Abstract
Recycling has been proposed as a promising potential source of supply to meet some of the US rare-earth demand for use in permanent magnets. The high growth rates of products that make use of rare-earth magnets, particularly wind turbines and electric and hybrid vehicles, show that their stock in use is on the rise and in the near term will become available as scrap feed for recycling. This study presents an overview of magnet recycling technologies and focuses on the technoeconomic analysis of liquid metal leaching and distillation, including the effect of a new continuous gravity-driven multiple effect thermal system (G-METS) metal distillation technology on energy use and overall cost. The G-METS system can potentially reduce the energy consumption of the overall process to 64 kWh/kg, which is about 30% less than metal production from ore and 61–67% less than the process using conventional distillation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of permanent magnets has evolved from lodestone in the 1920s to modern neodymium-iron-boron (NdFeB, or “neo”) magnets because of the need to obtain a good combination of maximum energy product and high Curie temperature. The high energy product value of rare-earth and other permanent magnets enables the use of smaller magnets and higher magnetic strength applications. Although there are generally two types of rare-earth magnetie, i.e., neo magnets and samarium cobalt (SmCo) magnets, neo magnets are the strongest and most affordable. Dysprosium (Dy) and cobalt addition can improve the magnet’s high-temperature characteristics.1 Their applications are seen in technologies that require potent magnets such as wind turbine generators, vehicle traction motors in hybrid and electric vehicles (HEVs/EVs), speakers and headphones, magnetic resonance imaging (MRI) scanners, computer hard disk drives, and high-performance alternating current (AC) servo motors, among others. According to a forecast by Adamas Intelligence,2 an independent research and advisory company on strategic metals and minerals, there will be an increase in the global demand for NdFeB alloys and powders at a compound annual growth rate (CAGR) of 9.7% from 2020 through 2030, and thus an increase in the need for rare-earth elements (i.e., neodymium, praseodymium, dysprosium, and terbium). Meanwhile, global production will increase at a slower CAGR of 7.1%, leading to a supply market struggling to keep up with growing demand. NdFeB alloy global shortage is predicted to amount to 48,000 tonnes annually by 2030, estimated as the amount needed to produce 25–30 million electric vehicle traction motors. Didymium oxide (NdPr oxide) will also experience a shortage rising to 16,000 tonnes in 2030, equivalent to roughly three times the total annual output of Lynas Corporation and MP Materials.2 Therefore, there is a need to ensure a sustainable supply of these elements by methods that include recycling. In achieving this, it is essential to ascertain the availability of the raw materials for recycling and the economic viability of the methods employed.
Several studies have reported various methods for recycling rare earths from end-of-life (EoL) products. Still, there are limited detailed technoeconomic analysis (TEA) studies on these methods. This study presents a review of various processes for recycling rare earths from EoL magnet products and a technoeconomic analysis of a proposed low-cost alternative using a new distillation process.
Availability of Rare-Earth Permanent Magnet (REPM)
An essential aspect for understanding the recycling potential of rare-earth elements (REEs) from EoL products is evaluating the quantities of these elements in secondary sources, as studies show that a maximum recycling rate of 20% can be reached in at least 10 years with perfect recovery and a continuous growth trend.3 Nd, Fe, and B primarily make up rare-earth permanent magnets, although praseodymium can be added partially (~ 5%) to offset Nd. Meanwhile, Dy is a common alloying material added to increase the magnet’s operational temperature for applications that involve high temperatures, such as EVs and wind turbines. In lower-temperature applications, such as magnetic refrigeration systems, very minimal (0.5% maximum) or no Dy is used. For room-temperature applications, such as in computer hard disk drives, the Dy content is typically around 1.5%, or none at all.4 About 31–32% of RE permanent magnets is REEs,5 with Nd accounting for as much as 30%.6 Thus, knowledge of the mass of REPM used in secondary sources allows an estimation of the quantity of REEs available for recovery.
