Abstract
Purpose
Polycystic ovarian syndrome (PCOS) is a complex and not fully elucidated pathology. This prevalent endocrinopathy affects patients in reproductive age, impacts on estrogen-dependent diseases, as well as in infertility. In this context, Kisspeptin (KP) may be considered a potential biomarker for PCOS diagnosis and follow-up. Here, we aimed to verify the levels of KP in obese and non-obese patients with PCOS, their relationship with other hormones, in comparison to healthy controls.
Methods
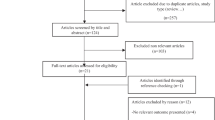
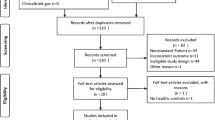
A systematic review and meta-analysis were performed according to the PRISMA guidelines. We searched MEDLINE, EMBASE, PsycINFO, Global Health, The Cochrane Library, Health Technology Assessment Database, and Web of Science for eligible studies. A random effects model meta-analysis of standardized mean difference (SMD) was conducted and the I2 was used to assess heterogeneity. Meta-regression was conducted through mixed-effects model.
Results
A total of 12 studies were included, comprising 660 PCOS patients and 600 controls. The KP levels were lower in the control group (0.76: 0.17–1.35; 95% CI). In the subgroup analyses, patients were divided in non-overweight/obese (BMI < 25) and overweight/obese (BMI ≥ 25) groups. The meta-regression revealed a difference between the obese and non-obese groups (z = 2.81; p = 0.0050).
Conclusions
PCOS patients showed higher KP levels than control, and obese non-PCOS patients also showed altered KP levels. All studies had poor descriptions of sample collection, pre-analytical and analytical procedures, which is critical considering structural characteristics of the KP molecule.






Similar content being viewed by others
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Giampaolino P, Della Corte L, De Rosa N, Mercorio A, Bruzzese D, Bifulco G (2017) Ovarian volume and PCOS: a controversial issue. Gynecol Endocrinol 34:229–232. https://doi.org/10.1080/09513590.2017.1391205
Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47. https://doi.org/10.1016/j.fertnstert.2003.10.004
Laganà AS, Rossetti P, Buscema M, La Vignera S, Condorelli RA, Gullo G, Granese R, Triolo O (2016) Metabolism and ovarian function in PCOS women: a therapeutic approach with inositols. Int J Endocrinol 2016:6306410. https://doi.org/10.1155/2016/6306410
Mani H, Levy MJ, Davies MJ, Morris DH, Gray LJ, Bankart J, Blackledge H, Khunti K, Howlett TA (2013) Diabetes and cardiovascular events in women with polycystic ovary syndrome: a 20-year retrospective cohort study. Clin Endocrinol (Oxf) 78:926–934. https://doi.org/10.1111/cen.12068
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF (2006) Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J Clin Endocrinol Metab 91:4237–4245. https://doi.org/10.1210/jc.2006-0178
Palomba S, Falbo A, Russo T, Tolino A, Orio F, Zullo F (2010) Pregnancy in women with polycystic ovary syndrome: the effect of different phenotypes and features on obstetric and neonatal outcomes. Fertil Steril 94:1805–1811. https://doi.org/10.1016/j.fertnstert.2009.10.043
Laganà AS, Garzon S, Casarin J, Franchi M, Ghezzi F (2018) Inositol in polycystic ovary syndrome: restoring fertility through a pathophysiology-based approach. Trends Endocrinol Metab 29:768–780. https://doi.org/10.1016/j.tem.2018.09.001
Matsuzaki T, Tungalagsuvd A, Takeshi I, Munkhzaya M, Yanagihara R, Tokui T, Yano K, Mayila Y, Kato T, Kuwahara A, Matsui S, Irahara M (2017) Kisspeptin mRNA expression is increased in the posterior hypothalamus in the rat model of polycystic ovary syndrome. Endocr J 64:7–14. https://doi.org/10.1507/endocrj.EJ16-0282
Albalawi FS, Daghestani MH, Daghestani MH, Eldali A, Warsy AS (2018) rs4889 polymorphism in KISS1 gene, its effect on polycystic ovary syndrome development and anthropometric and hormonal parameters in Saudi women. J Biomed Sci 25:50. https://doi.org/10.1186/s12929-018-0452-2
Cortés ME, Carrera B, Rioseco H, Pablo del Rio J, Vigil P (2015) The role of kisspeptin in the onset of puberty and in the ovulatory mechanism: a mini-review. J Pediatr Adolesc Gynecol 28:286–291. https://doi.org/10.1016/j.jpag.2014.09.017
