Abstract
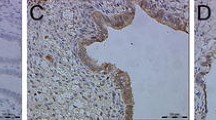
Endometriosis is characterized by the presence of ectopic endometrial tissues. Mechanisms of tissue dissemination in endometriosis may be similar to those involved in tumor metastasis. We hypothesize that dysregulation of kisspeptin (KISS1), a metastasis suppressor in endometrial carcinoma, may contribute to the pathogenesis of endometriosis. In this study, we characterized the immunoreactivity of kisspeptin and its receptor, KISS1R, in eutopic and ectopic endometrial tissue of women with and without endometriosis, in proliferative and secretory menstrual cycle phases. Immunohistochemistry was performed using KISS1 and KISS1R antibodies on samples from women with (n = 35) and without (n = 14) endometriosis. Samples from women with endometriosis included eutopic endometrium (n = 20) samples, superficial endometriotic implants (SUP, n = 10) deep infiltrating endometriotic implants (DIE, n = 15), and ovarian endometriomas (OMA, n = 15). Immunoreactivity was quantified using histoscores. KISS1 and KISS1R immunoreactivity was significantly lower in eutopic endometrial stroma of women with versus without endometriosis, regardless of the menstrual cycle phase (P = 0.001 and P = 0.015 respectively). In endometriotic implants, KISS1 levels were significantly lower in both glandular and stromal components of DIE (P < 0.01) and OMA (P < 0.01) compared to SUP. KISS1R immunoreactivity was lower in the glandular component of OMA (P = 0.035) compared to SUP. KISS1 and KISS1R levels are lower in eutopic endometrial stroma from women with versus without endometriosis, consistent with a role for decreased KISS1 expression in the pathogenesis of endometriosis. As deeply invasive lesions showed lower KISS1 levels than superficial lesions, downregulation of KISS1 levels may contribute to implant invasiveness.






Similar content being viewed by others
References
Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best practice & research. Clin Obstet Gynaecol. 2004;18:177–200.
Sinaii N, Plumb K, Cotton L, Lambert A, Kennedy S, Zondervan K, et al. Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil Steril. 2008;89:538–45.
Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 14:422–69.
Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–4.
Filigheddu N, Gregnanin I, Porporato PE, et al. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol. 2010;2010:369549.
Bulun SE, Monsivais D, Kakinuma T, Furukawa Y, Bernardi L, Pavone ME, et al. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. 2015;33:220–4.
Mizumoto H, Saito T, Ashihara K, Nishimura M, Takehara M, Tanaka R, et al. Expression of matrix metalloproteinases in ovarian endometriomas: immunohistochemical study and enzyme immunoassay. Life Sci. 2002;71:259–73.
Szamatowicz J, Laudański P, Tomaszewska I. Matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1: a possible role in the pathogenesis of endometriosis. Hum Reprod. 2002;17:284–8.
Wolber E-M, Kressin P, Meyhöfer-Malik A, Diedrich K, Malik E. Differential induction of matrix metalloproteinase 1 and 2 in ectopic endometrium. Reprod BioMed Online. 6:238–43.
Chung HW, Lee JY, Moon HS, Hur SE, Park MH, Wen Y, et al. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil Steril. 2002;78:787–95.
Uzan C, Cortez A, Dufournet C, Fauvet R, Siffroi JP, Darai E. Eutopic endometrium and peritoneal, ovarian and bowel endometriotic tissues express a different profile of matrix metalloproteinases-2, −3 and −11, and of tissue inhibitor metalloproteinases-1 and -2. Virchows Archiv : an international journal of pathology. 2004;445:603–9.
Pitsos M, Kanakas N. The role of matrix metalloproteinases in the pathogenesis of endometriosis. Reprod Sci (Thousand Oaks, Calif). 2009;16:717–26.
Visse R, Nagase H, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73.
Nomura H, Sato H, Seiki M, Mai M, Okada Y. Expression of membrane-type matrix metalloproteinase in human gastric carcinomas. Cancer Res. 1995;55:3263–6.
West A, Vojta PJ, Welch DR, Weissman BE. Chromosome localization and genomic structure of the KiSS-1 metastasis suppressor gene (KISS1). Genomics. 1998;54:145–8.
Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–6.
Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–7.
Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–75.
Lee DK, Nguyen T, O'Neill GP, Cheng R, Liu Y, Howard AD, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446:103–7.
Navarro VM, Fernandez-Fernandez R, Castellano JM, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–86.
Navarro VM, Castellano JM, Fernandez-Fernandez R, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–74.
Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–56.
Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–34.
Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–7.