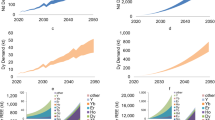
Dynamic variables considered to ascertain the quantity of REPM scrap available include yearly sales of potential sources, product lifespan, and quantity of REPM available. The most promising urban mining resources that contain rare-earth permanent magnets are automobiles, home appliances, acoustic equipment, magnetic resonance imaging (MRI) machines, factory automation tools, and electronic devices.7 For example, a conventional gasoline engine sedan contains approximately 0.44 kg of REEs, 80% of which is in permanent magnets. An entire hybrid electric vehicle (HEV) that uses nickel metal hydride batteries contains 4.5 kg of REEs, and an HEV with a lithium-ion battery contains approximately 1 kg of REEs.8 In the USA, HEV sales began in 1999 with an annual sale of 9400 vehicles and reached a peak of 495,500 sales in 2013. US plug-in HEV and EV sales started in 2010 with a yearly sale of 7700 and 10,100 vehicles, and since then annual sales have increased at an average rate of 34.8% and 48.8%, respectively, reaching 122,800 vehicles in 2018 and 242,000 vehicles in 2019, respectively.9
According to a market analysis report on REEs in Europe, EVs which typically have an average lifespan of 15 years can provide more than 11 kt of Nd and close to 1 kt of Dy likely to be harvested over the next two decades. Also, electric power steering motors of conventional vehicles can supply an estimated 5 kt of Nd content. Residential air conditioners could provide approximately 2 kt of Nd. This represents approximately 20% of the total annual demand.2,10
Offshore wind turbines that use direct-drive permanent magnet generators (DDPMGs) each contain about 160–650 kg NdFeB per MW of wind power capacity, with 51–208 kg of REEs/MW. Due to the enormous power capacity and lower part count, DDPMGs are the top design choice for US offshore wind applications. Currently, just seven offshore wind turbines in the USA with 42 MW of wind power capacity have been installed and commissioned.
In 2019, the US offshore wind project pipeline grew to a potential generating capacity of 28,521 MW from 25,824 MW in 2016 across 13 states. Also, an estimate of the onshore wind turbines with DDPMGs in the USA indicates the potential of supplying ~ 37 tonnes/year of REEs less than two decades from now, considering their average lifespan of 20 years.11,12,13
Other devices such as microphones, loudspeakers, earbuds, and headphones also contain REEs that can be recovered.14
A study on RE availability for recycling reported that REEs from US hard disk drives (HDDs) alone could meet ~ 5.2% of global demand for Nd-Fe-B magnets (excluding China).15 Swarfs and slags generated from magnet manufacturing also contain a high concentration of REEs that are recoverable. An estimated 30% of an original PM material can be generated as waste in plants.16 Also, secondary sources such as phosphogypsum, nickel metal hydride batteries, red mud, and coal ash can provide ~ 11× the total global demand for Nd, with phosphogypsum accounting for the most significant portion.17,18
Recycling Processes for REPM
There are multiple processes for producing new REPMs from old ones. Figure 1 shows the approximate cumulative energy and cost of the value chain for virgin REPMs from mining to magnet production. In this graph, one can group recycling processes in terms of how many “steps back” they take in the value chain to recover rare earths. The most efficient, which loses the least energy and value, is the magnet-to-magnet approach. Recycled magnets bring in all the impurities from scrap inputs and must be blended with other materials to create different magnet grades. Introducing a decrepitation agent, generally hydrogen, can degrade the properties somewhat, requiring small additions of unalloyed rare-earth metals in order to recover properties similar to virgin magnets. On the other end, solvent extraction and sulfuric acid baking recover a mixed rare-earth oxide that can be inserted into the separations process, recovering pure oxides that can be made into any magnet alloy with properties indistinguishable from virgin magnets. However, this requires the most energy, as the separation, reduction to metal, and alloy production steps must be redone. Liquid metal leaching lies between these two: it recovers a rare-earth magnet mischmetal, preserving the energy of metal reduction but making it difficult to tailor the REPM alloy.
The magnet-to-magnet approach has recently seen commercial deployment, as Urban Mining Company has set up operations in Texas. However, an industry which makes magnets only from scrap magnets will need to use one of the other two recycling approaches to provide the pure rare-earth metal inputs required to achieve good properties.