Trevisan CM, Montagna E, de Oliveira R, Christofolini DM, Barbosa CP, Crandall KA, Bianco B (2018) Kisspeptin/GPR54 system: what do we know about its role in human reproduction? Cell Physiol Biochem 49:1259–1276. https://doi.org/10.1159/000493406
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350:g7647. https://doi.org/10.1136/bmj.g7647
National Center for Biotechnology Information. Available at https://pubchem.ncbi.nlm.nih.gov/compound/71306396. Accessed 27 Feb 2019
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
Wells G, Shea B, O’Connell D, Peterson G, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 27 Feb 2019
Borenstein M, Hedges LV, Higgins JPT, Rothstein RH (2009) Introduction to meta-analysis. Wiley, West Sussex
Higgins JPT (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, Chew EY, Bosch J, Collins R, Lewington S, Armitage J, Clarke R, Omega-3 Treatment Trialists’ Collaboration (2018) Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol 3:225–234. https://doi.org/10.1001/jamacardio.2017.5205
Sterne JAC, Egger M (2001) Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54:1046–1055. https://doi.org/10.1016/S0895-4356(01)00377-8
Baujat B, Mahé C, Pignon J-P, Hill C (2002) A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials: graphical method for exploring heterogeneity in meta-analyses. Stat Med 21:2641–2652. https://doi.org/10.1002/sim.1221
Estébanez N, Gómez-Acebo I, Palazuelos C, Llorca J, Dierssen-Sotos T (2018) Vitamin D exposure and risk of breast cancer: a meta-analysis. Sci Rep 8:9039. https://doi.org/10.1038/s41598-018-27297-1
Schwarzer G, Carpenter JR, Rücker G (2015) Meta-analysis with R. Springer International Publishing, Switzerland
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48. https://doi.org/10.18637/jss.v036.i03
Branavan U, Muneeswaran K, Wijesundera WSS, Senanayake A, Chandrasekharan NV, Wijeyaratne CN (2019) Association of KISS1 and GPR54 gene polymorphisms with polycystic ovary syndrome among sri lankan women. Biomed Res Int 2019:6235680. https://doi.org/10.1155/2019/6235680
Umayal B, Jayakody SN, Chandrasekharan NV, Wijesundera WS, Wijeyaratne CN (2019) Polycystic ovary syndrome (PCOS) and kisspeptin: a Sri Lankan study. J Postgrad Med 65:18–23. https://doi.org/10.4103/jpgm.JPGM_683_17
Panidis D, Rousso D, Koliakos G, Kourtis A, Katsikis I, Farmakiotis D, Votsi E, Diamantikandarakis E (2006) Plasma metastin levels are negatively correlated with insulin resistance and free androgens in women with polycystic ovary syndrome. Fertil Steril 85:1778–1783. https://doi.org/10.1016/j.fertnstert.2005.11.044
Chen X, Mo Y, Li L, Chen Y, Li Y, Yang D (2010) Increased plasma metastin levels in adolescent women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 149:72–76. https://doi.org/10.1016/j.ejogrb.2009.11.018
Jeon YE, Lee KE, Jung JA, Yim SY, Kim H, Seo SK, Cho S, Choi YS, Lee BS (2013) Kisspeptin, leptin, and retinol-binding protein 4 in women with polycystic ovary syndrome. Gynecol Obstet Invest 75:268–274. https://doi.org/10.1159/000350217
Yilmaz SA, Kerimoglu OS, Pekin AT, Incesu F, Dogan NU, Celik C, Unlu A (2014) Metastin levels in relation with hormonal and metabolic profile in patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 180:56–60. https://doi.org/10.1016/j.ejogrb.2014.06.004
Emekci Ozay O, Ozay AC, Acar B, Cagliyan E, Seçil M, Küme T (2016) Role of kisspeptin in polycystic ovary syndrome (PCOS). Gynecol Endocrinol 32:718–722. https://doi.org/10.3109/09513590.2016.1161019
Nyagolova PV, Mitkov MD, Orbetzova MM, Terzieva DD (2016) Kisspeptin and galanin-like peptide (galp) levels in women with polycystic ovary syndrome. Int J Pharmaceut Med Res 4:6–12
Gorkem U, Togrul C, Arslan E, Sargin Oruc A, Buyukkayaci Duman N (2018) Is there a role for kisspeptin in pathogenesis of polycystic ovary syndrome? Gynecol Endocrinol 34:157–160. https://doi.org/10.1080/09513590.2017.1379499
