commercial extractant Cyanex 272 (for representative case only) can be explained using the Eqs. 13–15 given below. In Eqs. 13–15, the double over bar and single over bar represents the dimeric Cyanex 272 and monomeric Cyanex 272 species, respectively. In Eq. 13–15, the Cyanex 272 can be replaced with other extractants like D2EPHA and PC 88A.
Precipitation stripping of REM from explained loaded extractants in Eqs. 10–12 using oxalic acid and further valorization of REM oxalate to REM oxide can be explained using Chemical Eqs. 16–17 given below.
Based on the above studies the entire REPM recycling process flow diagram can be summarized as presented in Fig. 4. In the REPM reclining process like other e-waste recycling processes, the physical separation of EOL equipment is essential for the initial separation of metal, nonmetal, and reusable and to access the target material for recycling. Followed by the isolation, the isolated REPM can be recycled either by hydrometallurgical process or pyrometallurgical process. The generalized process flowsheet presented in Fig. 4 indicates the employed processes are mainly hybrid processes. Whereas several authors interpret the hybrid process either as a pure pyrometallurgical process or a pre-hydrometallurgical process, can be interpreted as inaccurate.
Based on Fig. 4, rather the process flowsheet should be classified as pyrometallurgy dominated hybrid process and as hydrometallurgy dominated hybrid process. In the pyrometallurgical-dominated hybrid process, the isolated REPM is either glass slagged or alloyed using various reagents, then the glass slag or alloy passes through the hydrometallurgical process. Similarly, In the hydrometallurgical dominated hybrid process, the isolated REPM is roasted or calcinated for demagnetization followed by the hydrometallurgical process being followed. In either process after glass slagging or alloying or demagnetizing the processed material gets leached mainly using mineral acids like HCl, HNO3, or H2SO4. Then the leached metal from leach liquor was purified mainly through solvent extraction using a selective extractant. Finally, from purified/separated metal solution either metal salt or metals recovered through precipitation or metallization, respectively.
4.1.2 REM recovery from EOL batteries
With the emergence of the lithium-ion battery, although the use of nickel-metal hydride (NiMH) batteries has gradually decreased, currently the NiMH batteries are being used in hybrid electric vehicles (HEV) significantly (http://www3.cec.org/2015). Driven by demand, stringent policies against fossil fuel-powered (internal combustion engine) automotive, and policy supports for hybrid electric vehicle (HEV) and electric vehicles (EV), the HEV and EV revolution getting accelerated. Though HEV and EV have their inherent advantages and disadvantages, considering the requirement either of higher power density or energy density, batteries like the lithium-ion battery or NiMH get selected for the purpose. HEVs are taking advantage of batteries with a higher power density and the gasoline rechargeable option and use preferably NiMH batteries (http://www3.cec.org/2015). The use of HEV continues to increase due to the higher demand and hence NiMH batteries attract attention for the efficient and cost-effective recycling process. Secondary portable nickel-metal hydride (NiMH) batteries contain a significant amount of REM, which varies in the range of 7–18% depending upon the type of cells. REM content in different types of NiMH batteries is summarized in Table 5 (Rodrigues and Mansur 2010). The anode material of NiMH batteries uses REM in the form of metal hydride (MH). In the anode material of NiMH batteries, the REMs are present either as A2B7 (LaCePrNdNiCoMnAl) or AB5 (LaCePrNdNiCoMnAl) or as AB2 (VTiZr-NiCrCoMnAlSn). Where the 'ABx' represents the ratio of the A metals (LaCePrNd or TiZr) to B metals (VNiCrCoMnAlSn) (Ruetschi et al. 1995).
The summarized report presented in Table 5 indicated that a significant portion of the anode in NiMH contains REM, hence recycling of REM from EOL NiMH batteries can also address supply chain contraptions. Like other waste valorization processes NiMH battery is also associated with the integration of processes like the removal of labels, the opening of casings, destruction of seals, separation of cathode and anode materials by mechanical cutting chopping/ vacuum milling/ cryo-grinding/ pyrolysis, and metal value recovery through hardcore metallurgy process (Meshram et al. 2019). Spent portable NiMH batteries have often been recycled using high-temperature/pyro-metallurgical processes to recover the Ni and Co. However, the high-temperature processes lead to a metal loss in the slag. Metals like Ni, Cr, and Mn find applications in steel production, whereas other metals like Zn and Cd owed to high temperature, lead to ash/ dust. The pure elements can either be supplied for the re-manufacturing of batteries or any other chemical/ metallurgical applications, and the slag/ash/dust is mostly used for construction purposes. In the pyro-metallurgical processes, only base metal gets recycled whence REMs have rarely been recycled and rather slagged, which is counterproductive in the context of REM scarcity (Binnemans et al. 2013). Considering the disadvantages like; energy-intensive processes, high cost for infrastructure, and negative carbon-footprint processes, pyrometallurgy, in general, is not preferable. Hence, further recovery of REMs from slag generally hydrometallurgy process being employed because of flexibility and versatility preference.
In the reported hydrometallurgy process leaching is the elementary step that uses mineral acid preferably sulfuric acid (Pietrelli et al. 2005; Rodrigues and Mansur 2010; Meshram et al. 2016) or hydrochloric acid (Zhang et al. 1998; Tzanetakis and Scott 2004) to extract metal values including REM from NiMH battery for further purification. Several hydro-metallurgical technologies reported in the literature recover metals from the spent NiMH batteries on a laboratory scale. Inorganic reagents such as hydrochloric acid (Zhang et al. 1998) and sulfuric acid (Pietrelli et al. 2002, 2005) or sulfuric acid with the addition of hydrogen peroxide as a reduction agent (Mantuano et al. 2006) were used for the dissolution of the black mass of the dismantled batteries. Pietrelli et al. have reported extraction of metals values from spent NiMH batteries using 2 M sulfuric acid, pulp density 100 g/L, leaching temperature 20 ℃, and leaching time of 2 h. Followed by REM recovery from the leached solution precipitation REM through pH control has been reported by Pietrelli et al. (Pietrelli et al. 2002). Like Pietrelli et al. process, Luiz et al. also have reported a very similar REM recovery route through sequential sulfuric acid leaching and REM precipitation through PH control using NaOH. Unlike Pietrelli et al. process, Luiz et al. have recovered Co and Ni by the solvent extraction process. After REM precipitation in the solvent extraction process, metal impurities were scrubbed out using di-2-Ethylhexyl phosphoric acid (D2EHPA), then used bis(2,4,4-trimethylpentyl) phosphinic acid (Cyanex 272) to separate Ni/Co from the solution containing Ni and Co (Rodrigues and Mansur 2010). Meshram et al. have reported quantitative leaching of both base battery metal and REMs using 2 M H2SO4 and pulp density of 100 g/L As reported through H2SO4 leaching 98.1% 98.4%, 95.5%, and 89.4% of Nd, Sm, Pr, and Ce has been achieved along with base metal recovery with 90% efficiency (Meshram et al. 2016). Meshram et al. have reported roasting power spent NiMH battery powder material followed by 2 stages of leaching for selective leaching of REMs and base metals. In the first stage REM, Ni, and Zn were selectively water leached with 91–94% efficiency. In the second stage, 74% Mn and 55% Co were leached using NaHSO3 with H2SO4 as lixiviant (Meshram et al. 2017). Abreu et al. and Namil et al. have recovered REM as NaREM(SO4)‧xH2O adding NaOH solution followed REM oxide recovery through metathesis (Abreu and Morais 2010; Um and Hirato 2016). Similar to Abreu et al. and Namil et al. report, Ahn et al. have also reported REM recovery from spent NiMH through sequential H2SO4 leaching followed by REM precipitation as (NaREM(SO4)2‧H2O) using concentrated NaOH (Ahn et al. 2020). Provazi et al. have reported metal value recovery from mixed spend batteries, where through a series of processes like grinding the waste of mixed batteries, reducing, and volatile metals elimination using an electric furnace, acid leaching, selective precipitation, and solvent extraction (Provazi et al. 2011). In their process spent batteries were dismantled and fed into a shredder to separate non-metal, metals, and active battery powder material. The separated black powder was acid leached to extract metal values, then sequentially followed two-stage precipitation and solvent extraction. Though mixed battery clinging through hydrometallurgy was an innovative attempt, an adverse result was observed for REM recovery (Provazi et al. 2011).
Reported research elsewhere (Ahn et al. 2020) indicates in the waste NiMH battery the active material mainly consists of nickel-oxo compound Ni(OH)2, NiO, NiOOH, Ni-REM alloys like Ni7Ce2, Ni7Nd2, and Co3Nd, Co-REM alloy like Co5Nd, and Co3Nd and La2O3. The leaching chemistry of nickel-oxo compound and La2O3 can be expressed using chemical Eqs. 18–21 (Ahn et al. 2019).
Similarly, leaching chemistry for Ni-REM alloys like, Ni7Ce2, Ni7Nd2, and Co3Nd, and Co-REM alloy like Co5Nd, and Co3Nd can be summarized by Eqs. 22 and 23, respectively.
Solvent extraction chemistry using D2EHPA and Cyanex 272 for extraction and purification Co and Ni can be explained using Eqs. 24–25 given below.
where \(\overline{\overline{{\left[ {\left( {{\text{HL}}} \right)_{2} } \right]}}}_{org}\) stands for D2EHPA and Cyanex 272 as both exist as a dimer in solution and \(\overline{L}\) stands for monomer anion of D2EHPA and Cyanex 272.
Similarly, precipitation reaction chemistry for REM using NaOH can be explained using Eq. 26 below.
From elsewhere reported studies (Ahn et al. 2019, 2020) and based on explained chemistry the entire NiMH recycling process flow diagram can be summarized as presented in Fig. 5. The process follows sheet follows the two-stage process, i.e., (i) mechanical sorting/separation process to access the electrode material, followed by a chemical metallurgy process to recover the metals values from the electrode material. The mechanical process follows: (i) remaining energy discharge, (ii) crushing and calcination, (iii) classification based on metal, non-metal, and electrode material, and finally access to the electrode material. From the electrode, material REM was recovered through a selective sequential chemical metallurgy process as follows(i) mineral acid leaching dominated by H2SO4, (ii) selective precipitation of REM as sulfate salt from Ni/Co sulfate solution (using NaOH), (iii) further dissolution of REM sulfate for carbocation and (iv) REM oxide recovery by calcination. Followed by REM recovery, from leach liquor Co and Ni were recovered by solvent extraction.