Liquid Metal Leaching and Distillation
This liquid metal extraction process uses molten magnesium or other group IIA metals such as Ca or Ba for rare-earth magnet recycling. Previous studies have shown that this technology best extracts rare-earth elements (Nd, Dy) from removed magnets at a temperature of 1273 K and a Mg/magnet ratio of 10,23 with Nd and Dy extraction yields of 100% and 60%, respectively. Furthermore, the addition of calcium suppressed the oxidation of Dy, thus increasing the extraction efficiency.24 The use of bismuth (Bi) instead of Mg can increase the Dy recovery above 90%, and using both Mg and Bi enables some separation of light and heavy rare earths.25
Proposed Leaching and Distillation Process
The proposed process is similar to the two-stage leaching using Mg and Bi as described by Ott and McCallum,25 but with a new efficient distillation step called the gravity-driven multiple effect thermal system (G-METS).26 It consists of the following steps:
Crushing and Sorting
The magnet scrap is first processed to remove nonmagnetic components, including nickel or other coatings, for example by preferential degradation,27 and if necessary is further ground to the particle size required for the leaching operation.
Leaching RE Metals into Molten Mg
Crushed magnets and magnesium are fed into a leaching vessel and heated to the leaching temperature of 700–1000°C. Neodymium is soluble in liquid magnesium, but iron and boron are relatively insoluble, and the Mg–B compounds that form remain as solids. Various studies have used Mg:magnet mass ratios from 1:1 to 10:1; here, our model considers a reference case of 10:1 and a best case of 5:1.
The diffusion coefficient of Nd in liquid Mg was measured to be (4.61 ± 1.32) × 10–8 cm2/s at a leaching temperature of 700°C, increasing with leaching temperature to (8.98 ± 3.50) × 10–8 cm2/s at 800°C.28 Here we assume a leaching temperature of 1000°C and a duration of 6 h, following the work of Akahori et al. (Ref. 23). At this temperature, the Nd recovery rate is close to 100% while the Dy recovery rate is 60%.
Separating the Melt from Fe-B Alloy Particles
After leaching, liquid Mg-RE and scrap particles are transported to the separation unit. The denser Fe-B alloy particles settle to the crucible bottom, thus the Mg-Re liquid alloy can be easily separated from the melt. A filtration or centrifugal separation unit completes the separation of liquid Mg-RE from Fe-B alloy particles. We nonetheless assume that a small amount of Mg remains with the Fe-B alloy particles. Note that, owing to the limited leaching recovery rate of heavy rare-earth elements such as dysprosium, this alloy particle stream contains a large fraction of those elements from the scrap.
G-METS and Vacuum Distillation of the Mg-RE Melt
The G-METS Mg distiller concept uses gravity to create a pressure difference without moving parts. The pressure in the evaporation chamber is raised by the weight of the column of liquid metal above it. Also, the pressure in the condenser below the evaporation chamber is raised as shown in Fig. 2. Heat flows upwards from the main heater, and each condenser feeds heat to the evaporator above it. In recycling, solid or liquid alloys are introduced into the top melter and the melted Mg alloy flows at a controlled rate downward into the top evaporator, where a portion of the Mg alloy evaporates. The evaporated portion rises to the top condenser and condenses, while the remaining Mg that does not evaporate here flows down through the liquid standpipe into the next evaporator, where a portion of it evaporates, and so on. Each evaporator–condenser pair is called an “effect.” Heat flows upward from effect to effect, from lower condensers to the evaporators above them. Liquid Mg flows downward between evaporators from effect to effect. Mg vapor flows upward within each effect from its evaporator into its condenser above it. Standpipes control the height of liquid Mg alloy in each evaporator.
In contrast, conventional Mg distillation is a slower batch process that produces solid metal in the condenser. Because of the pyrophoric nature of the high-surface-area condensed Mg crown product, it must be completely cooled before opening the vacuum vessel, resulting in a long cycle time (6–12 h) and a low process throughput.
