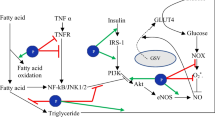
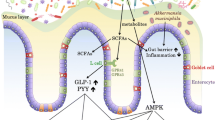
Abstract
Obesity is a global public health problem that results in chronic pathologies such as diabetes, cardiovascular diseases, and cancer. The treatment approach based on energy restriction and promotion of physical activity is ineffective in the long term. Due to the high prevalence of this pathology, complementary treatments such as brown adipose tissue activation (BAT) and white adipose tissue browning (WAT) have been proposed. Dietary polyphenols are plant secondary metabolites that can stimulate browning and thermogenesis of adipose tissue. They have also been shown to prevent body weight gain, and decrease systemic inflammation produced by high-fat diets. Ingested dietary polyphenols that reach the colon are metabolized by the gut microbiota (GM), regulating its composition and generating a great array of metabolites. GM is involved in the production of short chain fatty acids and secondary bile salts that regulate energetic metabolism. The alteration in the composition of GM observed in metabolic diseases such as obesity and type 2 diabetes can be attenuated by polyphenols. Recent studies support the hypothesis that GM would mediate WAT browning and BAT thermogenesis activation induced by polyphenol administration. Together, these results indicate that GM in the presence of polyphenols plays a fundamental role in the control of obesity possible through BAT activation.



Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- AT:
-
adipose tissue
- AMPK:
-
adenosine monophosphate-activated protein kinase
- BAT:
-
brown adipose tissue activation
- CC:
-
Camu-Camu
- cAMP:
-
cyclic adenosine monophosphate
- C/EBPα:
-
CCAAT/enhancer-binding protein α
- CPT1α:
-
carnitine palmitoyltransferase Iα.
- CREB:
-
co-activator cAMP response element-binding protein
- COX2:
-
cyclooxygenase 2
- DIO2:
-
deiodinase 2
- EE:
-
energy expenditure
- FIAF:
-
fasting-induced adipocyte factor
- FXR:
-
farnesoid X receptor
- GE:
-
grape extract
- GSP:
-
Guarana seed powder
- GM:
-
gut microbiota
- HFD:
-
high-fat diets
- iWAT:
-
inguinal WAT
- LDL:
-
low density lipoprotein
- LPL:
-
lipoprotein lipase
- LPS:
-
lipopolysaccharide
- MAPKs:
-
mitogen activated protein kinases
- NLE:
-
Nitzschia laevis extract
- BE:
-
polyphenol-rich blueberry extract
- PKA:
-
protein kinase A
- PE:
-
Pu-erh tea after its fermentation
- PEBC:
-
Pu-erh tea bioactive compounds
- PGC1-α:
-
peroxisome proliferator-activated receptor gamma coactivator 1-α
- PPARα/β /γ:
-
peroxisome proliferator-activated receptor α/β /γ
- PRDM16:
-
PR domain zinc finger protein 16
- SCFAs:
-
short-chain volatile fatty acids
- SIRT1:
-
sirtuin 1
- SREBP1c:
-
sterol regulatory element-binding protein-1c
- TFAM:
-
mitochondrial transcription factor A
- TGR5:
-
membrane-type receptor for bile acids
- TLR4:
-
toll-like receptor 4
- UCP-1:
-
uncoupling protein 1
- VA:
-
vanillic acid
- WAT:
-
white adipose tissue browning.
References
Zukiewicz-Sobczak W, Wroblewska P, Zwolinski J, Chmielewska-Badora J, Adamczuk P, Krasowska E, et al. Obesity and poverty paradox in developed countries. Ann Agric Environ Med. 2014;21(3):590–4. https://doi.org/10.5604/12321966.1120608.
Yudkin JS. Inflammation, obesity, and the metabolic syndrome. Horm Metab Res. 2007;39(10):707–9. https://doi.org/10.1055/s-2007-985898.
Concha F, Prado G, Quezada J, Ramirez A, Bravo N, Flores C, et al. Nutritional and non-nutritional agents that stimulate white adipose tissue browning. Rev Endocr Metab Disord. 2019;20(2):161–71. https://doi.org/10.1007/s11154-019-09495-y.