4.1.3 REM recovery from phosphor powders
Cathode ray tubes (CRT), fluorescent lamps (FL), and compact Fluorescent Lamps (CFL) contain tri-band REM phosphors which are responsible for white light emission from the lamp (Khazova and O'Hagan 2008; Safari et al. 2015). As CRTs are phased out, the use of CFLs and FLs has risen by > 90% (Tan et al. 2014). The REM content in REM phosphors of CFLs and FLs accounts for 23% amounts to 230 kg/t (Binnemans et al. 2013) (Tan et al. 2014). The REM phosphors are primarily a mixture of red, blue, and green (~ 55% red, 35% green, and 10% blue), though variation exists. Depending on the manufacturer and application requirements, different quantities of REMs are used in different phosphors. Y (mainly) and Eu constitute the red phosphor. The green phosphor contains ~ 10% Tb, and < 5% Eu is in the blue phosphor (Balachandran 2014; Lucas et al. 2015). As the mass of REMs in the phosphors of the waste FLs is high, which may reach up to 30–40 wt %, it is a potential source of REM and inherent potential recycling value. Commonly, the spent FLs/CFLs value recovery follows the separation of individual components of phosphors and the aqueous processing of the material in mineral acid/ alkali. As the scope for pyro-process is limited mainly hydro process being investigated to complete REM value recovery from REM phosphors. Leaching/selective leaching using mineral acid mainly HCl followed by solvent extraction processes using D2EHPA, PC-88A (2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester), or Cyanex 272 are often explored to separate and recover the REMs from REM phosphors.
Although limited, a couple of investigation on REM value recovery from REM phosphors has been reported, mainly of the hydrometallurgical route. Verma et al. have reported a two-stage selective acid leaching process, where 96% and 94% of Eu and Y, respectively, have been leached using 2 M HCl and 30% pulp density. In the second stage La, Ce and Tb have been extracted with 95%, 90%, and 70% efficiency respectively, using 6 M HCl from the first stage residue. The leached metals were subsequently separated by a solvent extraction process using a reagent, Cyanex 272 (Verma et al. 2013). Ippolito et al. have proposed REM recovery from FLs through alkali fusion followed by sulfuric acid leaching to recover REMs (Ippolito et al. 2017). Achievement of 99% of Y and Eu, 80% of Tb, 65% of La, 63% of Ga, and 60% of Ce recovery from the leach liquor by oxalic acid precipitation has been proposed (Ippolito et al. 2017). Tunsu et al. (Tunsu et al. 2016) have studied REM recovery through the leaching solvent extraction route in a bench-scale investigation. In their study, the powder sample of FLs followed an I2/KI leaching process to remove the Hg contaminant before the above-explained acid leaching and solvent extraction route. For Hg leaching, 0.025/0.05 M of I2/KI solution with pulp density of 20 and 40% w/v were employed. Followed by the residual sample was leached using nitric acid and from leach-liquor REM was separated by solvent extraction using Cyanex 923 (Tunsu et al. 2016).
The entire REM phosphors CRT/FL/CFL recycling process flow diagram can be summarized as presented in Fig. 6a. Like other waste recycling processes, the REM phosphors track process flow as follows, (i) mechanical separation process for accessing REM phosphors, pretreatment, leaching, solvent extraction, and REM recovery process. Figure 6a represents two different routes for REM oxalate recovery from leach liquor. In one process, the leach liquor was directedly precipitated using oxalic acid and further calcinated to get REM oxide. In another process, followed by leaching, the leach liquor gets treated by solvent extraction for better purification and selectivity then REM recovery follows the same REM oxalate precipitating and REM oxide recovery route.
Mei et al. (2009) reported an innovative approach for the separation of red, blue, and green phosphors in a two-stage selective solvent extraction process using 2-thenoyltrifluoroacetone (TTA) and chloroform in 1-pentanol (Fig. 6b). In the first stage, TTA is dissolved in heptane to extract the blue powder at alkaline pH. Potassium sodium tartrate (PST) and Na2CO3 were used as regulators. In the second stage, chloroform was used to extract the green phosphor into the organic phase leaving the red phosphor in the aqueous phase. The addition of a small quantity of 1-pentanol improved the separation efficiency. A report regarding the application of novel green extractants such as N, N-dioctyldiglycolamic acid (DODGAA) for recovery of REMs from fluorescent lamps has also been reported (Van Loy et al. 2017).
4.2 REM recovery from industrial waste
4.2.1 REM recovery from Red Mud
Red mud is a solid waste produced in Bayer's process of alumina production from bauxite contains major metal oxides like Fe2O3, Al2O3, and TiO2 and minor metals like V, Ga, Cr, P, Mn, Cu, Cd, Ni, Zn, Pb, Mg, Zr, Hf, Nb, U, Th, K, Ba, Sr, and REMs. Red mud contains REMs like La, Ce, Pr, Nd, Sm, Eu, Sc, and Y with a varied ratio depending upon geological origins (Swain et al. 2020b). Bauxite is the principal ore utilized for aluminum production through Bayer’s process, usually generating 4 to 6 tons of red mud for each ton of aluminum production (Kumar et al. 2006; Sinha et al. 2016). It is reasonable to explain here that the Bayer process primarily produces alumina, followed by the produced alumina used for aluminum production(Swain 2022). Red mud is a rich source of poly-metals that include rare elements such as Ti, V, Ga, and REMs such as Sc, La, Ce, Y, and Nd (Zhang et al. 2019). Typical red mud contains 121, 76, 114, 368, 28, 99, 21, 5, 22, 4, 17, 14, 4, 2, 14, and 3 g/ton of REMs like Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Er, Ho, Tm, Yb, and Lu, respectively. In general, typical red mud contains REMs ranging from 500 to 1700 g/ton. Though quantitatively minor, the value perspective of REMs in RM is significant. In comparison to the currently mined bastnäsite (carbonate-fluorides) and monazite (phosphates) ores, where the average REMs contents vary from 3000 to 13,000 g/ton (calculated from Binnemans et al. (Binnemans et al. 2018)). Compared to REM content in mined bastnäsite, the REMs concentrations in red mud are significant, and hardly could be neglected from a comparative quantity perspective. In the absence of efficient technology, most of the red mud remains stockpiled. Global red mud inventory is about 4 billion tons by 2020 and is generated 120–150 million tons per year worldwide. Analysis indicated that industrial-scale valorization could unlock approximately $4.3 trillion worth of REMs from stockpiled red mud (Swain et al. 2020b). A thorough investigation suggests that, currently, a sustainable industrial process for REM value recovery from RM is mostly non-existence though part of the problem is being addressed for base metal only on a laboratory scale.
Literature investigation indicated that several reports for recovery Sc, Y, and La have been reported mainly because of the cost of those metals and content, still, other REMs get any attention. Selective Sc recovery from red mud leach liquor by solvent extraction using D2EHPA (Di-(2-Ethylhexyl) phosphoric acid) has been reported by Wang et al. (Wang et al. 2013). The proposed process by Wang et al. mainly consists of sequential sulfuric acid leaching and then two stages of solvent extraction. As reported after red mud leaching with dilute sulfuric acid, Ti and Zr were separated by solvent extraction using Primene JMT. After Ti and Zr recovery, Fe and Ti were scrubbed using D2EHPA. Finally by controlling pH through NaOH solution, Sc(OH)3 was isolated. The isolated materials were dissolved in an acidic solution and then precipitated as Sc-oxalate. By calcination of Sc-oxalate, Sc2O3 has been recovered (Wang et al. 2013). Ochsenkühn et al. (Ochsenkühn et al. 1995) have also reported Sc as well as Y and La recovery from the red mud leach solution by combination sequential two stages, i.e., (i) ion exchange and (ii) solvent extraction process in lab-scale and then in a pilot scale. The process reported by Ochsenkühn et al. consists of pretreatment, acid leaching, ion exchanges, solvent extraction, and precipitation stripping. Before 1.5 M HCl leaching, the red mud was roasted with a mixture of NaKCO3 and Na2B4O7. From leach liquor, Fe, Al, Ca, Si, Ti and Na were separated by ion-exchange using Dowex 50W-X8 (ion exchange) resin and elution using 1.75 M HCl. In the second stage the loaded Dowex 50W-X8 resin was eluted using 6 M HCl to separate Sc, Y, and La. From elute chloride Sc, Y, and La mixture solution Sc was separated by solvent extraction using D2EPHA. From laded D2EPHA Sc(OH)3 precipitation was stripped by precipitation stripping. Through Ochsenkühn et al. not extended the investigation but taking strength from wang et al. (Wang et al. 2013) investigation, the Sc can be recovered as Sc2O3 from Sc(OH)3 precipitate through the same route. The reported process is quiet about Y and La separation/recovery, but reasonably those could be separated and recovered by solvent extraction as discussed elsewhere (Swain and Otu 2011; Saito et al. 2015; Swain and Tanaka 2018). Ochsenkühn et al. (Ochsenkühn et al. 2002) have also reported a pilot-scale Sc recovery from the red mud through a similar process investigated on the lab scale. The process developed by Ochsenkühn et al. and Wang et al. (2013) has been discussed extensively elsewhere (Swain et al. 2020b). In the context of REM recovery from, focusing on REM recovery from red mud, the process developed by Ochsenkühn et al. and Wang et al. (2013) discussed extensively elsewhere by Swain et al. (2020a) can be modified as presented in Fig. 7. An important drawback of the reported process is, that the process is limited to Sc only, other REM still gets neglected and enters to waste stream. The leaching, solvent extraction, Sc oxalate recovery and Sc oxide valorization chemistry from red mud can also be explained using chemical Eqs. 27–30 given below (Swain et al. 2020a).
Flow sheet for recovery of REM from red mud. The flow sheet has been borrowed from Swain et al. (2020a) and modified for the REM recovery perspective
4.2.2 REM recovery from blast furnace slag
In the ironmaking making when iron ore is mixed with flux (limestone and/or dolomite) and fuel (coke) in reducing conditions at a high temperature of more than 1500 °C, molten iron, and molten slag are produced. The molten slag knows as BFS. In the steelmaking process, 20–25% of materials slagged out as BFS. Approximately, 0.4 tons of BFS produced per ton of steel production. Worldwide BFS generation was 320–384 million tons during the year 2019 (Kim and Azimi 2020). Currently, 90% of produced BFS get utilized mainly in cement making and the rest of 10% (32–38 million tons annually) of BFS is disposed of in landfills (Kim and Azimi 2020). Several authors in the literature have reported BFS to contain REM along with other metals like niobium, tantalum, and titanium (Kim and Azimi 2020; 2021). Hence, BFS can be a contributor to REM supply chain scarcity.