Dhar DK, Naora H, Kubota H, Maruyama R, Yoshimura H, Tonomoto Y, et al. Downregulation of KiSS-1 expression is responsible for tumor invasion and worse prognosis in gastric carcinoma. Int J Cancer. 2004;111:868–72.
Yao HL, Yang ZL, Li YG, Liu GW. [In situ hybridization study on the expression of Kiss-1 and KAI-1 metastasis suppressor genes in gastric cancer]. Zhonghua wei chang wai ke za zhi =. Chinese Journal of Gastrointestinal Surgery. 2007;10:274–7.
Guan-Zhen Y, Ying C, Can-Rong N, Guo-Dong W, Jian-Xin Q, Jie-Jun W. Reduced protein expression of metastasis-related genes (nm23, KISS1, KAI1 and p53) in lymph node and liver metastases of gastric cancer. Int J Exp Pathol. 2007;88:175–83.
Hata K, Dhar DK, Watanabe Y, Nakai H, Hoshiai H. Expression of metastin and a G-protein-coupled receptor (AXOR12) in epithelial ovarian cancer. Eur J Cancer. 2007;43:1452–9.
Jiang Y, Berk M, Singh LS, Tan H, Yin L, Powell CT, et al. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin Exp Metastasis. 2005;22:369–76.
Jiang T, Zhang SL, Lin B, Meng LR, Gao H. Expression and clinical significance of KISS-1 and GPR54 mRNA in endometrial carcinoma. Zhonghua Zhong Liu Za Zhi. 2005;27:229–31.
Sanchez-Carbayo M, Capodieci P, Cordon-Cardo C. Tumor suppressor role of KiSS-1 in bladder cancer: loss of KiSS-1 expression is associated with bladder cancer progression and clinical outcome. Am J Pathol. 2003;162:609–17.
Yan C, Wang H, Boyd DD. KiSS-1 represses 92-kDa type IV collagenase expression by down-regulating NF-kappa B binding to the promoter as a consequence of Ikappa Balpha -induced block of p65/p50 nuclear translocation. J Biol Chem. 2001;276:1164–72.
Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, et al. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–28.
Takino T, Koshikawa N, Miyamori H, Tanaka M, Sasaki T, Okada Y, et al. Cleavage of metastasis suppressor gene product KiSS-1 protein/metastin by matrix metalloproteinases. Oncogene. 2003;22:4617–26.
Mead EJ, Maguire JJ, Kuc RE, Davenport AP. Kisspeptins: a multifunctional peptide system with a role in reproduction, cancer and the cardiovascular system. Br J Pharmacol. 2007;151:1143–53.
Timologou A, Zafrakas M, Grimbizis G, et al. Immunohistochemical expression pattern of metastasis suppressors KAI1 and KISS1 in endometriosis and normal endometrium. Eur J Obstet Gynecol Reprod Biol. 2016;199:110–5.
Makri A, Msaouel P, Petraki C, et al. KISS1/KISS1R expression in eutopic and ectopic endometrium of women suffering from endometriosis. In vivo (Athens, Greece). 2012;26:119–27.
Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–3.
Koninckx PR, Martin D. Treatment of deeply infiltrating endometriosis. Curr Opin Obstet Gynecol. 1994;6:231–41.
Peng B, Zhu H, Leung PC. Gonadotropin-releasing hormone regulates human trophoblastic cell invasion via TWIST-induced N-cadherin expression. J Clin Endocrinol Metab. 2015;100:E19–29.
Browne H, Taylor H. HOXA10 expression in ectopic endometrial tissue. Fertil Steril. 2006;85:1386–90.
Sharpe-Timms KL, Ricke EA, Piva M, Horowitz GM. Differential expression and localization of de-novo synthesized endometriotic haptoglobin in endometrium and endometriotic lesions. Hum Reprod (Oxford, England). 2000;15:2180–5.
Hauge-Evans AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A role for kisspeptin in islet function. Diabetologia. 2006;49:2131–5.
Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertility and sterility. 1997;67:817–821.
Baba T, Kang HS, Hosoe Y, Kharma B, Abiko K, Matsumura N, et al. Menstrual cyclic change of metastin/GPR54 in endometrium. Med Mol Morphol. 2015;48:76–84.
Gao GL, Liu LD, Zou XS, Chen WX. Expression of KiSS-1, matrix metalloproteinase-9, nuclear factor-kappaBp65 in ovarian tumour. Zhonghua fu chan ke za zhi. 2007;42:34–8.
Balkowiec M, Maksym RB, Wlodarski PK. The bimodal role of matrix metalloproteinases and their inhibitors in etiology and pathogenesis of endometriosis (review). Mol Med Rep. 2018;18:3123–36.