Bohn T. Dietary factors affecting polyphenol bioavailability. Nutr Rev. 2014;72(7):429–52. https://doi.org/10.1111/nure.12114.
Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1–24. https://doi.org/10.1007/s00394-017-1445-8.
Harakeh SM, Khan I, Kumosani T, Barbour E, Almasaudi SB, Bahijri SM, et al. Gut microbiota: a contributing factor to obesity. Front Cell Infect Microbiol. 2016;6:95. https://doi.org/10.3389/fcimb.2016.00095.
Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. The Firmicutes/Bacteroidetes ratio: a relevant marker of gut Dysbiosis in obese patients? Nutrients. 2020;12(5). https://doi.org/10.3390/nu12051474.
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. https://doi.org/10.2337/db07-1403.
Gasaly NRK, Gotteland M. Phytochemicals: a new class of prebiotics. Rev Chil Nutr. 2020;47(2).
Pan P, Lam V, Salzman N, Huang YW, Yu J, Zhang J, et al. Black raspberries and their anthocyanin and Fiber fractions Alter the composition and diversity of gut microbiota in F-344 rats. Nutr Cancer. 2017;69(6):943–51. https://doi.org/10.1080/01635581.2017.1340491.
Etxeberria U, Arias N, Boque N, Macarulla MT, Portillo MP, Martinez JA, et al. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J Nutr Biochem. 2015;26(6):651–60. https://doi.org/10.1016/j.jnutbio.2015.01.002.
Cani PD, Van Hul M, Lefort C, Depommier C, Rastelli M, Everard A. Microbial regulation of organismal energy homeostasis. Nat Metab. 2019;1(1):34–46. https://doi.org/10.1038/s42255-018-0017-4.
Hartroft WS. The pathology of obesity. Bull N Y Acad Med. 1960;36:313–22.
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–61. https://doi.org/10.1172/JCI21625.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. https://doi.org/10.1186/1471-2458-9-88.
Gonzalez-Barroso MDM, Ricquier D, Cassard-Doulcier AM. The human uncoupling protein-1 gene (UCP1): present status and perspectives in obesity research. Obesity reviews. 2000;1(2):61–72.
Vázquez-Vela MEF, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res. 2008;39(8):715–28.
Fenzl A, Kiefer FW. Brown adipose tissue and thermogenesis. Horm Mol Biol Clin Invest. 2014;19(1):25–37.
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. https://doi.org/10.1152/physrev.00015.2003.
Green AL, Bagci U, Hussein S, Kelly PV, Muzaffar R, Neuschwander-Tetri BA, et al. Brown adipose tissue detected by PET/CT imaging is associated with less central obesity. Nucl Med Commun. 2017;38(7):629–35.
Morton GJ, Muta K, Kaiyala KJ, Rojas JM, Scarlett JM, Matsen ME, et al. Evidence that the sympathetic nervous system elicits rapid, coordinated, and reciprocal adjustments of insulin secretion and insulin sensitivity during cold exposure. Diabetes. 2017;66(4):823–34. https://doi.org/10.2337/db16-1351.
Blondin DP, Tingelstad HC, Noll C, Frisch F, Phoenix S, Guérin B, et al. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nat Commun. 2017;8(1):1–9.
Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–63. https://doi.org/10.1038/nm.3361.
Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, et al. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol. 2004;24(7):3057–67.
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–39.
Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10(1):24–36. https://doi.org/10.1038/nrendo.2013.204.
Zhang X, Zhang QX, Wang X, Zhang L, Qu W, Bao B, et al. Dietary luteolin activates browning and thermogenesis in mice through an AMPK/PGC1alpha pathway-mediated mechanism. Int J Obes. 2016;40(12):1841–9. https://doi.org/10.1038/ijo.2016.108.
Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150(3):620–32. https://doi.org/10.1016/j.cell.2012.06.027.
Silvester AJ, Aseer KR, Yun JW. Dietary polyphenols and their roles in fat browning. J Nutr Biochem. 2019;64:1–12. https://doi.org/10.1016/j.jnutbio.2018.09.028.
Li AN, Li S, Zhang YJ, Xu XR, Chen YM, Li HB. Resources and biological activities of natural polyphenols. Nutrients. 2014;6(12):6020–47. https://doi.org/10.3390/nu6126020.
Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–47. https://doi.org/10.1093/ajcn/79.5.727.
Marin L, Miguelez EM, Villar CJ, Lombo F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. Biomed Res Int. 2015;2015:905215–8. https://doi.org/10.1155/2015/905215.
Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13(10):572–84.
Deby-Dupont G, Mouithys-Mickalad A, Serteyn D, Lamy M, Deby C. Resveratrol and curcumin reduce the respiratory burst of chlamydia-primed THP-1 cells. Biochem Biophys Res Commun. 2005;333(1):21–7. https://doi.org/10.1016/j.bbrc.2005.05.073.
Braunlich M, Slimestad R, Wangensteen H, Brede C, Malterud KE, Barsett H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients. 2013;5(3):663–78. https://doi.org/10.3390/nu5030663.
Chu AJ. Antagonism by bioactive polyphenols against inflammation: a systematic view. Inflamm Allergy Drug Targets. 2014;13(1):34–64.
Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 1999;70(6):1040–5. https://doi.org/10.1093/ajcn/70.6.1040.
Decorde K, Teissedre PL, Sutra T, Ventura E, Cristol JP, Rouanet JM. Chardonnay grape seed procyanidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol Nutr Food Res. 2009;53(5):659–66. https://doi.org/10.1002/mnfr.200800165.
Arias N, Macarulla MT, Aguirre L, Milton I, Portillo MP. The combination of resveratrol and quercetin enhances the individual effects of these molecules on triacylglycerol metabolism in white adipose tissue. Eur J Nutr. 2016;55(1):341–8. https://doi.org/10.1007/s00394-015-0854-9.
Boque N, Campion J, de la Iglesia R, de la Garza AL, Milagro FI, San Roman B, et al. Screening of polyphenolic plant extracts for anti-obesity properties in Wistar rats. J Sci Food Agric. 2013;93(5):1226–32. https://doi.org/10.1002/jsfa.5884.
Kim NH, Jegal J, Kim YN, Heo JD, Rho JR, Yang MH, et al. Chokeberry extract and its active polyphenols suppress Adipogenesis in 3T3-L1 adipocytes and modulates fat accumulation and insulin resistance in diet-induced obese mice. Nutrients. 2018;10(11). https://doi.org/10.3390/nu10111734.
Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Churruca I, Portillo MP. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 2013;141(2):1530–5. https://doi.org/10.1016/j.foodchem.2013.03.085.
Andrade JM, Frade AC, Guimaraes JB, Freitas KM, Lopes MT, Guimaraes AL, et al. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr. 2014;53(7):1503–10. https://doi.org/10.1007/s00394-014-0655-6.
Wang S, Liang X, Yang Q, Fu X, Zhu M, Rodgers BD, et al. Resveratrol enhances brown adipocyte formation and function by activating AMP-activated protein kinase (AMPK) alpha1 in mice fed high-fat diet. Mol Nutr Food Res. 2017;61(4). https://doi.org/10.1002/mnfr.201600746.
Carrasco-Pozo C, Cires MJ, Gotteland M. Quercetin and Epigallocatechin Gallate in the prevention and treatment of obesity: from molecular to clinical studies. J Med Food. 2019;22(8):753–70. https://doi.org/10.1089/jmf.2018.0193.
Forbes-Hernandez TY, Giampieri F, Gasparrini M, Afrin S, Mazzoni L, Cordero MD, et al. Lipid accumulation in HepG2 cells is attenuated by strawberry extract through AMPK activation. Nutrients. 2017;9(6). https://doi.org/10.3390/nu9060621.
Giampieri F, Alvarez-Suarez JM, Cordero MD, Gasparrini M, Forbes-Hernandez TY, Afrin S, et al. Strawberry consumption improves aging-associated impairments, mitochondrial biogenesis and functionality through the AMP-activated protein kinase signaling cascade. Food Chem. 2017;234:464–71. https://doi.org/10.1016/j.foodchem.2017.05.017.