Kim et. al. have reported Sc and Nd recovery from BFS by H2SO4 baking-water leaching, where BFS was mixed with concentrated H2SO4, and the mixture was baked. Then the acid-baked sample was water leached where 82.5% and 80.6% of Sc and Nd were extracted from BFS (Kim and Azimi 2020). The same author also reported a sequential carbothermic reduction-acid baking-water leaching process for REM recovery along with other metals (Kim and Azimi 2021). In their investigation, the optimum condition for carbothermic reduction was explored through Carbon to BFS mass ratio variation and temperature variation followed by the above-explained acid baking-water leaching process route were followed (Kim and Azimi 2021). Abhilash et al. have reported hot H2SO4 leaching followed by solvent extraction to extract and purify REMs. La, Ce, Nd, and Er recovery with 92%, 36%, 35%, and 52% efficiency were achieved using 1 M hot (95 °C) H2SO4 for pulp density of 1:5 (w/v) BSF versus H2SO4 from 17, 16, 44 and 4 mg/kg of La, Ce, Nd, and Er, respectively (Abhilash et al. 2017). Bisaka et al. have reported REM recovery from BSF by three classical leaching routes, (i) Caustic crack followed by REM leaching with HCl, (ii) H2SO4 baking followed by a water leaching, and (iii) Direct HCl leaching and compared all three-leaching process. Bisaka et al. have indicated direct HCl leaching was efficient and can leach 94–96% of total REM whence other leaching processes hardly can leach 49% of total REM (Bisaka 2016). Kasina et al. have investigated BSF to find out whether the BFS can be a potential source for REM and indicated the concentrations of REM in BFS could be inadequate for profitable recovery (Kasina and Michalik 2016).
4.2.3 REM recovery from Coal, coal mines, and coal by-products
Coal, coal mines, and coal byproducts such as coal combustion bottom ash are potential sources of REMs, particularly heavy REMs (HREMs). The concentration of total REMs in coal, coal mines, and coal byproducts is relatively lower than commonly used primary resources for commercial production. But the concentration of HREMs could be comparable to that found in current commercial sources. REM content in coal, coal mines and coal byproducts vary and depend upon geological origin. Franus et al. have reported coal fly ash in Poland's REM content vary from 101 to 543 ppm (Franus et al. 2015). Kolker et al. have reported US coal combustion fly ash contains 192–1667 ppm (Kolker et al. 2017). Baron has reported the average worldwide REM content in hard coal could be 60 ppm (Scott and Kolker 2019; Baron 2020). Kumari et al. have reported 1035 g.ton−1 of REM content in Indian-origin coal bottom ash (Kumari et al. 2019). Nevertheless, REM extraction from these resources is daunting and challenging. Considering current demand, the criticality of REM, REM content, and the unavailability of technology, researchers have contradicting views on the economic potential of REM in coal, coal mines, and coal byproducts. Although report regarding REM recovery of such resources is scarce, still some research report has been reported in the literature. Wen et al. have reported REM recovery from coal fly ash through mechanical grinding and alkali solution treatment followed by acid leaching, where 95% leaching efficiency has been achieved (Wen et al. 2020). Pan et al. have reported recovery of REM from coal fly ash through sequential magnetic separation, roosting, and acid leaching (Pan et al. 2020). A complete REM recovery from coal bottom ash process technology has been developed through HCl leaching- solvent extraction using Tris-2-ethylhexyl amine-precipitation using aqueous ammonia by Kumari et al. (2019). Similar to Kumari et al. (2019) reported process Tuan et al. have also reported REM recovery by leaching-solvent extraction route from coal bottom ash (Tuan et al. 2019). Zhang et al. have reviewed comprehensively REM recovery from coal-related material (Zhang et al. 2020a).
Based on the above reports proposed flow sheet for REM recovery from coal fly ash and coal bottom ash can be summarized as presented in Fig. 8a and b, respectively. Figure 8a exhibits recovery of REM from the coal fly ash by Sequential pretreatment-leaching-solvent extraction-acid stripping-oxalate precipitation- REM oxide calcination route. Figure 8a indicated REM enrichment in the coal fly ash is the rudimentary stage where REM in the coal fly ash can be enriched either by mineral floatation or by mechanical separation, preferably magnetic separation. Followed by REM enrichment calcination or roasting using Na2CO3 is essential which can help oxidation of REM content in enriched REM powder from coal fly ash. After Na2CO3 roasting, REM can be leached either by acid leaching or by caustic leaching using mineral acid or NaOH. As the commonly used process in hydrometallurgy, after leaching from an enriched sample the REM can easily be extracted or purified by solvent extraction using D2EPHA, Cyanex 272, or analogous. Purified REM from organic phases of the solvent extraction process can be stripped into the acid solution, and from REM oxalate can be recovered using oxalic acid. If oxalic acid is not desired product, the REM oxalate can be calcinated to isolate REM oxides for various end uses. Figure 8b exhibits that REM recovery from coal bottom ash can be achieved by leaching-solvent extraction-stripping-oxalate precipitation-REM oxide recovery route. Similarly, to REM recovery from coal fly ash, from the coal bottom ash REM recovery follows acid leaching using mineral acids for recovery of REM followed by solvent extraction using D2EPHA, Cyanex 272, or analogous extractant. Followed by, the purified REM in the organic phase, the REM gets stripped by acid and then precipitated as REM oxalate using oxalic acid. As explained the REM oxalate successfully can be converted to REM oxide for various end uses as required by the industry.
4.2.4 REM recovery from fuel cracking catalyst (FCC)
For FCC catalysts mainly CeO2 and La2O3 are used, (Zhao et al. 2017; Sposato et al. 2021) hence, several authors have reported both these metal recoveries from spent FCC catalysts (Innocenzi et al. 2016a; Zhao et al. 2017; Nguyen et al. 2018). Considering pollution and mass of FCC generation, Ce and La value recovery through the hydrometallurgical route mainly has been investigated. Zhao et al. have reported REM value recovery from spent FCC through sequential roasting-HCl leaching-solvent extraction (Zhao et al. 2017). In their process the FFC was first roasted at 750 °C followed by the roasted FCC was leached using 2 M HCl as lixiviant at 60 °C (achieved through optimization), where respectively 72.8% and86.4% leaching efficiency for La and Ce were achieved. From the FCC leach liquor, La and Ce were recovered through solvent extraction using D2EHPA and stripping using HCl. The author has also compared the extraction efficiency of D2EHPA and HEHEHP (2-ethylhexylphosphonic mono-2-ethylhexyl ester) and concluded D2EHPA is comparatively a better extractant than HEHEHP in the context of La and Ce recovery from leach liquor of FCC catalyst (Zhao et al. 2017). Ye et al. have reported REM recovery from FCC by solvent extraction using saponified 2-ethylhexyl phosphoric acid-2-ethylhexyl ester (EHEHPA)(Ye et al. 2017). Ye et al. have also followed sequential HCl leaching-solvent extraction using EHEHPA-stripping using the HCl route to recover REM from spent FCC (Ye et al. 2017). Lu et al. have reported La and Ce recovery from spent FCC by leaching and solvent extraction route, where HCl has been used as lixiviant and H2O2 as reductant. From leach FCC catalyst leach liquor using P507 as extractant and HCl stripping agent 90% recovery efficiency has been achieved (Lu et al. 2020). Nguyen et al. have reported La and Ce recovery from FCC by nitric acid leaching followed by solvent extraction using D2EHPA. Unlike Zhao et al. and Lu et al., Nguyen et al. have used 2 M nitric acid as lixiviant and leached at 80 °C. From nitrate leach liquor using a two-step solvent extraction process, the extraction efficiency of 89% and 72% for Ce and La, respectively (Nguyen et al. 2018). Sposato et al. have investigated La leaching behavior using all three mineral acids and concluded that acid type has hardly any effect rather than acidity and temperature of lixiviant play a vital role (Sposato et al. 2021).
Wang et al. have investigated REM recovery from FCC waste slag by acid leaching and selective precipitation (Wang et al. 2017a). Wang et al. have recovered REM through HCl leaching and followed a composite salt precipitation process and recovered NaRE(SO4)2. From the NaRE(SO4)2 salt through substitution reaction using NaOH followed by HCl in a two-step process, have recovered RECl3. Wang et al. also investigated an alternative route for REM recovery from FCC waste slag, where using a caustic conversion route by NaOH, initially, Al selectively recovered as NaAlO2 zeolite (Wang et al. 2017b). The residual REM bearing FCC waste slag has been leached by HCl. From the leach, liquor REM has been recovered as REM oxalate using oxalic acid. Sadeghi et al. have reviewed the available recycling technique for spent FCC catalyst and indicated in the literature the main two approaches, i.e., (i) acid leaching followed by solvent extraction and (ii) acid leaching followed by precipitation either as oxalate or sulfate followed by REM oxide and RECl3, has been used for recover REM values either from spent FCC catalyst or FCC waste slag (Sadeghi et al. 2018). Based on the above studies, the possible flow sheet for REM recovery from spent FCC catalysts can be summarized as given in Fig. 9. The process flow sheet depicted in Fig. 9 and the literature investigation indicated that hydrometallurgy plays a vital role in REM value recovery from the spent catalyst and the summarized process is also a proficient process for industrial application as the process follows a versatile leching-solvent extraction approach.
5 Valorization of REM bearing anthological waste: opportunities
Precise global quantitative information regarding various anthropogenic waste gets generated has hardly been reported in the literature. In the current study, from available information reported elsewhere, through a couple of assumptions, the mass of REM getting scrapped annually along with various REM bearing anthropogenic waste has been estimated and presented in Fig. 10. For the estimation of REM bearing EOL waste generation following assumptions were made, i.e., (i) ideally no EOL waste being recycled (one < 1% waste being recycled (Graedel et al. 2011), hence neglected), (ii) ideally total mass of REM gets consumed for REPM, battery, phosphorous and FCC manufacturing ultimately enters EOL waste stream (time lag between consumption to EOL has been neglected as progressively it would follow the consumption), and (iii) mass of REM consumption for each sector was calculated from primary production multiplying with the fraction being consumed for the respective sector. REM mine production data reported by USGS for the year 2020 has been considered for the purpose (Gambogi 2021). Sector-wise worldwide REM consumption data for the year 2020, which were 0.38, 0.23,0.13, 0.08, 0.09, 0.05, and 0.03 fraction of total mine production were used for the estimation purpose (https://www.nrcan.gc.ca/2020). Individual REM bearing EOL waste generation is equal to the REM being consumed in each sector in an ideal case considering the above assumption. Similarly, for REM bearing industrial waste generation following assumptions were made, i.e., (i) Conservative side of total REM content 11.94 mg/kg in SMS (Table 3) and 180 million tons of SMS generation for the year 2020 as reported by USGS data (Ober 2021) was considered for the estimation, (ii) Conservative side of total REM content 127 mg/kg in BFS generation (Table 3) and 310 million tons of iron slag generation USGS data (Ober 2021) was considered for calculation, (iii) EAF and LF have not considered for calculation as hardly classified information about their generation is available, and (iv) as total REM content in coal fly ash varies drastically, the average value of REM content in fly ash 322 mg/kg (Table 3) was considered for estimation using total coal fly ash generation data 1.1 billion ton reported by Harris and Feuerborn (2020). For the red mud, worldwide 120 million tons generation which is a conservative estimation has been reported elsewhere (Swain et al. 2020a) considered, and the total REM 909.3 mg/kg (Table 3) in the red mud was used for calculation purposes. As red mud and coal fly ash are generated mainly from mega plants, waste generation data is assumed to be the same for the year 2020, though the reference year is different.