This flow sheet uses both G-METS and conventional distillation. G-METS efficiently removes most of the Mg from the rare-earth metals, producing a liquid product with about 30 wt.% RE. Conventional distillation then removes nearly all the remaining Mg, producing a product suitable for use in magnet production. It uses these same two processes to separate the heavy rare-earth product from the second Bi metal leaching agent.
The Mg-Re melt is distilled to recover the Mg and RE separately, based on the difference in vapor pressure. This proposed method distills this melt in two steps. The first uses the new G-METS process for distilling most of the Mg. Studies have shown that this process can reduce the energy required to distill magnesium by as much as 90% versus today’s batch distillation process.26 This process flows liquid Mg-RE alloy through a steel distiller, so the maximum rare-earth content is limited to 20–30 wt.% to prevent corrosion of the steel. That said, if the material comes in with 90–97 wt.% Mg, G-METS can efficiently remove 65–85% of the magnesium. The second step is less-efficient conventional vacuum distillation using Mg sublimation, leaving behind solid metal consisting almost entirely of rare earths. As a result, the rare-earth extraction efficiency mainly depends on the Mg:magnet ratio.23, 24,28,29
Liquid Bismuth Leaching of Fe-B Particles
Leaching in liquid bismuth is used to recover more dysprosium from the Fe-B particle stream after the magnesium leaching process, following.30 This again selectively removes rare earths, with low iron. However, as with 85% target recovery of magnesium using G-METS, the target bismuth recovery will be 80%, with the remainder recovered using traditional vacuum distillation.
Technoeconomic Analysis
Studies carried out on the technoeconomic analysis (TEA) of rare-earth recycling have focused on secondary sources such as clay waste,31 coal ash,32 and fluidized catalytic cracking waste.33 Another study on the TEA of recycling HDD magnets considered other precious materials including Au, Ag, and Pd alongside REE recovery.15 This study focused on magnet scrap recycling from EoL NdFeB magnets to obtain RE alloy as a precursor for magnet production.
A process flow diagram was developed to estimate the economic feasibility of the magnesium and bismuth leaching process. The TEA was carried out using the ARPA-E METALS tool spreadsheet v1.0 from the US Department of Energy.34 A complete material and energy balance was developed and used in the ARPA-E METALS tool. Information was pulled from these to determine the variable cost, including raw materials, utilities, and miscellaneous materials. The mass balance contained a total of 12 elements from the magnet scrap and leaching metal.
Various adjustable parameters such as the Mg/scrap feed ratio, Bi/leaching vessel waste ratio, and Mg and Bi losses to waste streams were modeled to determine their effects on the overall target recovery and energy use. Due to high uncertainty in scrap acquisition cost, this was omitted from this analysis; this cost can be added to the analysis separately. The Streams and Compositions sheets in the ARPA-E METALS tool calculated the value flows for each input and output stream based on their compositions and verified perfect closure of the mass balance for all 12 elements. Various online vendors were used to determine the values for each compound/element, and their best- and worst-case scenarios were found.
The energy requirement for the leaching vessel was calculated from the equation:
In this equation, \(\dot{m}_{sc}\) is the mass flow rate of the scrap, \(\dot{m}_{Mg}\) is the mass flow rate of the Mg refill, and \( \dot{m}_{r}\) is the mass flow rate of the liquid Mg recycled from the distillation operation. Moreover, \(c_{psc}\), \(c_{pMg }\), and \(c_{pr}\) are the specific heat capacities of the scrap, magnesium, and Mg recycle, respectively. \(T_{0}\) is the room temperature at which the scrap will be fed into the leaching vessel, \(T_{r}\) is the weighted average temperature of Mg recycle exiting the G-METS and conventional distillation processes, and \(T_{f}\) is the temperature of extraction of the leaching vessel. This was similarly used to obtain the energy required for the second leaching process with bismuth.
