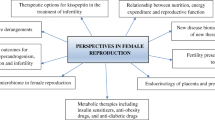
Abstract
Endometriosis and polycystic ovary syndrome (PCOS) are two common female reproductive disorders with a significant impact on the health and quality of life of women affected. A novel hypothesis by evolutionary biologists suggested that these two diseases are inversely related to one another, representing a pair of diametrical diseases in terms of opposite alterations in reproductive physiological processes but also contrasting phenotypic traits. However, to fully explain the phenotypic features observed in women with these conditions, we need to establish a potential nexus system between the reproductive system and general biological functions. The recent discovery of kisspeptin as pivotal mediator of internal and external inputs on the hypothalamic–pituitary–gonadal axis has led to a new understanding of the neuroendocrine upstream regulation of the human reproductive system. In this review, we summarize the current knowledge on the physiological roles of kisspeptin in human reproduction, as well as its involvement in complex biological functions such as metabolism, inflammation and pain sensitivity. Importantly, these functions are known to be dysregulated in both PCOS and endometriosis. Within the evolving scientific field of “kisspeptinology”, we critically discuss the clinical relevance of these discoveries and their potential translational applications in endometriosis and PCOS. By exploring the possibilities of manipulating this complex signaling system, we aim to pave the way for novel targeted therapies in these reproductive diseases.





Similar content being viewed by others
Abbreviations
- PCOS:
-
Polycystic ovary syndrome
- HPG:
-
Hypothalamic–pituitary–gonadal
- KP:
-
Kisspeptin
- GnRH:
-
Gonadotropin Releasing Hormone
- SANRA:
-
Scale for the Assessment of Narrative Review Articles
- KPR:
-
Kisspeptin receptor
- KISS1:
-
Kisspeptin (gene)
- KISS1R:
-
Kisspeptin receptor (gene)
- RF-amide peptide:
-
Arginine-Phenylalanine amide peptide (sequence at C-terminus)
- GPR54:
-
G protein-coupled receptor 54
- PLC:
-
Phospholipase C
- DAG:
-
Diacylglycerol
- IP3:
-
Inositol-(1,4,5)-triphosphate
- LH:
-
Luteinizing hormone
- FSH:
-
Follicle stimulating hormone
- KNDy:
-
Kisspeptin-Neurokinin B-Dynorphin neurons
- NKB:
-
Neurokinin B
- E2:
-
Estradiol
- POA:
-
Preoptic area
- POI:
-
Premature ovarian insufficiency
- AMH:
-
Anti-Mullerian hormone
- KO:
-
Knockout
- GCs:
-
Granulosa cells,
- CCs:
-
Cumulus cells
- ESCs:
-
Endometrial stromal cells
- LIF:
-
Leukemia inhibitory factor
- MMPs:
-
Matrix metalloproteinases
- VEGFA:
-
Vascular endothelial growth factor A
- BMI:
-
Body mass index
- EP:
-
Ectopic pregnancy
- PTB:
-
Preterm birth
- FGR:
-
Fetal growth restriction
- HDP:
-
Hypertensive disorders of pregnancy
- GDM:
-
Gestational diabetes mellitus
- GTD:
-
Gestational trophoblastic disease
- CH:
-
Chronic hypertension
- PIH:
-
Pregnancy induced hypertension
- PE:
-
Pre-eclampsia
- GSIS:
-
Glucose‐stimulated insulin secretion
- LPS:
-
Lipopolysaccharide
- TNF:
-
Tumor necrosis factor
- WHR:
-
Waist-to-hip ratio
- NK3Ra:
-
Neurokinin-3-receptor antagonists
- BBB:
-
Blood-brain-barrier
References
Dinsdale NL, Crespi BJ. Endometriosis and polycystic ovary syndrome are diametric disorders. Evol Appl. 2021;14(7):1693–715. https://doi.org/10.1111/eva.13244.
Dinsdale N, Nepomnaschy P, Crespi B. The evolutionary biology of endometriosis. Evol Med Public Health. 2021;9(1):174–91. https://doi.org/10.1093/emph/eoab008.
Fabozzi G, Rebuzzini P, Cimadomo D, Allori M, Franzago M, Stuppia L, Garagna S, Ubaldi F, Zuccotti M, Rienzi L. Endocrine-disrupting chemicals, gut microbiota, and human (in)Fertility—It is time to consider the triad. Cells. 2022;11(21):3335. https://doi.org/10.3390/cells11213335.
Fontana R, Torre SD. The deep correlation between energy metabolism and reproduction: A view on the effects of nutrition for women fertility. Nutrients. 2016;8(2):87. https://doi.org/10.3390/nu8020087.
Fujiwara T, Ono M, Mieda M, Yoshikawa H, Nakata R, Daikoku T, Sekizuka-Kagami N, Maida Y, Ando H, Fujiwara H. Adolescent dietary habit-induced obstetric and gynecologic disease (ADHOGD) as a new Hypothesis—Possible involvement of clock system. Nutrients. 2020;12(5):1294. https://doi.org/10.3390/nu12051294.
Shao S, Zhao H, Lu Z, Lei X, Zhang Y. Circadian rhythms within the female HPG axis: From physiology to etiology. Endocrinolog. 2021;162(8):bqab117. https://doi.org/10.1210/endocr/bqab117.
de Roux N, Genin E, Carel J, Matsuda F, Chaussain J, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–6. https://doi.org/10.1073/pnas.1834399100.
Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–27. https://doi.org/10.1056/NEJMoa035322.
Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20(4):485–500. https://doi.org/10.1093/humupd/dmu009.
Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92(3):1235–316. https://doi.org/10.1152/physrev.00037.2010.
Cejudo Roman A, Pinto FM, Dorta I, Almeida TA, Hernández M, Illanes M, Tena-Sempere M, Candenas L. Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract. Fertil Steril. 2012;97(5):1213–9. https://doi.org/10.1016/j.fertnstert.2012.02.021.
Masumi S, Lee EB, Dilower I, Upadhyaya S, Chakravarthi VP, Fields PE, Rumi MAK. The role of Kisspeptin signaling in Oocyte maturation. Front Endocrinol (Lausanne). 2022;13:917464. https://doi.org/10.3389/fendo.2022.917464.
León S, Fernandois D, Sull A, Sull J, Calder M, Hayashi K, Bhattacharya M, Power S, Vilos GA, Vilos AG, Tena-Sempere M, Babwah AV. Beyond the brain-Peripheral kisspeptin signaling is essential for promoting endometrial gland development and function. Sci Rep. 2016;6:29073. https://doi.org/10.1038/srep29073.