Estimation of a current REM getting scrapped with anthropogenic waste, b REM getting scrapped along with various anthropogenic waste as a function of primary mine production, and c comparison of the projected year-by-year REM demand versus REM scrapped along with anthropogenic waste in a typical year (the year 2020)
The amount of REM gets scrapped along with REPM, battery, FCC and phosphorus was calculated using mathematical Eq. 33, and the amount of REM get scrapped along with red mud, SMS, BFS, and coal fly ash was calculated using mathematical Eq. 33 and 34, respectively, given below. The amount of REM scrapped along with individual anthropogenic waste accounted for a fraction or factor of total mine production were calculated using mathematical Eqs. 35 and 36 respectively, given below. Using data obtained from Eqs. 33 and 34, a graph for REM in the waste versus anthropogenic waste was constructed and presented in Fig. 10a. Similarly, using data obtained from Eqs. 35 and 36, a graph for % REM in the waste versus anthropogenic waste was constructed and presented in Fig. 10b.
Figure 10(a) depicts global scrapped REM along with various anthropogenic waste typically per annum, in this particular case, it is for the year 2020. The figure indicated that REM gets scrapped along with industrial waste far greater than EOL consumer waste. In the EOL consumer goods like REPM, battery, FCC, and phosphorous typically 91,000, 22,000, 55,000, and 7000 tons of REM get scrapped, respectively, in a conservative estimation. The estimation is representative only of the mass of REM getting scrapped along with EOL worldwide. As the presented data in Fig. 10a for the year 2020 only, and projected demand for the years 2022–2025 (presented in Fig. 3a) are estimated to be increasing progressively and significantly, the REM bearing EOL waste should follow the exact trend as production of REM from primary mine resources and EOL life scrap generation is a function of REM bearing consumer goods demand. Currently, the stated correlation of demand driven EOL waste generation and primary mine REM productions holds good because hardly any REM bearing EOL waste gets recycled and contribution from the circular REM metal economy is non-existent. Figure 10a reveals an opportunity for REM metal circular economy from EOL urban resources, which could be conservatively 173,000 tons per year. The same opportunity can be progressively followed by REPM and battery demand as these sectors are projected to expand rapidly. The same figure also indicated that significantly higher REM mass gets scrapped with various industrial waste compared to REM-bearing EOL waste. In industrial waste like red mud, SMS, BFS, and coal fly ash typically 109,000, 2000, 39,000, and 354,000 tons of REM get scrapped, respectively, in a conservative estimation. The estimation of REM bearing industrial waste is representative only in a conservative calculation. The estimated REM mass in REM bearing industrial waste should hold good because the represented waste is getting generated in the mega metallurgical plant and mega power generation plant. Unlike EOL waste generation, the possibility of significant change in REM bearing industrial waste generation is limited. Figure 10a also revealed that the total REM mass gets scrapped annually with REM bearing industrial waste could be 504,000 tons which are significantly higher than REM bearing EOL waste, the same is true for annual mine REM production. As explained earlier in the year 2020 and 2021, respectively, 240,000 and 280,000 tons of REM were produced by mine production contrary to 504,000 tons of REM that were scrapped with REM bearing industrial waste. Hence, Fig. 10a, quantitatively reveals the wealth of REM gets scrapped along with anthropogenic waste in the absence of feasible industrial-scale REM recovery technology which is a huge opportunity for the REM circular economy. Figure 10b otherwise quantifies the REM circular economy potential from anthropogenic industrial waste in an ideal quantitative resources recycling scenario. Figure 10b indicates the ratio of REM getting scrapped with anthropogenic waste versus primary REM mine production (the year 2020) was 2.84 times. The same figure also indicated that the said ratio for REPM, battery, FCC, phosphorus, red mud, SMS, BFS, and coal fly ash are 0.38, 0.09, 0.23, 0.03, 0.46, 0.01, 0.16, and 1.48 respectively. Figure 10c compares the mass of projected REM demand for the years 2022–25 versus the current mass of REM (the year 2020) getting scrapped with anthropogenic waste. Figure 10c exhibits that even though the current rate of REM waste generation remains constant, still projected demand for REM could remain far less than the current rate of REM waste generation. One should not confuse it with the constant total REM value which is chosen for comparison only. In the realistic scenario, the total REM waste generation should be progressive mainly driven by EOL waste generation. Considering the nature of the mega plant, in the absence of the new metallurgical plant and power generation plant, the REM generation could remain constant. Considering the size, large capital investment and time required for the commission of mega plants, the near future estimation of REM bearing industrial waste has been given in Fig. 10c and should hold good. The calculation for current total REM getting scrapped with anthropogenic waste versus projected REM demand for the years 2022, 2023, 2024, and 2025 (Fig. 3a) could be standing at 2.66, 2.51, 2.37 and 2.23, respectively.
The strength of the above estimation is in absence of precise information about REM-bearing anthropogenic waste and its REM content, it can provide overall information regarding REM wealth being scrapped with these wastes. As the estimation considers the conservative approach and the previous year's data, the chance of data discrepancy would be minimum. The weakness of the estimation is the accuracy of anthropogenic waste generation and their REM content on each waste considered from minimally diversified resources, in the event of error assorted with information resources can add uncertainties to the estimation significantly. Figure 10 revealed the following information, i.e., (i) anthropogenic waste can be an absolute alternative to primary mine resources for REM demand, (ii) Ideally, in resources recycling and a perfect circular economy scenario, anthropogenic waste potentially can resolve REM supply chain bottleneck, and (iii) anthropogenic waste are potent and prudent resources can address the REM criticality issue and in an ideal situation turns supply chain bottleneck to supply chain abundance.
6 Advanced and sustainable alternative technology for valorization REM bearing anthropogenic waste
All REM recovery processes discussed in the above section are mainly associated with pyrometallurgy, hydrometallurgy, chemical metallurgy, or hybrid processes. The pyrometallurgical process for recovery from REM from anthropogenic waste is an energy and capital-intensive process which have a higher carbon footprint and is not sustainable from the circular economy and waste valorization perspective. Similarly, the hydrometallurgical process or chemical metallurgical process has a higher proportion of chemical use which has adverse effects on the environment and occupational safety. Hence, various researchers are looking for advanced and sustainable alternative technology for REM bearing anthropogenic waste valorization. Literature suggests that biohydrometallurgy and solvometallurgy could be sustainable alternatives for REM valorization from REM bearing anthropogenic waste if industrially feasible, economically viable, and sustainable. The research report on biohydrometallurgy for REM recovery from anthropogenic waste is limited in the open literature and is progressively being investigated extensively by several authors, only the current scenario appropriate for REM recovery from anthropogenic waste has been discussed in the section on biohydrometallurgy of REM, below. As solvometallurgy for REM recovery from anthropogenic waste has rarely been investigated extensively, only the current scenario for the same has been discussed in the section solvometallurgy for REM, below.
6.1 REM valorization from anthropogenic waste by biohydrometallurgy
In biohydrometallurgy, extracting metals from either primary or secondary resources like REM bearing anthropogenic waste, bioleaching is a rudimentary process and potentially environment friendly and economically favorable (Jin et al. 2019). In bioleaching, microbial release/solubilize/extract the metal either from primary resources like ore or alternative sources like anthropogenic wastes. Unlike the hydrometallurgy process, the application, and utilization of microbial for bioleaching rarely needs any hazardous chemical or environment-deteriorating chemicals. The same process is also less energy-intensive and cost-effective. Unlike the pyrometallurgy process, the bioleaching process does not have a higher carbon footprint, energy efficient contrary to the energy-intensive process. Like the pyrometallurgical process, the bioleaching process does not need capital-intensive plant construction, hence, the bioleaching process is more sustainable than the pyrometallurgical process. Hence, several authors have reported bioleaching of REM from various anthropogenic wastes which have been reviewed in the next paragraph (Castro et al. 2021).
Table 6 summarizes the bioleaching studies of REM extraction from various anthropogenic wastes. In general, in bioleaching Pikovskaya phosphate medium is used as a medium for growing the microbial and bioleaching activities. For the specific purpose of REM extraction from various anthropogenic waste various modified mediums were used for purpose of sustainability and reviewed in Table 6. Table 6 also summarizes the advantages and disadvantages of respective studies. Table 6 reviews have been reviewed over both EOL life waste and industrial waste. From the reported research through review, it has been observed that EOL anthropogenic waste is associated with various limitations like slow kinetics, substantially limited pup density utilization, poor leaching efficiency, lack of selective leaching, and lack of extensive investigation. Most of the REM bearing EOL anthropogenic waste bioleaching studies are limited to lab-scale study limited with extensive industrial scale investigation and operations. The review indicated that bioleaching studies from industrial waste like red mud are limited to only some geographical origins rather than extensively covered. Red mud bioleaching is also associated with challenges like limited pulp density utilization and lack of industrial scale investigation. The summary of the review depicted in Table 6 indicated issues like higher pulp density utilization, industrial scale investigation, REM recovery efficiency, and REM metal selectivity challenges needed to address for the successful sustainable valorization of REM from anthropogenic waste.
6.2 REM valorization from anthropogenic waste by solvometallurgy
Solvometallurgy is a branch of extractive metallurgy very similar to hydrometallurgy but unlike hydrometallurgy does not use a distinct aqueous phase for metal extraction. Solvometallurgy may not be an alternative technology to pyrometallurgy, hydrometallurgy, and chemical metallurgy rather complementary technology to existing technologies that use alternative green solvents can eliminate toxic and environmentally hazardous solvents (Binnemans and Jones 2017). Solvometallurgy is an emerging metallurgical technology being studied in limited laboratories around the world and being utilized for REM recovery from various resources including anthropogenic waste. As the report regarding REM recovery by solvometallurgy is limited, briefly the application solvometallurgy for REM has been discussed. Orefice et al. have reported REM recovery process flowsheet from NdFeB permanent magnets by solvometallurgical process (Orefice and Binnemans 2021). The reported solvometallurgical process was a sequential leaching and solvent extraction where pyridine hydrochloride was used as lixiviant followed by selective extraction of Dy and Nd extraction using 30 vol% PC 88A and 100% PC 88A, respectively.