The ARPA-E METALS tool was used to calculate the costs of utilities and inform best- and worst-case scenarios based on the data for average electricity costs and emissions per kWh for each state from the Energy Information Administration.35 Estimation of labor requirement was carried out based on the textbook by Peters, Timmerhaus, and West36 using Figure 6-9 on operating labor requirements in the chemical process industry. For a processing plant with an average condition of batch operations and continuous remelting process of Mg, the full-time equivalent (FTE) was obtained. This was based on an output rate of ~ 1460 kg/h, which is the sum of the mass flow rates out of the leaching vessels as shown in the material balance, and used correlations for multiple small units or completely batch operations for 100% traditional distillation, and for average conditions for the cases with mostly G-METS separation. The principal processing steps considered were crushing, heat transfer, reaction, and distillation. Without G-METS (that is, assuming only traditional distillation), the operating labor was estimated on the basis of completely batch operations, thus requiring more employee-hours per day. This resulted in an estimate of 60 employee-hours/day and 12 FTEs, for this case. With G-METS, the estimate is 40 employee-hours/day and 8 FTEs. These were used as best-case labor values, with 1.5 and 2 times these values for reference and worst-case labor values, respectively.
Other labor requirements, such as supervision, laboratory costs, and plant overhead, were estimated as a percentage of the operating labor. Maintenance, insurance, and royalties were estimated as a percentage of fixed capital investment. Indirect costs, such as research and development, distribution, marketing, and other administrative costs, were estimated as a percentage of a sum of the fixed and variable costs. Sensitivity analysis was carried out for the best-case, reference, and worst-case scenarios, and these percentages were varied according to the ranges shown in Table I. The cost implications of integrating the G-METS were studied. This considered an increase in the full-time equivalent because of an increase in the employee-hours as a result of complete traditional distillation.
Model Results
Figure 3 shows the proportions of each material flow compared with the product flow as calculated in the spreadsheet (Electronic Supplementary Material). Thus, for a plant with a target of 205 metric tons of RE alloy per year and an additional 11 tons of Dy metal, about 745 metric tons of scrap feed, ~ 149 metric tons of Mg, and 104 tons of bismuth will be required. Magnesium and bismuth are recycled after distillation.
The raw material costs can be calculated based on these flows as shown in Table II.
The energy requirement for the process can be seen in Fig. 4, with a total energy usage of 64.28 kWh/kg of RE (mixed rare-earth metals obtained). These are given for an Mg/scrap feed mass ratio and Bi/waste mass ratio of 10:1. This increases to 248.1 kWh/kg without G-METS. A decrease in these ratios will lead to an overall decrease in the energy used by the equipment. The energy consumption for the first G-METS distiller was estimated to be 15.3 kWh/kg of RE product and 6.58 kWh/kg of Dy metal based on the thermal model described in the G-METS distillation patent application.37 This is estimated for 85% Mg distillation and 80% Bi distillation, respectively. For the conventional distillation and the rotary evaporation, the energy usage was estimated at 25.36 kWh/kg of RE product and 15.75 kWh/kg of Dy, respectively, based on the fraction of Mg and Bi distilled.
Also, assuming a best- and worst-case energy cost of $0.06/kWh and $0.18/kWh, the annual electricity consumption is expected to be $900,993 and $2,702,980, respectively.
Discussion
The operating cost estimated here covers the flow sheet from scrap magnet powder input to rare-earth magnet mischmetal output, but does not include the cost of acquiring magnets or the cost of mechanical separation from coating and other materials. Prices for NdPr and DyFe alloys for magnets have been around $120/kg and $410/kg, respectively, during 2021,38 which would seem to imply high operating margins for all but the non-G-METS worst case above. However, these are relatively high; for example, in December 2020 they were around $60/kg to $65/kg and $300/kg to $350/kg, respectively,38 so reversion to historical average prices could strongly affect operating margins, as would high EoL magnet prices. That said, rare-earth prices are generally given FOB China on the Shanghai exchange, whereas these recycling operations could be closer to Western customers and free from supply risk concerns, so the products may command higher prices.
Capital cost was not discussed here, as there is currently limited information on some of the processes involved. It should differ considerably for the G-METS and non-G-METS cases due to the batch nature and long cycle time of conventional vacuum distillation, as opposed to the high-throughput, continuous nature of G-METS.