Sotnikova NY, Antsiferova YS, Posiseeva LV, Shishkov DN, Posiseev DV, Filippova ES. Mechanisms regulating invasiveness and growth of endometriosis lesions in rat experimental model and in humans. Fertil Steril. 2010;93:2701–5.
Collette T, Bellehumeur C, Kats R, et al. Evidence for an increased release of proteolytic activity by the eutopic endometrial tissue in women with endometriosis and for involvement of matrix metalloproteinase-9. Hum Reprod (Oxford, England). 2004;19:1257–64.
Collette T, Maheux R, Mailloux J, Akoum A. Increased expression of matrix metalloproteinase-9 in the eutopic endometrial tissue of women with endometriosis. Hum Reprod (Oxford, England). 2006;21:3059–67.
Di Carlo C, Bonifacio M, Tommaselli GA, Bifulco G, Guerra G, Nappi C. Metalloproteinases, vascular endothelial growth factor, and angiopoietin 1 and 2 in eutopic and ectopic endometrium. Fertil Steril. 2009;91:2315–23.
Banerjee SK, Ballard KD, Wright JT. Endometriomas as a marker of disease severity. J Minim Invasive Gynecol. 2008;15:538–40.
Gargett CE, Schwab KE, Brosens JJ, Puttemans P, Benagiano G, Brosens I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol Hum Reprod. 2014;20:591–8.
Kang HS, Baba T, Mandai M, Matsumura N, Hamanishi J, Kharma B, et al. GPR54 is a target for suppression of metastasis in endometrial cancer. Mol Cancer Ther. 2011;10:580–90.
Klemmt PAB, Starzinski-Powitz A. Molecular and cellular pathogenesis of endometriosis. Current women's health reviews. 2018;14:106–16.
Patel BG, Rudnicki M. Progesterone resistance in endometriosis: origins, consequences and interventions 2017;96:623–632.
Makri A, Pissimissis N, Lembessis P, Polychronakos C, Koutsilieris M. The kisspeptin (KiSS-1)/GPR54 system in cancer biology. Cancer Treat Rev. 2008;34:682–92.
Panidis D, Rousso D, Koliakos G, Kourtis A, Katsikis I, Farmakiotis D, et al. Plasma metastin levels are negatively correlated with insulin resistance and free androgens in women with polycystic ovary syndrome. Fertil Steril. 2006;85:1778–83.
Park DW, Lee SK, Hong SR, Han AR, Kwak-Kim J, Yang KM. Expression of Kisspeptin and its receptor GPR54 in the first trimester trophoblast of women with recurrent pregnancy loss. Am J Reprod Immunol (New York, NY : 1989). 2012;67:132–9.
Logie JJ, Denison FC, Riley SC, Ramaesh T, Forbes S, Norman JE, et al. Evaluation of kisspeptin levels in obese pregnancy as a biomarker for pre-eclampsia. Clin Endocrinol. 2012;76:887–93.
Gaytan M, Castellano JM, Roa J, Sanchez-Criado JE, Tena-Sempere M, Gaytan F. Expression of KiSS-1 in rat oviduct: possible involvement in prevention of ectopic implantation? Cell Tissue Res. 2007;329:571–9.
Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27.
de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–6.
Castellano JM, Navarro VM, Fernandez-Fernandez R, et al. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–10.
Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–9.
Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–15.
Blumenfeld Z. Investigational and experimental GnRH analogs and associated neurotransmitters. Expert Opin Investig Drugs. 2017;26:661–7.
Kuohung W, Burnett M, Mukhtyar D, Schuman E, Ni J, Crowley WF, et al. A high-throughput small-molecule ligand screen targeted to agonists and antagonists of the G-protein-coupled receptor GPR54. J Biomol Screen. 2010;15:508–17.
Acknowledgments
The authors thank Professor Eman M. S. Muhammad from the pathology department at Sohag University in Egypt and Dr. Julia Naso from the pathology department at The University of British Columbia in Canada for their help with the study.
Funding
This project was internally funded by the Department of Obstetrics and Gynecology, University of British Columbia, Vancouver, BC, Canada, and by the joint supervision scholarships program, Egyptian Ministry of higher education, Cairo, Egypt.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was completed at the British Columbia Women’s Hospital, Vancouver, Canada and the BC Children’s Hospital Research Institute, Vancouver, Canada.
Rights and permissions
About this article
Cite this article
Abdelkareem, A.O., Alotaibi, F.T., AlKusayer, G.M. et al. Immunoreactivity of Kisspeptin and Kisspeptin Receptor in Eutopic and Ectopic Endometrial Tissue of Women With and Without Endometriosis. Reprod. Sci. 27, 1731–1741 (2020). https://doi.org/10.1007/s43032-020-00167-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00167-w