The solvometallurgical process reported by Orefice et al. elsewhere has been borrowed and modified and depicted in Fig. 11. The figure indicated by pyridine hydrochloride non-aqueous lixiviant the Nd, Dy, and Fe can be quantitively leached from EOL NdFeB permanent magnet, followed by either Dy or Nd can be separated from pyridine hydrochloride non-aqueous leach liquor with limited selectivity by controlling PC 88A concentration, finally, from loaded PC 88A, the respective REM can be precipitated using the stochiometric amount of oxalic acid. After REM recovery, the Fe impurities from pyridine hydrochloride non-aqueous solution can be scrubbed using ammonia. Metal-free pyridine hydrochloride non-aqueous solution can be regenerated for reuse using HCl washing. Though the solvometallurgical process addressed REM extraction and lixiviant regeneration challenges but provide limited information about separation factor, selectivity, and scale-up challenges. The reported research is only limited to laboratory scale investigation using only 250 mg EOL NdFeB permanent magnet and 2.5 g of pyridine hydrochloride non-aqueous lixiviant. As quantitative leaching and quantitative extraction could be achieved at high temperatures and high extractant concentrations, respectively, several engineering and occupational challenges needed to address.
Flowsheet for the valorization of EOL NdFeB permanent magnet by solvometallurgy, borrowed from Orefice and Binnemans (2021) and modified
The same author (Orefice et al.) also reported metal value recovery from SmCO permanent magnet by solvometallurgy (Orefice et al. 2019). The uniqueness of the SmCO permanent magnet valorization study by solvometallurgy is the mixture of Ethylene glycol and HCl (wt 37%) was used for lixiviant leaching of metals including Sm. Followed by Alamine 336 was used as solvent extraction of Co, Fe, and Cu. Followed by Co, Fe, and Cu extraction, the Sm was extracted using Cyanex 272. From loaded Cyanex 272 Sm was recovered by precipitation stripping using oxalic acid. A complete flowsheet for the recovery process has been discussed by Orefice et al. (2019) and as solvometallurgical aspect and uniqueness is limited to leaching only, it has been discussed briefly only. Although research reports regarding solvometallurgy for REM are not rare in the context of REM, valorization of anthropogenic waste by solvometallurgy is limited. Hence, the current review has been kept limited to REM valorization from anthropogenic waste by solvometallurgy only.
7 Conclusion
Estimation of the mass of REM getting scrapped with anthropogenic waste indicated a far greater amount of REM getting scrapped concerning worldwide REM mine production annually. In the year 2020 total of 680,000 tons of REM was scrapped along with REM bearing anthropogenic waste whence in the same year total of 240,000 tons of REM was produced from primary mining resources. The ratio of global REM gets scrapped versus REM mine production was 2.84, which indicated the abundance of REM in anthropogenic waste despite the current supply chain bottleneck and criticality of REM. Ratios of current total REM getting scrapped with anthropogenic waste versus projected REM demand for the years 2022, 2023, 2024, and 2025 are 2.66, 2.51, 2.37, and 2.23, respectively, clearly indicating the REM bearing anthropogenic waste even can also address future REM demand, despite the REM demand increases progressively. Despite REM abundance in the anthropogenic waste in the current scenario concurrently three opposite factors i.e. (i) lack of industrial-scale recycling and proficient technology for REM valorization from waste resources, (ii) progressive demand for REM for consumer products, and (iii) geographical REM reserve and production disparity accelerate the REM supply chain criticality. The supply chain criticality of REM can efficiently be addressed through the techno-economical sustainable valorization of REM from anthropogenic waste. As REMs are essential without any alternative for future green and sustainable technologies, sustainable valorization of these secondary resources is essential. Review and above discussion over various technology available indicates broadly hybrid processes, either hydrometallurgical dominated process or pyrometallurgical dominated can address the technological gap and industrial-scale REM recovery issue. More precisely, considering REM content in the respective waste, volume of waste generation, cost, and energy, either a hydrometallurgical dominated process or pyrometallurgical-dominated hybrid process should be suitable for mass production scale industrial recovery of REM from REM bearing EOL waste. Whence, for REM bearing industrial waste, a pure hydrometallurgical process or hydrometallurgical dominated process should be preferable considering the volume of waste generation, REM content in the respective waste, and energy intensiveness to manage the massive industrial waste. REM bearing anthropogenic waste is prudent and a potential secondary resource for REM. The current investigation revealed that in an ideal resource recycling and circular economy scenario the REM-bearing anthropogenic waste like EOL urban waste and industrial waste can address the supply chain bottleneck, REM monopoly, and geological REM reserve discrepancies. Despite the potential, currently, REM recovery from secondary resources like anthropogenic waste has rarely been exploited, although future green and sustainable technologies are getting accelerated and project future promise. From the review challenges associated with accurate and precise data regarding REM bearing waste generation, precise data for REM content in the anthropogenic waste, waste generation data related to REM bearing EOL waste, and sincere effort for efficient industrial scale have been observed. Hence, extensive and diversified research for sustainable REM valorization technology development on the industrial scale for the circular economy of anthropogenic waste is recommended.
References
Abhilash A, Meshram P, Sarkar S, Venugopalan T (2017) Exploring blast furnace slag as a secondary resource for extraction of rare earth elements. Miner Metall Process 34:178–182. https://doi.org/10.19150/mmp.7857
Abreu RD, Morais CA (2010) Purification of rare earth elements from monazite sulphuric acid leach liquor and the production of high-purity ceric oxide. Miner Eng 23:536–540. https://doi.org/10.1016/j.mineng.2010.03.010
Ahn N-K, Swain B, Shim H-W, Kim D-W (2019) Recovery of rare earth oxide from waste NiMH batteries by simple wet chemical valorization process. Metals 9:1151. https://doi.org/10.3390/met9111151
Ahn NK, Shim HW, Kim DW, Swain B (2020) Valorization of waste NiMH battery through recovery of critical rare earth metal: a simple recycling process for the circular economy. Waste Manag 104:254–261. https://doi.org/10.1016/j.wasman.2020.01.014
Akcil A, Ibrahim YA, Meshram P, Panda S (2021) Hydrometallurgical recycling strategies for recovery of rare earth elements from consumer electronic scraps: a review. J Chem Technol Biotechnol 96:1785–1797. https://doi.org/10.1002/jctb.6739
Antonick PJ, Hu Z, Fujita Y et al (2019) Bio- and mineral acid leaching of rare earth elements from synthetic phosphogypsum. J Chem Thermodyn 132:491–496. https://doi.org/10.1016/j.jct.2018.12.034
Azevedo DMF, Silva JAS, Servulo EFC, Frescura VLA, Dognini J, Oliveira FJS (2019) Recovery of lanthanides from hydrocarbon cracking spent catalyst through chemical and biotechnological strategies. J Environ Sci Health A Tox Hazard Subst Environ Eng 54:686–693. https://doi.org/10.1080/10934529.2019.1579539
Balachandran G (2014) Case study 1—extraction of rare earths for advanced applications. In: Seetharaman S (ed) Treatise on process metallurgy. Elsevier, Boston, pp 1291–1340
Baron R (2020) Determination of rare earth elements content in hard coal type 31.1. Manag Syst Prod Eng 28:240–246. https://doi.org/10.2478/mspe-2020-0034
Behera SS, Parhi PK (2016) Leaching kinetics study of neodymium from the scrap magnet using acetic acid. Sep Purif Technol 160:59–66. https://doi.org/10.1016/j.seppur.2016.01.014
Bian Y, Guo S, Jiang L, Tang K, Ding W (2015) Extraction of rare earth elements from permanent magnet scraps by FeO–B2O3 flux treatment. J Sustain Metall 1:151–160. https://doi.org/10.1007/s40831-015-0009-5
Binnemans K, Jones PT (2017) Solvometallurgy: an emerging branch of extractive metallurgy. J Sustain Metall 3:570–600. https://doi.org/10.1007/s40831-017-0128-2
Binnemans K, Jones PT, Müller T, Yurramendi L (2018) Rare earths and the balance problem: how to deal with changing markets? J Sustain Metall 4:126–146. https://doi.org/10.1007/s40831-018-0162-8
Binnemans K, Jones PT, Blanpain B, Van Gerven T, Pontikes Y (2015) Towards zero-waste valorisation of rare-earth-containing industrial process residues: a critical review. J Clean Prod 99:17–38. https://doi.org/10.1016/j.jclepro.2015.02.089
Binnemans K, Jones PT, Blanpain B, Van Gerven T, Yang Y, Walton A, Buchert M (2013) Recycling of rare earths: a critical review. J Clean Prod 51:1–22. https://doi.org/10.1016/j.jclepro.2012.12.037
Castro L, Blazquez ML, Gonzalez F, Munoz JA (2021) Biohydrometallurgy for rare earth elements recovery from industrial wastes. Molecules. https://doi.org/10.3390/molecules26206200
Chinwego C, Wagner H, Giancola E, Jironvil J, Powell A (2022) Technoeconomic analysis of rare-earth metal recycling using efficient metal distillation. JOM 74:1296–1305. https://doi.org/10.1007/s11837-021-05045-7
Chowdhury NA, Deng S, Jin H, Prodius D, Sutherland JW, Nlebedim IC (2021) Sustainable recycling of rare-earth elements from NdFeB magnet swarf: techno-economic and environmental perspectives. ACS Sustain Chem Eng 9:15915–15924. https://doi.org/10.1021/acssuschemeng.1c05965
Commision E (2017) Study on the review of the list of Critical Raw Materials Critical Raw Materials Factsheets Publications Office of the European Union. European Commision, Brussels
Cordier DJ (2022) Mineral Commodity Summaries : Rare earths, 2022. U.S. Geological Survey, https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-rare-earths.pdf
Cordier DJ (2023) Mineral Commodity Summaries : Rare earths, 2022. U.S. Geological Survey, https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-rare-earths.pdf
Harris D, Feuerborn J (2020) Global aspect on coal comustionn products In Book Global aspect on coal comustionn products Vol 10. Editor, (eds). VGB Power tech, https://www.vgb.org/
David LO, Nwulu NI, Aigbavboa CO, Adepoju OO (2022) Integrating fourth industrial revolution (4IR) technologies into the water, energy & food nexus for sustainable security: a bibliometric analysis. J Clean Prod 363:132522. https://doi.org/10.1016/j.jclepro.2022.132522
Deng B, Wang X, Luong DX, Carter RA, Wang Z, Tomson MB, Tour JM (2022) Rare earth elements from waste. Sci Adv 8:eabm3132. https://doi.org/10.1126/sciadv.abm3132
Deng S, Perez-Cardona J, Huang A, Yih Y, Thompson VS, Reed DW, Jin H, Sutherland JW (2020) Applying design of experiments to evaluate economic feasibility of rare-earth element recovery. Procedia CIRP 90:165–170. https://doi.org/10.1016/j.procir.2020.02.005
Domenech T, Bahn-Walkowiak B (2019) Transition towards a resource efficient circular economy in Europe: policy lessons from the EU and the member states. Ecol Econ 155:7–19. https://doi.org/10.1016/j.ecolecon.2017.11.001