Daghestani MH (2018) Evaluation of biochemical, endocrine, and metabolic biomarkers for the early diagnosis of polycystic ovary syndrome among non-obese Saudi women. Int J Gynecol Obstet 142:162–169. https://doi.org/10.1002/ijgo.12527
Kaya C, Alay İ, Babayeva G, Gedikbaşı A, Ertaş Kaya S, Ekin M, Yaşar L (2019) Serum Kisspeptin levels in unexplained infertility, polycystic ovary syndrome, and male factor infertility. Gynecol Endocrinol 35:228–232. https://doi.org/10.1080/09513590.2018.1519792
Wang T, Han S, Tian W, Zhao M, Zhang H (2019) Effects of kisspeptin on pathogenesis and energy metabolism in polycystic ovarian syndrome (PCOS). Gynecol Endocrinol 5:1. https://doi.org/10.1080/09513590.2019.1597343 (Epub ahead of print)
Cela V, Obino MER, Alberga Y, Pinelli S, Sergiampietri C, Casarosa E, Simi G, Papini F, Artini PG (2018) Ovarian response to controlled ovarian stimulation in women with different polycystic ovary syndrome phenotypes. Gynecol Endocrinol 34(6):518–523. https://doi.org/10.1080/09513590.2017.1412429
Jamil AS, Alalaf SK, Al-Tawil NG, Al-Shawaf T (2016) Comparison of clinical and hormonal characteristics among four phenotypes of polycystic ovary syndrome based on the Rotterdam criteria. Arch Gynecol Obstet 293:447–456. https://doi.org/10.1007/s00404-015-3889-5
Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R (2016) Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 106:6–15. https://doi.org/10.1016/j.fertnstert.2016.05.003
Tolson KP, Garcia C, Yen S, Simonds S, Stefanidis A, Lawrence A, Smith JT, Kauffman AS (2014) Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J Clin Invest. 124:3075–3079. https://doi.org/10.1172/JCI71075
Holmes D (2014) Kisspeptin signalling linked to obesity. Nat Rev Endocrinol 10:511. https://doi.org/10.1038/nrendo.2014.106
Katulski K, Podfigurna A, Czyzyk A, Meczekalski B, Genazzani AD (2018) Kisspeptin and LH pulsatile temporal coupling in PCOS patients. Endocrine 61:149–157. https://doi.org/10.1007/s12020-018-1609-1
d’Anglemont de Tassigny X, Jayasena CN, Murphy KG, Dhillo WS, Colledge WH (2017) Mechanistic insights into the more potent effect of KP-54 compared to KP-10 in vivo. PLoS One 12:e0176821. https://doi.org/10.1371/journal.pone.0176821
Kolodziejski PA, Pruszynska-Oszmalek E, Korek E, Sassek M, Szczepankiewicz D, Kaczmarek P, Nogowski L, Mackowiak P, Nowak KW, Krauss H, Strowski MZ (2018) Serum levels of spexin and kisspeptin negatively correlate with obesity and insulin resistance in women. Physiol Res 67:45–56. https://doi.org/10.33549/physiolres.933467
Kasum M, Franulić D, Čehić E, Orešković S, Lila A, Ejubović E (2017) Kisspeptin as a promising oocyte maturation trigger for in vitro fertilisation in humans. Gynecol Endocrinol 33:583–587. https://doi.org/10.1080/09513590.2017.1309019
Decourt C, Robert V, Anger K, Galibert M, Madinier J-B, Liu X, Dardente H, Lomet D, Delmas AF, Caraty A, Herbison AE, Anderson GM, Aucagne V, Beltramo M (2016) A synthetic kisspeptin analog that triggers ovulation and advances puberty. Sci Rep 6:26908. https://doi.org/10.1038/srep26908
Acknowledgements
Dr. Bianca Bianco for the support in the manuscript preparation.
Funding
None.
Author information
Authors and Affiliations
Contributions
NP Assis Rodrigues: design of the study, literature review, data collection, data analysis and manuscript writing; AS Laganà: data analysis, manuscript writing and editing; V Zaia: data collection, data analysis and review of the manuscript; A Vitagliano: data analysis and review of the manuscript; CP Barbosa: review of the manuscript; R Oliveira: data analysis, review and manuscript writing; CM Trevisan: data collection, data analysis and review of the manuscript; E Montagna: design of the study, literature review, data analysis and manuscript writing/review. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Assis Rodrigues, N.P., Laganà, A., Zaia, V. et al. The role of Kisspeptin levels in polycystic ovary syndrome: a systematic review and meta-analysis. Arch Gynecol Obstet 300, 1423–1434 (2019). https://doi.org/10.1007/s00404-019-05307-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05307-5