Hu K, Chang H, Zhao H, Yu Y, Li R, Qiao J. Potential roles for the kisspeptin/kisspeptin receptor system in implantation and placentation. Hum Reprod Update. 2019;25(3):326–43. https://doi.org/10.1093/humupd/dmy046.
Millar RP, Newton CL. Current and future applications of GnRH, kisspeptin and neurokinin B analogues. Nat Rev Endocrinol. 2013;9(8):451–66. https://doi.org/10.1038/nrendo.2013.120.
Barabás K, Szabó-Meleg E, Ábrahám IM. Effect of inflammation on female gonadotropin-releasing hormone (GnRH) neurons: Mechanisms and consequences. Int J Mol Sci. 2020;21(2):529. https://doi.org/10.3390/ijms21020529.
Crespi B. Variation among human populations in endometriosis and PCOS A test of the inverse comorbidity model. Evol Med Public Health. 2021;9(1):295–310. https://doi.org/10.1093/emph/eoab029.
Lehman MN, Coolen LM, Steiner RA, Neal-Perry G, Wang L, Moenter SM, Moore AM, Goodman RL, Yeo S-, Padilla SL, Kauffman AS, Garcia J, Kelly MJ, Clarkson J, Radovick S, Babwah AV, Leon S, Tena-Sempere M, Comninos AN, Seminara S, Dhillo WS, Levine J, Terasawa E, Negron A, Navarro VM, Herbison AE. The 3rd world conference on kisspeptin, “Kisspeptin 2017: Brain and beyond”: Unresolved questions, challenges and future directions for the field. J Neuroendocrinol. 2018:e12600. https://doi.org/10.1111/jne.12600.
Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5. https://doi.org/10.1186/s41073-019-0064-8.
Dockray GJ. The expanding family of -RFamide peptides and their effects on feeding behaviour. Exp Physiol. 2004;89(3):229–35. https://doi.org/10.1113/expphysiol.2004.027169.
Lee J, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88(23):1731–7. https://doi.org/10.1093/jnci/88.23.1731.
West A, Vojta PJ, Welch DR, Weissman BE. Chromosome localization and genomic structure of the KiSS-1 metastasis suppressor gene (KISS1). Genomics. 1998;54(1):145–8. https://doi.org/10.1006/geno.1998.5566.
Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411(6837):613–7. https://doi.org/10.1038/35079135.
Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden J, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276(37):34631–6. https://doi.org/10.1074/jbc.M104847200.
Gottsch ML, Clifton DK, Steiner RA. From KISS1 to kisspeptins: An historical perspective and suggested nomenclature. Peptides. 2009;30(1):4–9. https://doi.org/10.1016/j.peptides.2008.06.016.
Navenot JM, Fujii N, Peiper SC. KiSS1 metastasis suppressor gene product induces suppression of tyrosine kinase receptor signaling to Akt, tumor necrosis factor family ligand expression, and apoptosis. Mol Pharmacol. 2009;75(5):1074–83. https://doi.org/10.1124/mol.108.054270.
Bai D, Ueno L, Vogt PK. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer. 2009;125(12):2863–70. https://doi.org/10.1002/ijc.24748.
Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16(1):80. https://doi.org/10.1186/s12958-018-0391-5.
Jeong K, Kaiser UB. Chapter 31 - Gonadotropin-Releasing Hormone Regulation of Gonadotropin Biosynthesis and Secretion. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3rd ed. Elsevier: Academic press; 2006. p. 1635–701.
Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149(9):4605–14. https://doi.org/10.1210/en.2008-0321.
Millar RP, Roseweir AK, Tello JA, Anderson RA, George JT, Morgan K, Pawson AJ. Kisspeptin antagonists: Unraveling the role of kisspeptin in reproductive physiology. Brain Res. 2010;1364:81–9. https://doi.org/10.1016/j.brainres.2010.09.044.
Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–9. https://doi.org/10.1523/JNEUROSCI.5740-08.2009.
Silveira LG, Noel SD, Silveira-Neto AP, Abreu AP, Brito VN, Santos MG, Bianco SDC, Kuohung W, Xu S, Gryngarten M, Escobar ME, Arnhold IJP, Mendonca BB, Kaiser UB, Latronico AC. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95(5):2276–80. https://doi.org/10.1210/jc.2009-2421.
Teles MG, Bianco SDC, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358(7):709–15. https://doi.org/10.1056/NEJMoa073443.
Constantin S, Bjelobaba I, Stojilkovic SS. Pituitary gonadotroph-specific patterns of gene expression and hormone secretion. Curr Opin Pharmacol. 2022;66:102274. https://doi.org/10.1016/j.coph.2022.102274.
Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92(10):3958–66. https://doi.org/10.1210/jc.2007-1116.
Narayanaswamy S, Jayasena CN, Ng N, Ratnasabapathy R, Prague JK, Papadopoulou D, Abbara A, Comninos AN, Bassett P, Bloom SR, Veldhuis JD, Dhillo WS. Subcutaneous infusion of kisspeptin-54 stimulates gonadotrophin release in women and the response correlates with basal oestradiol levels. Clin Endocrinol (Oxf). 2016;84(6):939–45. https://doi.org/10.1111/cen.12977.
Jayasena CN, Comninos AN, Veldhuis JD, Misra S, Abbara A, Izzi-Engbeaya C, Donaldson M, Ghatei MA, Bloom SR, Dhillo WS. A single injection of kisspeptin-54 temporarily increases luteinizing hormone pulsatility in healthy women. Clin Endocrinol (Oxf). 2013;79(4):558–63. https://doi.org/10.1111/cen.12179.
Navarro VM, Castellano JM, Fernández-Fernández R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146(1):156–63. https://doi.org/10.1210/en.2004-0836.
Gutiérrez-Pascual E, Martínez-Fuentes AJ, Pinilla L, Tena-Sempere M, Malagón MM, Castaño JP. Direct pituitary effects of kisspeptin: Activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J Neuroendocrinol. 2007;19(7):521–30. https://doi.org/10.1111/j.1365-2826.2007.01558.x.
Prashar V, Arora T, Singh R, Sharma A, Parkash J. Hypothalamic kisspeptin neurons: Integral elements of the GnRH system. Reprod Sci. 2023;30(3):802–22. https://doi.org/10.1007/s43032-022-01027-5.
Rometo AM, Krajewski SJ, Lou Voytko M, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92(7):2744–50. https://doi.org/10.1210/jc.2007-0553.
Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: Sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31(11):1984–98. https://doi.org/10.1111/j.1460-9568.2010.07239.x.
Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: Sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301–11. https://doi.org/10.1210/en.2009-0541.
Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy cells revisited. Endocrinology. 2018;159(9):3219–34. https://doi.org/10.1210/en.2018-00389.
Uenoyama Y, Nagae M, Tsuchida H, Inoue N, Tsukamura H. Role of KNDy neurons expressing kisspeptin, neurokinin B, and dynorphin A as a GnRH pulse generator controlling mammalian reproduction. Front Endocrinol (Lausanne). 2021;12:724632. https://doi.org/10.3389/fendo.2021.724632.
Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–67. https://doi.org/10.1210/en.2003-1305.
Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713–43. https://doi.org/10.1210/er.2009-0005.
Zhang C, Bosch MA, Qiu J, Rønnekleiv OK, Kelly MJ. 17β-estradiol increases persistent na+ current and excitability of AVPV/PeN Kiss1 neurons in female mice. Mol Endocrinol. 2015;29(4):518–27. https://doi.org/10.1210/me.2014-1392.
McCartney CR, Campbell RE. Abnormal GnRH pulsatility in polycystic ovary syndrome: Recent insights. Curr Opin Endocr Metab Res. 2020;12:78–84. https://doi.org/10.1016/j.coemr.2020.04.005.
Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77(4):332–7. https://doi.org/10.1016/j.steroids.2011.12.007.
Coyle C, Campbell RE. Pathological pulses in PCOS. Mol Cell Endocrinol. 2019;498:110561. https://doi.org/10.1016/j.mce.2019.110561.
Li Y, Li R, Ouyang N, Dai K, Yuan P, Zheng L, Wang W. Investigating the impact of local inflammation on granulosa cells and follicular development in women with ovarian endometriosis. Fertil Steril. 2019;112(5):882-891.e1. https://doi.org/10.1016/j.fertnstert.2019.07.007.
González-Fernández R, Peña Ó, Hernández J, Martín-Vasallo P, Palumbo A, Ávila J. Patients with endometriosis and patients with poor ovarian reserve have abnormal follicle-stimulating hormone receptor signaling pathways. Fertil Steril. 2011;95(7):2373–8. https://doi.org/10.1016/j.fertnstert.2011.03.030.
Chang RJ, Cook-Andersen H. Disordered follicle development. Mol Cell Endocrinol. 2013;373(1–2):51–60. https://doi.org/10.1016/j.mce.2012.07.011.
Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14(4):367–78. https://doi.org/10.1093/humupd/dmn015.
Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, Catteau-Jonard S, Collier F, Baroncini M, Dewailly D, Pigny P, Prescott M, Campbell R, Herbison AE, Prevot V, Giacobini P. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. 2016;7:10055. https://doi.org/10.1038/ncomms10055.
Garg D, Tal R. The role of AMH in the pathophysiology of polycystic ovarian syndrome. Reprod Biomed Online. 2016;33(1):15–28. https://doi.org/10.1016/j.rbmo.2016.04.007.
Owens LA, Kristensen SG, Lerner A, Christopoulos G, Lavery S, Hanyaloglu AC, Hardy K, Yding Andersen C, Franks S. Gene expression in granulosa cells from small antral follicles from women with or without polycystic ovaries. J Clin Endocrinol Metab. 2019;104(12):6182–92. https://doi.org/10.1210/jc.2019-00780.
de Carvalho BR, de Sá Rosa ACJ, Rosa-e-Silva JC, dos Reis RM, Ferriani RA, Silva-de-Sá MF. Increased basal FSH levels as predictors of low-quality follicles in infertile women with endometriosis. Int J Gynaecol Obstet. 2010;110(3):208–12. https://doi.org/10.1016/j.ijgo.2010.03.033.
Romanski PA, Brady PC, Farland LV, Thomas AM, Hornstein MD. The effect of endometriosis on the antimüllerian hormone level in the infertile population. J Assist Reprod Genet. 2019;36(6):1179–84. https://doi.org/10.1007/s10815-019-01450-9.
Dong Z, An J, Xie X, Wang Z, Sun P. Preoperative serum anti-müllerian hormone level is a potential predictor of ovarian endometrioma severity and postoperative fertility. Eur J Obstet Gynecol Reprod Biol. 2019;240:113–20. https://doi.org/10.1016/j.ejogrb.2019.06.024.
Rönnberg L, Kauppila A, Rajaniemi H. Luteinizing hormone receptor disorder in endometriosis. Fertil Steril. 1984;42(1):64–8. https://doi.org/10.1016/S0015-0282(16)47959-8.
Cahill DJ, Hull MGR. Pituitary–ovarian dysfunction and endometriosis. Hum Reprod Update. 2000;6(1):56–66. https://doi.org/10.1093/humupd/6.1.56.
Moore AM, Lohr DB, Coolen LM, Lehman MN. Prenatal androgen exposure alters KNDy neurons and their afferent network in a model of polycystic ovarian syndrome. Endocrinology. 2021;162(11):bqab158. https://doi.org/10.1210/endocr/bqab158.
Osuka S, Iwase A, Nakahara T, Kondo M, Saito A, Bayasula, Nakamura T, Takikawa S, Goto M, Kotani T, Kikkawa F. Kisspeptin in the hypothalamus of two rat models of polycystic ovary syndrome. Endocrinology. 2016;158(2):en.2016–1333. https://doi.org/10.1210/en.2016-1333.
Matsuzaki T, Tungalagsuvd A, Iwasa T, Munkhzaya M, Yanagihara R, Tokui T, et al. Kisspeptin mRNA expression is increased in the posterior hypothalamus in the rat model of polycystic ovary syndrome. Endocr J. 2017;64(1):7–14. https://doi.org/10.1507/endocrj.EJ16-0282.
Cernea M, Padmanabhan V, Goodman RL, Coolen LM, Lehman MN. Prenatal testosterone treatment leads to changes in the morphology of KNDy neurons, their inputs, and projections to GnRH cells in female sheep. Endocrinology. 2015;156(9):3277–91. https://doi.org/10.1210/en.2014-1609.
Filippou P, Homburg R. Is foetal hyperexposure to androgens a cause of PCOS? Hum Reprod Update. 2017;23(4):421–32. https://doi.org/10.1093/humupd/dmx013.
Esparza LA, Schafer D, Ho BS, Thackray VG, Kauffman AS. Hyperactive LH pulses and elevated kisspeptin and NKB gene expression in the arcuate nucleus of a PCOS mouse model. Endocrinology. 2020;161(4):1. https://doi.org/10.1210/endocr/bqaa018.