Emil-Kaya E, Polat B, Stopic S, Gürmen S, Friedrich B (2023) Recycling of NdFeB magnets employing oxidation, selective leaching, and iron precipitation in an autoclave. RSC Adv 13:1320–1332. https://doi.org/10.1039/D2RA06883D
Energy USDo (2011) U.S. Department of Energy Critical metal strategy. In Book U.S. Department of Energy Critical metal strategy. Editor, (ed)^(eds)
Ferella F, Innocenzi V, Maggiore F (2016) Oil refining spent catalysts: a review of possible recycling technologies. Resour Conserv Recycl 108:10–20. https://doi.org/10.1016/j.resconrec.2016.01.010
Ferronato N, Torretta V (2019) Waste mismanagement in developing countries: a review of global issues. Int J Environ Res Public Health 16:1060. https://doi.org/10.3390/ijerph16061060
Forti V. BCP, Kuehr R., Bel G. (2020) The Global E-waste Monitor 2020: Quantities, flows and the circular economy potential. United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR)—co-hosted SCYCLE Programme, International Telecommunication Union (ITU) & International Solid Waste Association (ISWA), Bonn/Geneva/Rotterdam
Franus W, Wiatros-Motyka MM, Wdowin M (2015) Coal fly ash as a resource for rare earth elements. Environ Sci Pollut Res Int 22:9464–9474. https://doi.org/10.1007/s11356-015-4111-9
Gambogi J (2018) 2017 Minerals Yearbook, Rare earths. U.S. Department of the Interior, U.S. Geological Survey, https://prd-wret.s3.us-west-2.amazonaws.com/assets/palladium/production/atoms/files/myb1-2017-raree.pdf
Gambogi J (2020) Mineral commodity summaries : rare earths, 2020. U.S. Geological Survey, https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-rare-earths.pdf
Gambogi J (2021) Mineral commodity summaries : rare earths, 2021. U.S. Geological Survey, https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-rare-earths.pdf
Garside M (2020) Rare earth reserves worldwide as of 2019, by country (in 1,000 metric tons REO). Statista Inc., New York, NY 10004 United States, https://www.statista.com/statistics/277268/rare-earth-reserves-by-country/
Garside M (2021) Distribution of rare earth element consumption worldwide in 2019, by end use. Statista Inc., https://www.statista.com/statistics/604190/distribution-of-rare-earth-element-consumption-worldwide-by-end-use/
Gergoric M, Barrier A, Retegan T (2019) Recovery of rare-earth elements from neodymium magnet waste using glycolic, maleic, and ascorbic acids followed by solvent extraction. J Sustain Metall 5:85–96. https://doi.org/10.1007/s40831-018-0200-6
Golev A, Scott M, Erskine PD, Ali SH, Ballantyne GR (2014) Rare earths supply chains: current status, constraints and opportunities. Resour Policy 41:52–59
Goll D, Kronmüller H (2000) High-performance permanent magnets. Naturwissenschaften 87:423–438. https://doi.org/10.1007/s001140050755
Graedel TE, Allwood J, Birat J-P, Buchert M, Hagelüken C, Reck BK, Sibley SF, Sonnemann G (2011) What do we know about metal recycling rates? J Ind Ecol 15:355–366. https://doi.org/10.1111/j.1530-9290.2011.00342.x
Mouna HM, Baral SS (2019) A bio-hydrometallurgical approach towards leaching of lanthanum from the spent fluid catalytic cracking catalyst using Aspergillus niger. Hydrometallurgy 184:175–182. https://doi.org/10.1016/j.hydromet.2019.01.007
Haque N, Hughes A, Lim S, Vernon C (2014) Rare earth elements: overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 3:614–635. https://doi.org/10.3390/resources3040614
Verma HR, Sahu KS, Pratima Meshram BD, Pandey P, Mankhand TR (2013) Kinetics of hydrometallurgical extraction of rare earth metals from waste phosphor. Int J Res Eng Technol 2:251–255
Hopfe S, Konsulke S, Barthen R, Lehmann F, Kutschke S, Pollmann K (2018) Screening and selection of technologically applicable microorganisms for recovery of rare earth elements from fluorescent powder. Waste Manag 79:554–563. https://doi.org/10.1016/j.wasman.2018.08.030
http://www3.cec.org/ (2015) Environmentally Sound Management of End-of-Life Batteries from Electric-Drive Vehicles in North America. In Book Environmentally Sound Management of End-of-Life Batteries from Electric-Drive Vehicles in North America. Editor, (ed)^(eds). Commission for Environmental Cooperation, Montreal, Canada
https://www.epo.org/ (2020) Fourth Industrial Revolution. https://www.epo.org/news-events/in-focus/ict/fourth-industrial-revolution.html
https://www.nrcan.gc.ca/ (2020) Rare earth elements facts. https://www.nrcan.gc.ca/our-natural-resources/minerals-mining/minerals-metals-facts/rare-earth-elements-facts/20522
https://www.nrcan.gc.ca/ (2021) Rare earth elements facts. https://www.nrcan.gc.ca/our-natural-resources/minerals-mining/minerals-metals-facts/rare-earth-elements-facts/20522
https://www.stanfordmagnets.com/ The Development of the NdFeB Magnet Industry. Stanford Magnet, https://www.stanfordmagnets.com/development-of-the-ndfeb-magnet-industry.html
https://www.statista.com/ (2022) Forecast rare earth element consumption value distribution worldwide in 2028, by application. Statista Inc., https://www.statista.com/statistics/1060478/rare-earth-consumption-value-forecast-worldwide-by-application/
https://www.statista.com/ (2023) Demand for neodymium-iron-boron (NdfeB) permanent magnets worldwide from 2018 to 2022. https://www.statista.com/, https://www.statista.com/statistics/1047263/neodymium-iron-boron-permanent-magnet-demand-worldwide/
Ilankoon IMSK, Dushyantha NP, Mancheri N et al (2022) Constraints to rare earth elements supply diversification: evidence from an industry survey. J Clean Prod 331:129932. https://doi.org/10.1016/j.jclepro.2021.129932
Innocenzi V, De Michelis I, Ferella F, Veglio F (2016a) Rare earths from secondary sources: profitability study. Adv Environ Res 5:125–140
Ippolito NM, Innocenzi V, De Michelis I, Medici F, Vegliò F (2017) Rare earth elements recovery from fluorescent lamps: a new thermal pretreatment to improve the efficiency of the hydrometallurgical process. J Clean Prod 153:287–298. https://doi.org/10.1016/j.jclepro.2017.03.195
Itakura T, Sasai R, Itoh H (2006) Resource recovery from Nd–Fe–B sintered magnet by hydrothermal treatment. J Alloy Compd 408–412:1382–1385. https://doi.org/10.1016/j.jallcom.2005.04.088
Jaffe RPJ, Ceder G, Eggert R, Graedel T, Gschneidner K, Hitzman M, Houle F, Hurd A, Kelley R, King A, Milliron DSB, Slakey F, Russo J (2011) Energy critical elements: securing materials for emerging technologies. APS Phys. http://www.aps.org/policy/reports/popa-reports/upload/elementsreport.pdf
Jin H, Reed DW, Thompson VS et al (2019) Sustainable bioleaching of rare earth elements from industrial waste materials using agricultural wastes. ACS Sustain Chem Eng 7:15311–15319. https://doi.org/10.1021/acssuschemeng.9b02584
Joseph CV, Mohd Yunus MY, Ismail NA, Kanthasamy R (2019) Economic potential assessment of neodymium recovery from Malaysia e-waste resource. Mater Today Proc 17:707–716. https://doi.org/10.1016/j.matpr.2019.06.354
Jyothi RK, Thenepalli T, Ahn JW, Parhi PK, Chung KW, Lee J-Y (2020) Review of rare earth elements recovery from secondary resources for clean energy technologies: Grand opportunities to create wealth from waste. J Clean Prod 267:122048. https://doi.org/10.1016/j.jclepro.2020.122048
K. Bisaka ICTaCP (2016) Extraction of Rare-Earth Elements from Iron-Rich Rare-Earth Deposits In Book Extraction of Rare-Earth Elements from Iron-Rich Rare-Earth Deposits Editor, (ed)^(eds). Southern African Institute of Mining and Metallurgy, Cape Town, pp 25–36
Kasina M, Michalik M (2016) Iron metallurgy slags as a potential source of critical elements - Nb, Ta and REE. Mineralogia 47:15–28. https://doi.org/10.1515/mipo-2017-0004
Kaya EE, Kaya O, Stopic S, Gürmen S, Friedrich B (2021) NdFeB magnets recycling process: an alternative method to produce mixed rare earth oxide from scrap NdFeB magnets. Metals 11:716. https://doi.org/10.3390/met11050716
Khazova M, O’Hagan JB (2008) Optical radiation emissions from compact fluorescent lamps. Radiat Prot Dosim 131:521–525. https://doi.org/10.1093/rpd/ncn234
Kim J, Azimi G (2020) Recovery of scandium and neodymium from blast furnace slag using acid baking–water leaching. RSC Adv 10:31936–31946. https://doi.org/10.1039/d0ra05797e
Kim J, Azimi G (2021) Valorization of electric arc furnace slag via carbothermic reduction followed by acid baking—water leaching. Resour Conserv Recycl 173:105710. https://doi.org/10.1016/j.resconrec.2021.105710
Kiskira K, Lymperopoulou T, Tsakanika L-A, Pavlopoulos C, Papadopoulou K, Ochsenkühn K-M, Lyberatos G, Ochsenkühn-Petropoulou M (2021) Study of microbial cultures for the bioleaching of scandium from alumina industry by-products. Metals 11:951. https://doi.org/10.3390/met11060951
Kitagawa J, Uemura R (2017) Rare earth extraction from NdFeB magnet using a closed-loop acid process. Sci Rep 7:8039. https://doi.org/10.1038/s41598-017-08629-z
Kolker A, Scott C, Hower JC, Vazquez JA, Lopano CL, Dai S (2017) Distribution of rare earth elements in coal combustion fly ash, determined by SHRIMP-RG ion microprobe. Int J Coal Geol 184:1–10. https://doi.org/10.1016/j.coal.2017.10.002
Kumar S, Kumar R, Bandopadhyay A (2006) Innovative methodologies for the utilisation of wastes from metallurgical and allied industries. Resour Conserv Recycl 48:301–314. https://doi.org/10.1016/j.resconrec.2006.03.003
Kumari A, Parween R, Chakravarty S, Parmar K, Pathak DD, Lee J-c, Jha MK (2019) Novel approach to recover rare earth metals (REMs) from Indian coal bottom ash. Hydrometallurgy 187:1–7. https://doi.org/10.1016/j.hydromet.2019.04.024
Li S, Cui Z, Li W, Wang D, Wang Z (2021) Technical actuality and prospect of NdFeB waste recycling. Mater Rep 35:3001–3009. https://doi.org/10.11896/cldb.20070004
Lie J, Liu J-C (2021) Selective recovery of rare earth elements (REEs) from spent NiMH batteries by two-stage acid leaching. J Environ Chem Eng 9:106084. https://doi.org/10.1016/j.jece.2021.106084
Liu F, Porvali A, Halli P, Wilson BP, Lundström M (2019) Comparison of different leaching media and their effect on REEs recovery from spent Nd-Fe-B magnets. Jom 72:806–815. https://doi.org/10.1007/s11837-019-03844-7
Lu G, Lu X, Liu P (2020) Recovery of rare earth elements from spent fluid catalytic cracking catalyst using hydrogen peroxide as a reductant. Min Eng 145:106104. https://doi.org/10.1016/j.mineng.2019.106104
Lucas J, Lucas P, Le Mercier T, Rollat A, Davenport W (2015) Chapter 16—applications of rare earth luminescent materials. In: Lucas J, Lucas P, Le Mercier T, Rollat A, Davenport W (eds) Rare earths. Elsevier, Amsterdam, pp 281–318
Mantuano DP, Dorella G, Elias RCA, Mansur MB (2006) Analysis of a hydrometallurgical route to recover base metals from spent rechargeable batteries by liquid–liquid extraction with Cyanex 272. J Power Sources 159:1510–1518. https://doi.org/10.1016/j.jpowsour.2005.12.056
Markets Ra (June 2018) Global Rare Earth Permanent Magnet Market 2018–2021 with Focus on the Chinese Industry Featuring 5 Global Players & 22 Chinese Companies. Research and Markets, http://www.researchandmarkets.com, https://www.prnewswire.com/news-releases/global-rare-earth-permanent-magnet-market-2018-2021-with-focus-on-the-chinese-industry-featuring-5-global-players--22-chinese-companies-300660906.html#:~:text=China%20has%20been%20the%20world's,produced%20in%20China%20in%202017.