The introduction of the G-METS system has the potential to reduce the energy required and thus the cost of utilities for the process by 67% for the reference case as shown in Table III. This thereby reduces the total annual cost and production cost by 40% compared with traditional distillation. This cost can be minimized further by reducing the Mg/scrap ratio, as can be observed by adjusting this parameter in the material balance spreadsheet in the ARPA-E METAL tool. Figure 5 shows three annual operating cost stack scenarios for an RE magnesium/bismuth leaching plant. The utilities cost is 14% of the total annual cost, while raw materials, which include the cost of the magnesium and bismuth metal, are ~ 15% of the total annual cost. The bulk of the cost is seen in the operating labor and other labor, i.e., the laboratory costs, plant overheads, and supervision, which amount to 56% of the total annual cost.
Given the high labor fraction in the overall operating costs, a note on process automation is warranted. Much of the labor is required for material handling between unit operations; the operations themselves should not require significant labor inputs. It may be possible to automate some of the material hand-off from magnet crushing, to liquid metal leaching, to centrifugal separation. But feeding scrap into the crushing operation, product recovery from the separation and G-METS operations, and feeding and safe product recovery from conventional distillation will be hard to automate. The relatively low production volume of this overall process at this scale would also likely make custom automation solutions require a long time to pay back labor savings.
That said, Peters, Timmerhaus, and West36 show a very low labor input scaling exponent of 0.2–0.25, such that a factor of 16–32 increase in scale results in only a doubling of labor. This reflects both automation and larger equipment sizing. The scale described here of roughly 200 tonnes/year of rare-earth metal production from recycled streams would likely increase considerably in the future. For example, 2020 US sales of 760,000 hybrid and electric vehicles, and 1 GW/year of wind turbine deployment with rare-earth permanent magnet generators, would result in roughly 760 t/year and 600 t/year of contained rare earths in magnet scrap, respectively, a total fivefold increase in scrap availability, when they reach end-of-life in 2030 to 2040.
Conclusion
TEA of recovery of REEs from NdFeB scrap using a novel G-METS distillation system was performed for a magnesium and bismuth leaching process. The G-METS system can potentially reduce the energy consumption of the overall process to 64 kWh/kg, which is about 30% less than metal production from ore and 61–67% less than the process using conventional distillation. The processing cost is also shown to be lowered from $34/kg to $115/kg for the best-case and worst-case scenarios without G-METS distillation, to $22/kg to $65/kg with it. The largest operating cost factor is labor, whose cost per kilogram of product would likely decline with increasing scale. These costs compare with today’s NdPr metal prices of $100–$120/kg, which would make operating margins much larger with G-METS than without it. The G-METS distillation process can therefore be considered a potential enabling technology for liquid metal leaching and distillation.
References
R. Ulewicz and E. Wyslocka, 24th International Conference on Metals and Material Proceedings, 1680 (2015).
Adamas Intelligence, UBS Rare Earth Forecasts Only Tell Half the Story (Adamas Intelligence, 2021). https://www.adamasintel.com/ubs-ndpr-forecasts-miss-mark/. Accessed Mar 27, 2021.
A.C. Powell, REWAS 2019, ed. G. Gaustad, C. Fleuriault, M. Gökelma, J. A. Howarter, R. Kirchain, K. Ma, C. Meskers, N. R. Neelamegham, E. Olivetti, A. C. Powell, F. Tesfaye, D. Verhulst, and M. Zhang, (Springer, 2019), p. 9. https://doi.org/10.1007/978-3-030-10386-6_2.
C. Booten, M. Mann, and A. Momen, O. Abdelaziz, Critical Material Supply Chain Analysis: Magnetocalorics Report No. DE-AC36-08GO28308, March 2020 https://www.nrel.gov/docs/fy20osti/75163.pdf
Y. Yang, A. Walton, R. Sheridan, K. Guth, R. GauB, O. Gutfleisch, M. Buchert, B. Steenari, T.V. Gerven, T.P. Jones, and K. Binnemans, REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 3, 2021. https://doi.org/10.1007/s40831-016-0090-4.AccessedApril1 (2017).