León S, Barroso A, Vázquez MJ, García-Galiano D, Manfredi-Lozano M, Ruiz-Pino F, Heras V, Romero-Ruiz A, Roa J, Schutz G, Kirilov M, Gaytan F, Pinilla L, Tena-Sempere M. Direct actions of Kisspeptins on GnRH neurons permit attainment of fertility but are insufficient to fully preserve gonadotropic axis activity. Sci Rep. 2016;6:19206. https://doi.org/10.1038/srep19206.
Terao Y, Kumano S, Takatsu Y, Hattori M, Nishimura A, Ohtaki T, Shintani Y. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta. 2004;1678(2–3):102–10. https://doi.org/10.1016/j.bbaexp.2004.02.005.
Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, Dieguez C, Aguilar E, Sánchez-Criado JE, Pellicer A, Pinilla L, Gaytan F, Tena-Sempere M. Expression of KiSS-1 in rat ovary: Putative local regulator of ovulation? Endocrinology. 2006;147(10):4852–62. https://doi.org/10.1210/en.2006-0117.
García-Ortega J, Pinto FM, Prados N, Bello AR, Almeida TA, Fernández-Sánchez M, Candenas L. Expression of tachykinins and tachykinin receptors and interaction with kisspeptin in human granulosa and cumulus Cells. Biol Reprod. 2016;94(6):1. https://doi.org/10.1095/biolreprod.116.139881.
García-Ortega J, Pinto FM, Fernández-Sánchez M, Prados N, Cejudo-Román A, Almeida TA, Hernández M, Romero M, Tena-Sempere M, Candenas L. Expression of neurokinin B/NK3 receptor and kisspeptin/KISS1 receptor in human granulosa cells. Hum Reprod. 2014;29(12):2736–46. https://doi.org/10.1093/humrep/deu247.
Hu K, Zhao H, Chang H, Yu Y, Qiao J. Kisspeptin/kisspeptin receptor system in the ovary. Front Endocrinol (Lausanne). 2017;8:365. https://doi.org/10.3389/fendo.2017.00365.
Shahed A, Young KA. Differential ovarian expression of KiSS-1 and GPR-54 during the estrous cycle and photoperiod induced recrudescence in Siberian hamsters (Phodopus sungorus). Mol Reprod Dev. 2009;76(5):444–52. https://doi.org/10.1002/mrd.20972.
Rehman R, Zafar A, Ali AA, Baig M, Alam F. Impact of serum and follicular fluid kisspeptin and estradiol on oocyte maturity and endometrial thickness among unexplained infertile females during ICSI. PLoS ONE. 2020;15(10):e0239142. https://doi.org/10.1371/journal.pone.0239142.
Merhi Z, Thornton K, Bonney E, Cipolla MJ, Charron MJ, Buyuk E. Ovarian kisspeptin expression is related to age and to monocyte chemoattractant protein-1. J Assist Reprod Genet. 2016;33(4):535–43. https://doi.org/10.1007/s10815-016-0672-x.
Babayev E, Duncan FE. Age-associated changes in cumulus cells and follicular fluid: The local oocyte microenvironment as a determinant of gamete quality. Biol Reprod. 2022;106(2):351–65. https://doi.org/10.1093/biolre/ioab241.
Gonnella F, Konstantinidou F, Di Berardino C, Capacchietti G, Peserico A, Russo V, Barboni B, Stuppia L, Gatta V. A systematic review of the effects of high-fat diet exposure on oocyte and follicular quality: A molecular point of view. Int J Mol Sci. 2022;23(16):8890. https://doi.org/10.3390/ijms23168890.
Mlyczyńska E, Kieżun M, Kurowska P, Dawid M, Pich K, Respekta N, Daudon M, Rytelewska E, Dobrzyń K, Kamińska B, Kamiński T, Smolińska N, Dupont J, Rak A. New aspects of corpus luteum regulation in physiological and pathological conditions: Involvement of adipokines and neuropeptides. Cells. 2022;11(6):957–1047. https://doi.org/10.3390/cells11060957.
Ruohonen ST, Gaytan F, Usseglio Gaudi A, Velasco I, Kukoricza K, Perdices-Lopez C, Franssen D, Guler I, Mehmood A, Elo LL, Ohlsson C, Poutanen M, Tena-Sempere M. Selective loss of kisspeptin signaling in oocytes causes progressive premature ovulatory failure. Hum Reprod. 2022;37(4):806–21. https://doi.org/10.1093/humrep/deab287.
Baba T, Kang HS, Hosoe Y, Kharma B, Abiko K, Matsumura N, Hamanishi J, Yamaguchi K, Yoshioka Y, Koshiyama M, Mandai M, Murphy SK, Konishi I. Menstrual cyclic change of metastin/GPR54 in endometrium. Med Mol Morphol. 2015;48(2):76–84. https://doi.org/10.1007/s00795-014-0081-0.
Kang HS, Baba T, Mandai M, Matsumura N, Hamanishi J, Kharma B, Kondoh E, Yoshioka Y, Oishi S, Fujii N, Murphy SK, Konishi I. GPR54 is a target for suppression of metastasis in endometrial cancer. Mol Cancer Ther. 2011;10(4):580–90. https://doi.org/10.1158/1535-7163.MCT-10-0763.
Calder M, Chan Y, Raj R, Pampillo M, Elbert A, Noonan M, Gillio-Meina C, Caligioni C, Bérubé NG, Bhattacharya M, Watson AJ, Seminara SB, Babwah AV. Implantation failure in female Kiss1−/− mice is independent of their hypogonadic state and can be partially rescued by leukemia inhibitory factor. Endocrinology. 2014;155(8):3065–78. https://doi.org/10.1210/en.2013-1916.
Radovick S, Babwah AV. Regulation of pregnancy: Evidence for major roles by the uterine and placental kisspeptin/KISS1R signaling systems. Semin Reprod Med. 2019;37(4):182–90. https://doi.org/10.1055/s-0039-3400966.
Tsoutsouki J, Patel B, Comninos AN, Dhillo WS, Abbara A. Kisspeptin in the prediction of pregnancy complications. Front Endocrinol (Lausanne). 2022;13:942664. https://doi.org/10.3389/fendo.2022.942664.
Abbara A, Al-Memar M, Phylactou M, Kyriacou C, Eng PC, Nadir R, Izzi-Engbeaya C, Clarke SA, Mills EG, Daniels E, Huo L, Pacuszka E, Yang L, Patel B, Tan T, Bech P, Comninos AN, Fourie H, Kelsey TW, Bourne T, Dhillo WS. Performance of plasma kisspeptin as a biomarker for miscarriage improves with gestational age during the first trimester. Fertil Steril. 2021;116(3):809–19. https://doi.org/10.1016/j.fertnstert.2021.04.031.