Matizamhuka W (2018) The Impact of magnetic materials in renewable energy-related technologies in the 21st century industrial revolution: the case of South Africa. Adv Mater Sci Eng 2018:1–9. https://doi.org/10.1155/2018/3149412
Mei G, Rao P, Matsuda M, Fujita T (2009) Separation of red (Y2O3:Eu3+), blue (BaMgAl10O17:Eu2+) and green (CeMgAl10O17:Tb3) rare earth phosphors by liquid/liquid extraction. J Wuhan Univ Technol Mater Sci Ed 24:603–607. https://doi.org/10.1007/s11595-009-4603-x
Meshram P, Pandey BD, Mankhand TR (2016) Process optimization and kinetics for leaching of rare earth metals from the spent Ni-metal hydride batteries. Waste Manag 51:196–203. https://doi.org/10.1016/j.wasman.2015.12.018
Meshram P, Pandey BD, Abhilash (2019) Perspective of availability and sustainable recycling prospects of metals in rechargeable batteries—a resource overview. Resources Policy 60:9–22, https://doi.org/10.1016/j.resourpol.2018.11.015
Meshram P, Somani H, Pandey BD, Mankhand TR, Deveci H, Abhilash (2017) Two stage leaching process for selective metal extraction from spent nickel metal hydride batteries. J Clean Prod 157:322–332, https://doi.org/10.1016/j.jclepro.2017.04.144
Muddanna MH, Baral SS (2021) Bioleaching of rare earth elements from spent fluid catalytic cracking catalyst using Acidothiobacillus ferrooxidans. J Environ Chem Eng 9:104848. https://doi.org/10.1016/j.jece.2020.104848
Nguyen L-P, Pham YTH, Ngo PT, Van Tran T, Tran LV, Le NTH, Nguyen LH, Dang TT, Nguyen DA, Wenzel M (2018) Production of high purity rare earth mixture from iron-rich spent fluid catalytic cracking (FCC) catalyst using acid leaching and two-step solvent extraction process. Korean J Chem Eng 35:1195–1202
Niskanen J, Lahtinen M, Perämäki S (2022) Acetic acid leaching of neodymium magnets and iron separation by simple oxidative precipitation. Clean Eng Technol 10:100544. https://doi.org/10.1016/j.clet.2022.100544
Ober JA (2021) Mineral Commodity Summaries :Iron and steel slag, 2021. U.S. Geological Survey, https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-iron-steel-slag.pdf
Ochsenkühn PM, Lyberopulu T, Parissakis G (1995) Selective separation and determination of scandium from yttrium and lanthanides in red mud by a combined ion exchange/solvent extraction method. Anal Chim Acta 315:231–237
Ochsenkühn P, Maria Th, Hatzilyberis KS, Mendrinos LN, Salmas CE (2002) Pilot-plant investigation of the leaching process for the recovery of scandium from red mud. Ind Eng Chem Res 41:5794–5801. https://doi.org/10.1021/ie011047b
Omodara L, Pitkäaho S, Turpeinen E-M, Saavalainen P, Oravisjärvi K, Keiski RL (2019) Recycling and substitution of light rare earth elements, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications—a review. J Cleaner Prod 236:117573. https://doi.org/10.1016/j.jclepro.2019.07.048
Önal MAR, Borra CR, Guo M, Blanpain B, Van Gerven T (2015) recycling of NdFeB magnets using sulfation, selective roasting, and water leaching. J Sustain Metall 1:199–215. https://doi.org/10.1007/s40831-015-0021-9
Orefice M, Binnemans K (2021) Solvometallurgical process for the recovery of rare-earth elements from Nd–Fe–B magnets. Sep Purif Technol 258:117800
Orefice M, Audoor H, Li Z, Binnemans K (2019) Solvometallurgical route for the recovery of Sm Co, Cu and Fe from SmCo permanent magnets. Sep Purif Technol 219:281–289. https://doi.org/10.1016/j.seppur.2019.03.029
Padhan E, Nayak AK, Sarangi K (2017) Recovery of neodymium and dysprosium from NdFeB magnet swarf. Hydrometallurgy 174:210–215. https://doi.org/10.1016/j.hydromet.2017.10.015
Palasyuk O, Onyszczak M, Kim T-H, Zhou L, Kramer MJ, Bud’ko SL, Canfield PC, Palasyuk A (2021) Structural and magnetic properties of hard magnetic system Ce(Co1-Fe )4.4Cu0.6 (0 ≤ x ≤ 0.19). J Alloys Compd 883:160866. https://doi.org/10.1016/j.jallcom.2021.160866
Palos R, Gutiérrez A, Arandes JM, Bilbao J (2018) Catalyst used in fluid catalytic cracking (FCC) unit as a support of NiMoP catalyst for light cycle oil hydroprocessing. Fuel 216:142–152
Pan J, Nie T, Vaziri Hassas B, Rezaee M, Wen Z, Zhou C (2020) Recovery of rare earth elements from coal fly ash by integrated physical separation and acid leaching. Chemosphere 248:126112. https://doi.org/10.1016/j.chemosphere.2020.126112
Pietrelli L, Bellomo B, Fontana D, Montereali MR (2002) Rare earths recovery from NiMH spent batteries. Hydrometallurgy 66:135–139. https://doi.org/10.1016/S0304-386X(02)00107-X
Pietrelli L, Bellomo B, Fontana D, Montereali M (2005) Characterization and leaching of NiCd and NiMH spent batteries for the recovery of metals. Waste Manag 25:221–226. https://doi.org/10.1016/j.wasman.2004.12.013
Provazi K, Campos BA, Espinosa DC, Tenorio JA (2011) Metal separation from mixed types of batteries using selective precipitation and liquid-liquid extraction techniques. Waste Manag 31:59–64. https://doi.org/10.1016/j.wasman.2010.08.021
Qu Y, Lian B (2013) Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour Technol 136:16–23. https://doi.org/10.1016/j.biortech.2013.03.070
Qu Y, Li H, Wang X, Tian W, Shi B, Yao M, Zhang Y (2019) Bioleaching of major, rare earth, and radioactive elements from red mud by using indigenous chemoheterotrophic bacterium Acetobacter sp. Minerals 9:67. https://doi.org/10.3390/min9020067
Rasoulnia P, Barthen R, Lakaniemi A-M (2020) A critical review of bioleaching of rare earth elements: The mechanisms and effect of process parameters. Crit Rev Environ Sci Technol 51:378–427. https://doi.org/10.1080/10643389.2020.1727718
Reed DW, Fujita Y, Daubaras DL, Jiao Y, Thompson VS (2016) Bioleaching of rare earth elements from waste phosphors and cracking catalysts. Hydrometallurgy 166:34–40. https://doi.org/10.1016/j.hydromet.2016.08.006
Rodrigues LEOC, Mansur MB (2010) Hydrometallurgical separation of rare earth elements, cobalt and nickel from spent nickel–metal–hydride batteries. J Power Sources 195:3735–3741. https://doi.org/10.1016/j.jpowsour.2009.12.071
Ruetschi P, Meli F, Desilvestro J (1995) Nickel-metal hydride batteries. The preferred batteries of the future? J Power Sources 57:85–91. https://doi.org/10.1016/0378-7753(95)02248-1
Sadeghi M, Jesus J, Soares H (2018) Recycling spent fluid cracking catalysts for rare earth metal recovery: a review. Reciklaza i Odrzivi Razvoj 11:43–52. https://doi.org/10.5937/ror1801043M
Safari S, Eshraghi Dehkordy S, Kazemi M, Dehghan H, Mahaki B (2015) Ultraviolet radiation emissions and illuminance in different brands of compact fluorescent lamps. Int J Photoenergy 2015:504674. https://doi.org/10.1155/2015/504674
Saito S, Ohno O, Igarashi S, Kato T, Yamaguchi H (2015) Separation and recycling for rare earth elements by homogeneous liquid-liquid extraction (HoLLE) using a pH-responsive fluorine-based surfactant. Metals 5:1543–1552. https://doi.org/10.3390/met5031543
Saito T, Sato H, Motegi T (2005) Extraction of Sm from Sm–Fe alloys by the glass slag method. J Alloy Compd 387:274–278. https://doi.org/10.1016/j.jallcom.2004.02.064
Saito T, Sato H, Motegi T (2006) Recovery of rare earths from sludges containing rare-earth elements. J Alloy Compd 425:145–147. https://doi.org/10.1016/j.jallcom.2006.01.011
Saito T, Sato H, Ozawa S, Yu J, Motegi T (2003) The extraction of Nd from waste Nd–Fe–B alloys by the glass slag method. J Alloy Compd 353:189–193. https://doi.org/10.1016/s0925-8388(02)01202-1
Salo M, Knauf O, Mäkinen J, Yang X, Koukkari P (2020) Integrated acid leaching and biological sulfate reduction of phosphogypsum for REE recovery. Min Eng 155:106408. https://doi.org/10.1016/j.mineng.2020.106408
Scott C, Kolker A (2019) Rare earth elements in coal and coal fly ash. In Book Rare earth elements in coal and coal fly ash. Editor, (ed)^(eds), Reston, VA, p 4
Sinha MK, Pramanik S, Kumari A, Sahu SK, Prasad LB, Jha MK, Yoo K, Pandey BD (2017) Recovery of value added products of Sm and Co from waste SmCo magnet by hydrometallurgical route. Sep Purif Technol 179:1–12. https://doi.org/10.1016/j.seppur.2017.01.056
Sinha S, Abhilash MP, Pandey BD (2016) Metallurgical processes for the recovery and recycling of lanthanum from various resources—a review. Hydrometallurgy 160:47–59. https://doi.org/10.1016/j.hydromet.2015.12.004
Sposato C, Catizzone E, Blasi A, Forte M, Romanelli A, Morgana M, Braccio G, Giordano G, Migliori M (2021) Towards the circular economy of rare earth elements: lanthanum leaching from spent FCC catalyst by acids. Processes 9:1369. https://doi.org/10.3390/pr9081369