J.H. Rademaker, R. Kleijn, and Y. Yang, Environ. Sci. Technol. 47, 10129. https://doi.org/10.1021/es305007w (2013).
N. Sekine, I. Daigo, and Y. Goto, J. Ind. Ecol. 21, 356. https://doi.org/10.1111/jiec.12458 (2017).
E. Alonso, T. Wallington, A. Sherman, M. Everson, F. Field, R. Roth, R. Kirchain, and S.A.E. Int, J. Mater. Manuf. 5, 473 (2012). https://www.jstor.org/stable/10.2307/26268481.
S. C. Davis and R. G. Boundy, Transportation Energy Data Book: Edition 39, (U.S. Department of Energy SciTech Connect, 2020). https://tedb.ornl.gov/wp-content/uploads/2021/02/TEDB_Ed_39.pdf. Accessed April 1 2020.
N. Akil, S. Loutatidou, D. Arslan, E. Festa, P. Circelli, and S. Colella, Report No. REE4EU- GA n° 680507, (REE4EU, Market Analysis Report), October 2019.
R. Wiser, M. Bolinger, G. Barbose, N. Darghouth, B. Hoen, A. Mills, J. Rand, D. Millstein, S. Jeong, K. Porter, N. Disanti, F. Oteri, Report No. GO-102019-5191, Department of Energy, Oak Ridge, Tennesse, August 2019. https://www.energy.gov/eere/wind/downloads/2018-wind-technologies-market-report
G. Prakash, H. Anuta, N. Wagner, G. Gallina, Deployment, Investment, Technology, Grid Integration and Socio-economic Aspects (A Global Energy Transformation paper), (International Renewable Energy Agency, Abu Dhabi, 2019) https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2019/Oct/IRENA_Future_of_wind_2019.pdf. Accessed July 9 2021.
D.D. Imholte, R.T. Nguyen, A. Vedantam, M. Brown, A. Iyer, B.J. Smith, J.W. Collins, C.G. Anderson, and B. O’Kelley, Energy Policy 113, 294. https://doi.org/10.1016/j.enpol.2017.11.001 (2018).
T. E. Lister, P. Wang, A. Anderko, Electrorecycling of Critical and Value Metals from Mobile Electronics (Canadian Institute of Mining, Metallurgy and Petroleum, 2014), https://inldigitallibrary.inl.gov/sites/sti/sti/6269294.pdf.
R.T. Nguyen, L.A. Diaz, D.D. Imholte, and T.E. Lister, JOM 69, 1546. https://doi.org/10.1007/s11837-017-2399-2 (2017).
I.C. Nlebedim, and A.H. King, JOM 70, 115. https://doi.org/10.1007/s11837-017-2698-7 (2018).
G. Gaustad, E. Williams, and A. Leader, Resour. Conserv. Recycl. 167, 105213. https://doi.org/10.1016/j.resconrec.2020.105213 (2021).
K. Binnemans, P.T. Jones, B. Blanpain, T. Van Gerven, and Y. Pontikes, J. Clean. Prod. 99, 17. https://doi.org/10.1016/j.jclepro.2015.02.089 (2015).
I. B. Fernandes, A. Abadías Llamas and M. A. Reuter, JOM 72(7), 2754. https://doi.org/10.1007/s11837-020-04185-6 (2020).
L. T. Peiró and G. V. Méndez, JOM 65(10). https://doi.org/10.1007/s11837-013-0719-8 (2013).
B. Inc, Rare Earth Elements to Make a Strong Comeback After 2020 Setback, Says Beroe Inc. https://www.prnewswire.com/news-releases/rare-earth-elements-to-make-a-strong-comeback-after-2020-setback-says-beroe-inc-301271188.html. Accessed Oct 01, 2021.
Statistica, REO price by type 2020, Statista. https://www.statista.com/statistics/617249/price-range-of-selected-rare-earth-oxides/. Accessed Oct 01 2021.