Szydełko-Gorzkowicz M, Poniedziałek-Czajkowska E, Mierzyński R, Sotowski M, Leszczyńska-Gorzelak B. The role of kisspeptin in the pathogenesis of pregnancy complications: A narrative review. Int J Mol Sci. 2022;23(12):6611. https://doi.org/10.3390/ijms23126611.
Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol. 2005;48(2):372–86. https://doi.org/10.1097/01.grf.0000160313.82606.d7.
Armstrong RA, Reynolds RM, Leask R, Shearing CH, Calder AA, Riley SC. Decreased serum levels of kisspeptin in early pregnancy are associated with intra-uterine growth restriction and pre-eclampsia. Prenat Diagn. 2009;29(10):982–5. https://doi.org/10.1002/pd.2328.
Abbara A, Al-Memar M, Phylactou M, Daniels E, Patel B, Eng PC, Nadir R, Izzi-Engbeaya C, Clarke SA, Mills EG, Hunjan T, Pacuszka E, Yang L, Bech P, Tan T, Comninos AN, Kelsey TW, Kyriacou C, Fourie H, Bourne T, Dhillo WS. Changes in circulating Kisspeptin levels during each trimester in women with antenatal complications. J Clin Endocrinol Metab. 2022;107(1):e71–83. https://doi.org/10.1210/clinem/dgab617.
Torricelli M, Galleri L, Voltolini C, Biliotti G, Florio P, De Bonis M, Petraglia F. Changes of placental kiss-1 mRNA expression and maternal/cord kisspeptin levels at preterm delivery. Reprod Sci. 2008;15(8):779–84. https://doi.org/10.1177/1933719108322442.
Wawrzkiewicz-Jałowiecka A, Kowalczyk K, Trybek P, Jarosz T, Radosz P, Setlak M, Madej P. In search of new Therapeutics—Molecular aspects of the PCOS pathophysiology: Genetics, hormones, metabolism and beyond. Int J Mol Sci. 2020;21(19):7054. https://doi.org/10.3390/ijms21197054.
Tang R, Ding X, Zhu J. Kisspeptin and Polycystic Ovary Syndrome. Front Endocrinol (Lausanne). 2019;10:298. https://doi.org/10.3389/fendo.2019.00298.
Hu KL, Zhao H, Min Z, He Y, Li T, Zhen X, Ren Y, Chang HM, Yu Y, Li R. Increased expression of KISS1 and KISS1 receptor in human granulosa lutein cells-potential pathogenesis of polycystic ovary syndrome. Reprod Sci. 2019;26(11):1429–38. https://doi.org/10.1177/1933719118818899.
Blasco V, Pinto FM, Fernández-Atucha A, Prados N, Tena-Sempere M, Fernández-Sánchez M, Candenas L. Altered expression of the kisspeptin/KISS1R and neurokinin B/NK3R systems in mural granulosa and cumulus cells of patients with polycystic ovarian syndrome. J Assist Reprod Genet. 2019;36(1):113–20. https://doi.org/10.1007/s10815-018-1338-7.
Timologou A, Zafrakas MM, Grimbizis G, Miliaras D, Kotronis K, Stamatopoulos P, Tarlatzis BC. Immunohistochemical expression pattern of metastasis suppressors KAI1 and KISS1 in endometriosis and normal endometrium. Eur J Obstet Gynecol Reprod Biol. 2016;199:110–5. https://doi.org/10.1016/j.ejogrb.2016.02.004.
Abdelkareem AO, Alotaibi FT, AlKusayer GM, Ait-Allah AS, Rasheed SM, Helmy YA, Allaire C, Peng B, Yong PJ, Bedaiwy MA. Immunoreactivity of kisspeptin and kisspeptin receptor in eutopic and ectopic endometrial tissue of women with and without endometriosis. Reprod Sci. 2020;27(9):1731–41. https://doi.org/10.1007/s43032-020-00167-w.
Önal M, Karli P, Özdemir AZ, Kocaman A, Katirci Y, Çoban G, Nakişli GK, Civil Y, Avci B. Serum kisspeptin levels in deep-infiltrating, ovarian, and superficial endometriosis: A prospective observational study. Medicine (Baltimore). 2022;101(45):e31529. https://doi.org/10.1097/MD.0000000000031529.
Blasco V, Pinto FM, Fernández-Atucha A, González-Ravina C, Fernández-Sánchez M, Candenas L. Female infertility is associated with an altered expression of the neurokinin B/neurokinin B receptor and kisspeptin/kisspeptin receptor systems in ovarian granulosa and cumulus cells. Fertil Steril. 2020;114(4):869–78. https://doi.org/10.1016/j.fertnstert.2020.05.006.
Matsuzaki S, Ueda Y, Nagase Y, Matsuzaki S, Kakuda M, Kakuda S, Sakaguchi H, Hisa T, Kamiura S. Placenta accreta spectrum disorder complicated with endometriosis: Systematic review and meta-analysis. Biomedicines. 2022;10(2):390. https://doi.org/10.3390/biomedicines10020390.
Salmeri N, Farina A, Candiani M, Dolci C, Bonavina G, Poziello C, Viganò P, Cavoretto PI. Endometriosis and impaired placentation: A prospective cohort study comparing uterine arteries doppler pulsatility index in pregnancies of patients with and without moderate-severe disease. Diagnostics (Basel). 2022;12(5):1024. https://doi.org/10.3390/diagnostics12051024.
Kelley AS, Smith YR, Padmanabhan V. A narrative review of placental contribution to adverse pregnancy outcomes in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(11):5299–315. https://doi.org/10.1210/jc.2019-00383.
Palomba S, Piltonen TT, Giudice LC. Endometrial function in women with polycystic ovary syndrome: A comprehensive review. Hum Reprod Update. 2021;27(3):584–618. https://doi.org/10.1093/humupd/dmaa051.
Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276(31):28969–75. https://doi.org/10.1074/jbc.M102743200.
Navarro VM. Metabolic regulation of kisspeptin — the link between energy balance and reproduction. Nat Rev Endocrinol. 2020;16(8):407–20. https://doi.org/10.1038/s41574-020-0363-7.
Reinehr T, Roth CL. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc Health. 2019;3(1):44–54. https://doi.org/10.1016/S2352-4642(18)30306-7.
Itriyeva K. The effects of obesity on the menstrual cycle. Curr Probl Pediatr Adolesc Health Care. 2022;52(8):101241. https://doi.org/10.1016/j.cppeds.2022.101241.