Swain B (2022) Red mud: an environmental challenge but overlooked treasure for critical rare earth metals. MRS Bull 47:289–302. https://doi.org/10.1557/s43577-022-00300-x
Swain B, Otu EO (2011) Competitive extraction of lanthanides by solvent extraction using Cyanex 272: analysis, classification and mechanism. Sep Purif Technol 83:82–90. https://doi.org/10.1016/j.seppur.2011.09.015
Swain B, Tanaka M (2018) Separation of yttrium from europium using a hollow fiber-supported liquid membrane with 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester as an extractant. Chem Eng Commun 205:1484–1493. https://doi.org/10.1080/00986445.2018.1458025
Swain B, Akcil A, Lee J-c (2020a) Red mud valorization an industrial waste circular economy challenge; review over processes and their chemistry. Crit Rev Environ Sci Technol 52:520–570. https://doi.org/10.1080/10643389.2020.1829898
Swain B, Akcil A, Lee J-c (2020b) Red mud valorization an industrial waste circular economy challenge; review over processes and their chemistry. Critical Reviews in Environmental Science and Technology:1–51, doi:https://doi.org/10.1080/10643389.2020b.1829898
Swain N, Mishra S (2019) A review on the recovery and separation of rare earths and transition metals from secondary resources. J Clean Prod 220:884–898. https://doi.org/10.1016/j.jclepro.2019.02.094
Takeda O, Okabe TH, Umetsu Y (2006) Recovery of neodymium from a mixture of magnet scrap and other scrap. J Alloy Compd 408–412:387–390. https://doi.org/10.1016/j.jallcom.2005.04.094
Tan Q, Li J, Zeng X (2014) Rare earth elements recovery from waste fluorescent lamps: a review. Crit Rev Environ Sci Technol 45:749–776. https://doi.org/10.1080/10643389.2014.900240
Tetsuji Saito HS, Ozawa S, Moteg T (2003) The extraction of Sm from Sm–Co alloys by the Glass Slag Method. Mater Trans 4:637–640
Thompson VS, Gupta M, Jin H et al (2017) Techno-economic and life cycle analysis for bioleaching rare-earth elements from waste materials. ACS Sustain Chem Eng 6:1602–1609. https://doi.org/10.1021/acssuschemeng.7b02771
Tuan L, Thenepalli T, Chilakala R, Vu H, Ahn J, Kim J (2019) Leaching characteristics of low concentration rare earth elements in Korean (Samcheok) CFBC bottom ash samples. Sustainability 11:2562. https://doi.org/10.3390/su11092562
Tuck CC (2022) Mineral Commodity Summaries :Iron and steel slag, 2022. U.S. Geological Survey, https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-iron-steel-slag.pdf
Tunsu C, Petranikova M, Ekberg C, Retegan T (2016) A hydrometallurgical process for the recovery of rare earth elements from fluorescent lamp waste fractions. Sep Purif Technol 161:172–186. https://doi.org/10.1016/j.seppur.2016.01.048
Tzanetakis N, Scott K (2004) Recycling of nickel–metal hydride batteries. I: dissolution and solvent extraction of metals. J Chem Technol Biotechnol 79:919–926. https://doi.org/10.1002/jctb.1081
Um N, Hirato T (2016) A hydrometallurgical method of energy saving type for separation of rare earth elements from rare earth polishing powder wastes with middle fraction of ceria. J Rare Earths 34:536–542. https://doi.org/10.1016/s1002-0721(16)60059-5
Van Loy S, Binnemans K, Van Gerven T (2017) Recycling of rare earths from lamp phosphor waste: enhanced dissolution of LaPO 4: Ce 3+, Tb 3+ by mechanical activation. J Clean Prod 156:226–234. https://doi.org/10.1016/j.jclepro.2017.03.160
Vander Hoogerstraete T, Blanpain B, Van Gerven T, Binnemans K (2014) From NdFeB magnets towards the rare-earth oxides: a recycling process consuming only oxalic acid. RSC Adv 4:64099–64111. https://doi.org/10.1039/C4RA13787F
Wang J, Huang X, Cui D, Wang L, Feng Z, Hu B, Long Z, Zhao N (2017a) Recovery of rare earths and aluminum from FCC waste slag by acid leaching and selective precipitation. J Rare Earths 35:1141–1148. https://doi.org/10.1016/j.jre.2017.05.011
Wang J, Xu Y, Wang L, Zhao L, Wang Q, Cui D, Long Z, Huang X (2017b) Recovery of rare earths and aluminum from FCC catalysts manufacturing slag by stepwise leaching and selective precipitation. J Environ Chem Eng 5:3711–3718. https://doi.org/10.1016/j.jece.2017.07.018
Wang S, Fang Y, Wang C, Wang L, Zhu M, Li W, Hadjipanayis GC (2020) Dependence of macromagnetic properties on the microstructure in high-performance Sm2Co17-type permanent magnets. J Magn Magn Mater 510:166942. https://doi.org/10.1016/j.jmmm.2020.166942
Wang W, Pranolo Y, Cheng CY (2013) Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep Purif Technol 108:96–102. https://doi.org/10.1016/j.seppur.2013.02.001
Wen Z, Zhou C, Pan J, Cao S, Hu T, Ji W, Nie T (2020) Recovery of rare-earth elements from coal fly ash via enhanced leaching. Int J Coal Prep Util. https://doi.org/10.1080/19392699.2020.1790537
Wu J, Wang D, Ye C, Wang Z, Hu X (2023) Selective extraction and separation of REEs from NdFeB magnets scrap using co-chlorination and water leaching. Sep Purif Technol 306:122452. https://doi.org/10.1016/j.seppur.2022.122452
Wübbeke J (2013) Rare earth elements in China: policies and narratives of reinventing an industry. Resour Policy 38:384–394
Yadav KK, Anitha M, Singh DK, Kain V (2018) NdFeB magnet recycling: dysprosium recovery by non-dispersive solvent extraction employing hollow fibre membrane contactor. Sep Purif Technol 194:265–271. https://doi.org/10.1016/j.seppur.2017.11.025
Yang Y, Lan C, Wang Y, Zhao Z, Li B (2020) Recycling of ultrafine NdFeB waste by the selective precipitation of rare earth and the electrodeposition of iron in hydrofluoric acid. Sep Purif Technol 230:115870. https://doi.org/10.1016/j.seppur.2019.115870
Yang Y, Walton A, Sheridan R et al (2016) REE recovery from end-of-life NdFeB permanent magnet scrap: a critical review. J Sustain Metall 3:122–149. https://doi.org/10.1007/s40831-016-0090-4
Ye S, Jing Y, Wang Y, Fei W (2017) Recovery of rare earths from spent FCC catalysts by solvent extraction using saponified 2-ethylhexyl phosphoric acid-2-ethylhexyl ester (EHEHPA). J Rare Earths 35:716–722. https://doi.org/10.1016/s1002-0721(17)60968-2
Yurramendi L, Gijsemans L, Forte F, Aldana JL, del Río C, Binnemans K (2019) Enhancing rare-earth recovery from lamp phosphor waste. Hydrometallurgy 187:38–44. https://doi.org/10.1016/j.hydromet.2019.04.030
Zhang C, Liu Z, Li M, Liu L, Li T, Chen R, Lee D, Yan A (2018) The evolution of phase constitution and microstructure in iron-rich 2:17-type Sm–Co magnets with high magnetic performance. Sci Rep 8:9103. https://doi.org/10.1038/s41598-018-27487-x
Zhang P, Yokoyama T, Itabashi O, Wakui Y, Suzuki TM, Inoue K (1998) Hydrometallurgical process for recovery of metal values from spent nickel-metal hydride secondary batteries. Hydrometallurgy 50:61–75. https://doi.org/10.1016/S0304-386X(98)00046-2
Zhang W, Noble A, Yang X, Honaker R (2020a) A comprehensive review of rare earth elements recovery from coal-related materials. Minerals 10:451. https://doi.org/10.3390/min10050451
Zhang X-k, Zhou K-g, Chen W, Lei Q-y, Huang Y, Peng C-h (2019) Recovery of iron and rare earth elements from red mud through an acid leaching-stepwise extraction approach. J Central South Univ 26:458–466. https://doi.org/10.1007/s11771-019-4018-6
Zhang Y, Gu F, Su Z, Liu S, Anderson C, Jiang T (2020b) Hydrometallurgical recovery of rare earth elements from NdFeB permanent magnet scrap: a review. Metals 10:841. https://doi.org/10.3390/met10060841
Zhao Z, Qiu Z, Yang J, Lu S, Cao L, Zhang W, Xu Y (2017) Recovery of rare earth elements from spent fluid catalytic cracking catalysts using leaching and solvent extraction techniques. Hydrometallurgy 167:183–188
Zheng H, Chen T, Rudolph V, Golding SD (2017) Biogenic methane production from Bowen Basin coal waste materials. Int J Coal Geol 169:22–27. https://doi.org/10.1016/j.coal.2016.09.006
Acknowledgements
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Basudev swain is the solo contributor for the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval and consent to participate
The work has been approved by relevant authorities for publication. All author has consented to the participation and publication of this research work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Swain, B. Challenges and opportunities for sustainable valorization of rare earth metals from anthropogenic waste. Rev Environ Sci Biotechnol 22, 133–173 (2023). https://doi.org/10.1007/s11157-023-09647-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-023-09647-2