T. Akahori, Y. Miyamoto, T. Saeki, M. Okamoto, and T. Okabe, Magnesium Technol. 2014, 35. https://doi.org/10.1007/978-3-319-48231-6_10 (2014).
T. W. Ellis and F. A. Schmidt, Recycling of Rare Earth Metals from Rare Earth-Transition Metal Alloy Scrap by Liquid Metal Extraction, (U.S. Patents, 5,437,709, 1995) https://patents.google.com/patent/US5437709A/en. Accessed May 17 2021.
R. T. Ott and R. W. McCallum, Recovering rare earth metals from magnet scrap, (U.S. Patent 10,323,299, Jun. 18, 2019. https://patents.google.com/patent/US10323299B2/en. Accessed May 17 2021
A.E. Telgerafchi, G. Espinosa, M. Rutherford, A. Powell, and D. Dussault, Magnesium 2021, 145. https://doi.org/10.1007/978-3-030-65528-0_22 (2021).
B. Ott, D. E. Spiller, and P. R. Taylor, (Springer, 2019), https://link.springer.com/content/pdf/10.1007%2F978-3-030-10386-6.pdf Accessed 24 Jun 2021
H. W. Na, Y. H. Kim, H. Taek Son, I. Ho Jung, H. Shin Choi, and T. Bum Kim, Curr. Nanosci. 10, 128. https://doi.org/10.2174/1573413709666131109002236 (2014).
Y. Xu, L.S. Chumbley, and F.C. Laabs, J. Mater. Res. 15, 2296. https://doi.org/10.1557/JMR.2000.0330 (2000).
R. T. Ott, R. W. Ralph, Recovering rare earth metals using bismuth extractant. (Ames lab.) http://isurftech.technologypublisher.com/technology/21024. Accessed May 17 2021.
S. Archambault, Economic Analysis of Rare Earth Elements Extraction from Clay Waste, University of Tennessee Honors Thesis Projects.https://trace.tennessee.edu/cgi/viewcontent.cgi?article=3086&context=utk_chanhonoproj. Accessed Sep 23 2020.
S. Das, G. Gaustad, A. Sekar, and E. Williams, J. Clean. Prod. 189, 539. https://doi.org/10.1016/j.jclepro.2018.03.252 (2018).
V.S. Thompson, M. Gupta, H. Jin, E. Vahidi, M. Yim, M.A. Jindra, V. Nguyen, Y. Fujita, J.W. Sutherland, Y. Jiao, W.D. Reed, and A.C.S. Sustain, Chem. Eng. 6, 1602. https://doi.org/10.1021/acssuschemeng.7b02771 (2018).
D. Matuszak, ARPA-E Metals Tool (Unpublished Spreadsheet Distributed to U.S, Department of Energy Grantees, 2013).
U.S. Energy Information Administration, State Electricity Profiles, https://www.eia.gov/electricity/state/. Accessed 1 October 2021.
M. Peters, K. Timmerhaus, and R. West, Plant Design and Economics for Chemical Engineers, 5th ed. McGraw-Hill Education, 2003.
A. C. Powell, D. M. Dussault, M. R. Earlam, A. Tajima, and C. Raymes, Method and Apparatus for Efficient Metal Distillation and Related Primary Production Process, (U.S. Patent Application 16/944,147, 2020 ), https://patents.google.com/patent/US20210040633A1/en. Accessed 26 Jun 2021.
Daily Rare Earth Metals price, Lme Comex Shfe Price of Rare Earth Metals live | SMM - China Metal Market. https://www.metal.com/Rare-Earth-Metals. Accessed Oct 01 2021.
Acknowledgements
This work was funded by the US DEVCOM Army Research Laboratory, Cooperative agreement W911NF-19-2-0108.
Funding
Army research laboratory, W911NF-19-2-0108.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chinwego, C., Wagner, H., Giancola, E. et al. Technoeconomic Analysis of Rare-Earth Metal Recycling Using Efficient Metal Distillation. JOM 74, 1296–1305 (2022). https://doi.org/10.1007/s11837-021-05045-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-05045-7