Tolson KP, Garcia C, Yen S, Simonds S, Stefanidis A, Lawrence A, Smith JT, Kauffman AS. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J Clin Invest. 2014;124(7):3075–9. https://doi.org/10.1172/JCI71075.
Castellano JM, Navarro VM, Roa J, Pineda R, Sánchez-Garrido MA, García-Galiano D, Vigo E, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Alterations in hypothalamic KiSS-1 system in experimental diabetes: Early changes and functional consequences. Endocrinology. 2009;150(2):784–94. https://doi.org/10.1210/en.2008-0849.
Plum L, Belgardt BF, Brüning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006;116(7):1761–6. https://doi.org/10.1172/JCI29063.
Hauge-Evans AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A role for kisspeptin in islet function. Diabetologia. 2006;49(9):2131–5. https://doi.org/10.1007/s00125-006-0343-z.
Bowe JE, Hill TG, Hunt KF, Smith LIF, Simpson SJS, Amiel SA, Jones PM. A role for placental kisspeptin in β cell adaptation to pregnancy. JCI Insight. 2019;4(20):e124540. https://doi.org/10.1172/jci.insight.124540.
Bowe JE, Foot VL, Amiel SA, Huang GC, Lamb M, Lakey J, Jones PM, Persaud SJ. GPR54 peptide agonists stimulate insulin secretion from murine, porcine and human islets. Islets. 2012;4(1):20–3. https://doi.org/10.4161/isl.18261.
Vikman J, Ahrén B. Inhibitory effect of kisspeptins on insulin secretion from isolated mouse islets. Diabetes Obes Metab. 2009;11(Suppl 4):197–201. https://doi.org/10.1111/j.1463-1326.2009.01116.x.
Chen J, Fu R, Cui Y, Pan J, Li Y, Zhang X, Evans SM, Cui S, Liu J. LIM-homeodomain transcription factor isl-1 mediates kisspeptin’s effect on insulin secretion in mice. Mol Endocrinol. 2014;28(8):1276–90. https://doi.org/10.1210/me.2013-1410.
Izzi-Engbeaya C, Comninos AN, Clarke SA, Jomard A, Yang L, Jones S, Abbara A, Narayanaswamy S, Eng PC, Papadopoulou D, Prague JK, Bech P, Godsland IF, Bassett P, Sands C, Camuzeaux S, Gomez-Romero M, Pearce JTM, Lewis MR, Holmes E, Nicholson JK, Tan T, Ratnasabapathy R, Hu M, Carrat G, Piemonti L, Bugliani M, Marchetti P, Johnson PR, Hughes SJ, James Shapiro AM, Rutter GA, Dhillo WS. The effects of kisspeptin on β-cell function, serum metabolites and appetite in humans. Diabetes Obes Metab. 2018;20(12):2800–10. https://doi.org/10.1111/dom.13460.
Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, Fujino M. Dramatic elevation of plasma metastin concentrations in human pregnancy: Metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88(2):914–9. https://doi.org/10.1210/jc.2002-021235.
Kołodziejski PA, Pruszyńska-Oszmałek E, Korek E, Sassek M, Szczepankiewicz D, Kaczmarek P, Nogowski L, Maćkowiak P, Nowak KW, Krauss H, Strowski MZ. Serum levels of spexin and kisspeptin negatively correlate with obesity and insulin resistance in women. Physiol Res. 2018;67(1):45–56. https://doi.org/10.33549/physiolres.933467.
Hestiantoro A, Astuti BPK, Muharam R, Pratama G, Witjaksono F, Wiweko B. Dysregulation of Kisspeptin and Leptin, as Anorexigenic Agents, Plays Role in the Development of Obesity in Postmenopausal Women. Int J Endocrinol. 2019;2019:1347208. https://doi.org/10.1155/2019/1347208.
True C, Verma S, Grove KL, Smith MS. Cocaine- and amphetamine-regulated transcript is a potent stimulator of GnRH and kisspeptin cells and may contribute to negative energy balance-induced reproductive inhibition in females. Endocrinology. 2013;154(8):2821–32. https://doi.org/10.1210/en.2013-1156.
Padilla SL, Qiu J, Nestor CC, Zhang C, Smith AW, Whiddon BB, Rønnekleiv OK, Kelly MJ, Palmiter RD. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci U S A. 2017;114(9):2413–8. https://doi.org/10.1073/pnas.1621065114.
Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18(4):298–303. https://doi.org/10.1111/j.1365-2826.2006.01417.x.
Donato J, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua J, Streamson, Coppari R, Zigman JM, Elmquist JK, Elias CF. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121(1):355–68. https://doi.org/10.1172/JCI45106.
Iwasa T, Matsuzaki T, Tungalagsuvd A, Munkhzaya M, Kawami T, Niki H, Kato T, Kuwahara A, Uemura H, Yasui T, Irahara M. Hypothalamic Kiss1 and RFRP gene expressions are changed by a high dose of lipopolysaccharide in female rats. Horm Behav. 2014;66(2):309–16. https://doi.org/10.1016/j.yhbeh.2014.06.007.
Lee CY, Li S, Li XF, Stalker DAE, Cooke C, Shao B, Kelestimur H, Henry BA, Conductier G, O’Byrne KT, Clarke IJ. Lipopolysaccharide reduces gonadotrophin-releasing hormone (GnRH) gene expression: Role of RFamide-related peptide-3 and kisspeptin. Reprod Fertil Dev. 2019;31(6):1134–43. https://doi.org/10.1071/RD18277.
Iwasa T, Matsuzaki T, Murakami M, Shimizu F, Kuwahara A, Yasui T, Irahara M. Decreased expression of kisspeptin mediates acute immune/inflammatory stress-induced suppression of gonadotropin secretion in female rat. J Endocrinol Invest. 2008;31(7):656–9. https://doi.org/10.1007/BF03345620.
Sarchielli E, Comeglio P, Squecco R, Ballerini L, Mello T, Guarnieri G, Idrizaj E, Mazzanti B, Vignozzi L, Gallina P, Maggi M, Vannelli GB, Morelli A. Tumor necrosis factor-α impairs kisspeptin signaling in human gonadotropin-releasing hormone primary neurons. J Clin Endocrinol Metab. 2017;102(1):46–56. https://doi.org/10.1210/jc.2016-2115.
Fergani C, Routly JE, Jones DN, Pickavance LC, Smith RF, Dobson H. KNDy neurone activation prior to the LH surge of the ewe is disrupted by LPS. Reproduction. 2017;154(3):281–92. https://doi.org/10.1530/REP-17-0191.
Sato K, Shirai R, Hontani M, Shinooka R, Hasegawa A, Kichise T, Yamashita T, Yoshizawa H, Watanabe R, Matsuyama T, Ishibashi-Ueda H, Koba S, Kobayashi Y, Hirano T, Watanabe T. Potent vasoconstrictor kisspeptin-10 induces atherosclerotic plaque progression and instability: Reversal by its receptor GPR54 antagonist. J Am Heart Assoc. 2017;6(4):e005790. https://doi.org/10.1161/JAHA.117.005790.
Wang D, Wu Z, Zhao C, Yang X, Wei H, Liu M, Zhao J, Qian M, Li Z, Xiao J. KP-10/Gpr54 attenuates rheumatic arthritis through inactivating NF-κB and MAPK signaling in macrophages. Pharmacol Res. 2021;171:105496. https://doi.org/10.1016/j.phrs.2021.105496.
Akdag T, Uca AU, Altas M, Odabas FO, Aktas F. Level of kisspeptin-10 in patients with multiple sclerosis and the association between third ventricle diameter size and vitamin D level. Physiol Int. 2021;2021:00179. https://doi.org/10.1556/2060.2021.00179.
Luedde M, Spehlmann ME, Hippe H, Loosen SH, Roy S, Vargas Cardenas D, Vucur M, Frey N, Koch A, Luedde T, Trautwein C, Tacke F, Roderburg C. Serum levels of kisspeptin are elevated in critically ill patients. PLoS ONE. 2018;13(10):e0206064. https://doi.org/10.1371/journal.pone.0206064.
Aczél T, Kun J, Szőke É, Rauch T, Junttila S, Gyenesei A, Bölcskei K, Helyes Z. Transcriptional alterations in the trigeminal ganglia, nucleus and peripheral blood mononuclear cells in a rat orofacial pain model. Front Mol Neurosci. 2018;11:219. https://doi.org/10.3389/fnmol.2018.00219.
Csabafi K, Bagosi Z, Dobó É, Szakács J, Telegdy G, Szabó G. Kisspeptin modulates pain sensitivity of CFLP mice. Peptides. 2018;105:21–7. https://doi.org/10.1016/j.peptides.2018.04.018.
Spampinato S, Trabucco A, Biasiotta A, Biagioni F, Cruccu G, Copani A, Colledge WH, Sortino MA, Nicoletti F, Chiechio S. Hyperalgesic activity of kisspeptin in mice. Mol Pain. 2011;7:90. https://doi.org/10.1186/1744-8069-7-90.
Elhabazi K, Humbert J, Bertin I, Schmitt M, Bihel F, Bourguignon J, Bucher B, Becker JAJ, Sorg T, Meziane H, Petit-Demoulière B, Ilien B, Simonin F. Endogenous mammalian RF-amide peptides, including PrRP, kisspeptin and 26RFa, modulate nociception and morphine analgesia via NPFF receptors. Neuropharmacology. 2013;75:164–71. https://doi.org/10.1016/j.neuropharm.2013.07.012.
Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618–37. https://doi.org/10.1093/humupd/dms030.
Wang F, Wu Y, Zhu Y, Ding T, Batterham RL, Qu F, Hardiman PJ. Pharmacologic therapy to induce weight loss in women who have obesity/overweight with polycystic ovary syndrome: a systematic review and network meta-analysis. Obes Rev. 2018;19(10):1424–45. https://doi.org/10.1111/obr.12720.
Adali E, Yildizhan R, Kurdoglu M, Kolusari A, Edirne T, Sahin HG, Yildizhan B, Kamaci M. The relationship between clinico-biochemical characteristics and psychiatric distress in young women with polycystic ovary syndrome. J Int Med Res. 2008;36(6):1188–96. https://doi.org/10.1177/147323000803600604.
Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, Di Fede G, Rini G. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab. 2007;92(7):2500–5. https://doi.org/10.1210/jc.2006-2725.
Carmina E, Guastella E, Longo RA, Rini GB, Lobo RA. Correlates of increased lean muscle mass in women with polycystic ovary syndrome. Eur J Endocrinol. 2009;161(4):583–9. https://doi.org/10.1530/EJE-09-0398.
Backonja U, Hediger ML, Chen Z, Lauver DR, Sun L, Peterson CM, Buck Louis GM. Beyond body mass index: Using anthropometric measures and body composition indicators to assess odds of an endometriosis diagnosis. J Womens Health (Larchmt). 2017;26(9):941–50. https://doi.org/10.1089/jwh.2016.6128.
Liu Y, Zhang W. Association between body mass index and endometriosis risk: A meta-analysis. Oncotarget. 2017;8(29):46928–36. https://doi.org/10.18632/oncotarget.14916.
Backonja U, Buck Louis G, Lauver D. Overall adiposity, adipose tissue distribution, and endometriosis: A systematic review. Nurs Res. 2016;65(2):151–66. https://doi.org/10.1097/NNR.0000000000000146.
Byun J, Peterson CM, Backonja U, Taylor RN, Stanford JB, Allen-Brady KL, Smith KR, Louis GMB, Schliep KC. Adiposity and endometriosis severity and typology. J Minim Invasive Gynecol. 2020;27(7):1516–23. https://doi.org/10.1016/j.jmig.2020.01.002.
Geronikolou SA, Pavlopoulou A, Cokkinos DV, Bacopoulou F, Chrousos GP. Polycystic οvary syndrome revisited: An interactions network approach. Eur J Clin Invest. 2021;51(9):e13578. https://doi.org/10.1111/eci.13578.
Sarray S, Madan S, Saleh LR, Mahmoud N, Almawi WY. Validity of adiponectin-to-leptin and adiponectin-to-resistin ratios as predictors of polycystic ovary syndrome. Fertil Steril. 2015;104(2):460–6. https://doi.org/10.1016/j.fertnstert.2015.05.007.
de Medeiros SF, Rodgers RJ, Norman RJ. Adipocyte and steroidogenic cell cross-talk in polycystic ovary syndrome. Hum Reprod Update. 2021;27(4):771–96. https://doi.org/10.1093/humupd/dmab004.
Schüler-Toprak S, Ortmann O, Buechler C, Treeck O. The complex roles of adipokines in polycystic ovary syndrome and endometriosis. Biomedicines. 2022;10(10):2503. https://doi.org/10.3390/biomedicines10102503.
Kalaitzopoulos DR, Lempesis IG, Samartzis N, Kolovos G, Dedes I, Daniilidis A, Nirgianakis K, Leeners B, Goulis DG, Samartzis EP. Leptin concentrations in endometriosis: A systematic review and meta-analysis. J Reprod Immunol. 2021;146:103338. https://doi.org/10.1016/j.jri.2021.103338.
Choi YS, Oh HK, Choi J. Expression of adiponectin, leptin, and their receptors in ovarian endometrioma. Fertil Steril. 2013;100(1):135,141.e2. https://doi.org/10.1016/j.fertnstert.2013.03.019.
Kim TH, Bae N, Kim T, Hsu AL, Hunter MI, Shin J, Jeong J. Leptin stimulates endometriosis development in mouse models. Biomedicines. 2022;10(9):2160. https://doi.org/10.3390/biomedicines10092160.
Styer AK, Sullivan BT, Puder M, Arsenault D, Petrozza JC, Serikawa T, Chang S, Hasan T, Gonzalez RR, Rueda BR. Ablation of leptin signaling disrupts the establishment, development, and maintenance of endometriosis-like lesions in a murine model. Endocrinology. 2008;149(2):506–14. https://doi.org/10.1210/en.2007-1225.
Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70. https://doi.org/10.1038/27376.
Ferrero S, Anserini P, Remorgida V, Ragni N. Body mass index in endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;121(1):94–8. https://doi.org/10.1016/j.ejogrb.2004.11.019.
Lafay Pillet M, Schneider A, Borghese B, Santulli P, Souza C, Streuli I, de Ziegler D, Chapron C. Deep infiltrating endometriosis is associated with markedly lower body mass index: A 476 case–control study. Hum Reprod. 2012;27(1):265–72. https://doi.org/10.1093/humrep/der346.
Bedaiwy MA, Falcone T, Goldberg JM, Sharma RK, Nelson DR, Agarwal A. Peritoneal fluid leptin is associated with chronic pelvic pain but not infertility in endometriosis patients. Hum Reprod. 2006;21(3):788–91. https://doi.org/10.1093/humrep/dei376.
Pantelis A, Machairiotis N, Lapatsanis DP. The formidable yet unresolved interplay between endometriosis and obesity. Sci World J. 2021;2021:6653677. https://doi.org/10.1155/2021/6653677.
Gruber TM, Mechsner S. Pathogenesis of Endometriosis: The origin of Pain and Subfertility. Cells. 2021;10(6):1381. https://doi.org/10.3390/cells10061381.
Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O’Byrne KT. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS ONE. 2009;4(12):e8334. https://doi.org/10.1371/journal.pone.0008334.
Young J, George JT, Tello JA, Francou B, Bouligand J, Guiochon-Mantel A, Brailly-Tabard S, Anderson RA, Millar RP. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: Physiological, pathophysiological and therapeutic implications. Neuroendocrinology. 2013;97(2):193–202. https://doi.org/10.1159/000336376.
Garg A, Patel B, Abbara AA, Dhillo WS. Treatments targeting neuroendocrine dysfunction in polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2022;97(2):156–64. https://doi.org/10.1111/cen.14704.
Romero-Ruiz A, Skorupskaite K, Gaytan F, Torres E, Perdices-Lopez C, Mannaerts BM, Qi S, Leon S, Manfredi-Lozano M, Lopez-Rodriguez C, Avendaño MS, Sanchez-Garrido MA, Vazquez MJ, Pinilla L, van Duin M, Kohout TA, Anderson RA, Tena-Sempere M. Kisspeptin treatment induces gonadotropic responses and rescues ovulation in a subset of preclinical models and women with polycystic ovary syndrome. Hum Reprod. 2019;34(12):2495–512. https://doi.org/10.1093/humrep/dez205.
Fraser GL, Obermayer-Pietsch B, Laven J, Griesinger G, Pintiaux A, Timmerman D, Fauser BCJM, Lademacher C, Combalbert J, Hoveyda HR, Ramael S. Randomized controlled trial of neurokinin 3 receptor antagonist fezolinetant for treatment of polycystic ovary syndrome. J Clin Endocrinol Metab. 2021;106(9):E3519–32. https://doi.org/10.1210/clinem/dgab320.
George JT, Kakkar R, Marshall J, Scott ML, Finkelman RD, Ho TW, Veldhuis J, Skorupskaite K, Anderson RA, McIntosh S, Webber L. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: A randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4313–21. https://doi.org/10.1210/jc.2016-1202.
Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Kisspeptin and neurokinin B interactions in modulating gonadotropin secretion in women with polycystic ovary syndrome. Hum Reprod. 2020;35(6):1421–31. https://doi.org/10.1093/humrep/deaa104.
Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM. Selective progesterone receptor modulators and progesterone antagonists: Mechanisms of action and clinical applications. Hum Reprod Update. 2005;11(3):293–307. https://doi.org/10.1093/humupd/dmi002.
Pawsey S, Mills EG, Ballantyne E, Donaldson K, Kerr M, Trower M, Dhillo WS. Elinzanetant (NT-814), a neurokinin 1,3 receptor antagonist, reduces estradiol and progesterone in healthy women. J Clin Endocrinol Metab. 2021;106(8):e3221–34. https://doi.org/10.1210/clinem/dgab108.
d’Anglemont de Tassigny X, Jayasena CN, Murphy KG, Dhillo WS, Colledge WH. Mechanistic insights into the more potent effect of KP-54 compared to KP-10 in vivo. PLoS One. 2017;12(5):e0176821. https://doi.org/10.1371/journal.pone.0176821.
Matsui H, Asami T. Effects and therapeutic potentials of kisspeptin analogs: Regulation of the hypothalamic-pituitary-gonadal axis. Neuroendocrinology. 2014;99(1):49–60. https://doi.org/10.1159/000357809.
Funding
This study was (partially) funded by Italian Ministry of Health- Current research IRCCS.
Author information
Authors and Affiliations
Contributions
Conceptualization, N.S. and P.V.; methodology, N.S.; software, N.S.; validation, N.S., P.V., P.C., R.M., M.C; investigation, N.S.; resources, N.S.; data curation, N.S.; writing—original draft preparation, N.S.; writing—review and editing, P.V, P.C.; visualization, N.S., P.V., P.C., R.M., M.C; supervision, P.V.; project administration, N.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no commercial or financial ties that could be seen as a conflict of interest in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salmeri, N., Viganò, P., Cavoretto, P. et al. The kisspeptin system in and beyond reproduction: exploring intricate pathways and potential links between endometriosis and polycystic ovary syndrome. Rev Endocr Metab Disord 25, 239–257 (2024). https://doi.org/10.1007/s11154-023-09826-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09826-0