Mosqueda-Solis A, Sanchez J, Portillo MP, Palou A, Pico C. Combination of capsaicin and hesperidin reduces the effectiveness of each compound to decrease the adipocyte size and to induce Browning features in adipose tissue of Western diet fed rats. J Agric Food Chem. 2018;66(37):9679–89. https://doi.org/10.1021/acs.jafc.8b02611.
Neyrinck AM, Bindels LB, Geurts L, Van Hul M, Cani PD, Delzenne NM. A polyphenolic extract from green tea leaves activates fat browning in high-fat-diet-induced obese mice. J Nutr Biochem. 2017;49:15–21. https://doi.org/10.1016/j.jnutbio.2017.07.008.
Peng CH, Liu LK, Chuang CM, Chyau CC, Huang CN, Wang CJ. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J Agric Food Chem. 2011;59(6):2663–71. https://doi.org/10.1021/jf1043508.
Prior RL, Wu X, Gu L, Hager TJ, Hager A, Howard LR. Whole berries versus berry anthocyanins: interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J Agric Food Chem. 2008;56(3):647–53. https://doi.org/10.1021/jf071993o.
Han X, Guo J, You Y, Yin M, Liang J, Ren C, et al. Vanillic acid activates thermogenesis in brown and white adipose tissue. Food Funct. 2018;9(8):4366–75. https://doi.org/10.1039/c8fo00978c.
Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–70. https://doi.org/10.1016/j.cell.2012.01.035.
Dinan TG, Cryan JF. Microbes, immunity, and behavior: psychoneuroimmunology meets the microbiome. Neuropsychopharmacology. 2017;42(1):178–92. https://doi.org/10.1038/npp.2016.103.
Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623–67. https://doi.org/10.1146/annurev-immunol-030409-101330.
Ringel-Kulka T, Cheng J, Ringel Y, Salojarvi J, Carroll I, Palva A, et al. Intestinal microbiota in healthy U.S. young children and adults--a high throughput microarray analysis. PLoS One. 2013;8(5):e64315. https://doi.org/10.1371/journal.pone.0064315.
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8. https://doi.org/10.1126/science.1110591.
Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–12. https://doi.org/10.1093/jn/125.6.1401.
Kim E, Coelho D, Blachier F. Review of the association between meat consumption and risk of colorectal cancer. Nutr Res. 2013;33(12):983–94. https://doi.org/10.1016/j.nutres.2013.07.018.
Fujio-Vejar S, Vasquez Y, Morales P, Magne F, Vera-Wolf P, Ugalde JA, et al. The gut microbiota of healthy Chilean subjects reveals a high abundance of the phylum Verrucomicrobia. Front Microbiol. 2017;8:1221. https://doi.org/10.3389/fmicb.2017.01221.
Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–5. https://doi.org/10.1073/pnas.0504978102.
Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–43.
Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab. 2015;26(9):493–501. https://doi.org/10.1016/j.tem.2015.07.002.
Cummings JH, Macfarlane GT. Role of intestinal bacteria in nutrient metabolism. JPEN J Parenter Enteral Nutr. 1997;21(6):357–65. https://doi.org/10.1177/0148607197021006357.
Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858–76. https://doi.org/10.3390/nu3100858.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. https://doi.org/10.1038/nature05414.
Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–23. https://doi.org/10.1073/pnas.0407076101.
Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51(5):1101–12. https://doi.org/10.1194/jlr.M002774.
Mandard S, Zandbergen F, van Straten E, Wahli W, Kuipers F, Muller M, et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem. 2006;281(2):934–44. https://doi.org/10.1074/jbc.M506519200.
Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104(3):979–84. https://doi.org/10.1073/pnas.0605374104.
Jenner AM, Rafter J, Halliwell B. Human fecal water content of phenolics: the extent of colonic exposure to aromatic compounds. Free Radic Biol Med. 2005;38(6):763–72. https://doi.org/10.1016/j.freeradbiomed.2004.11.020.
Braune A, Engst W, Blaut M. Identification and functional expression of genes encoding flavonoid O- and C-glycosidases in intestinal bacteria. Environ Microbiol. 2016;18(7):2117–29. https://doi.org/10.1111/1462-2920.12864.
Rechner AR, Smith MA, Kuhnle G, Gibson GR, Debnam ES, Srai SK, et al. Colonic metabolism of dietary polyphenols: influence of structure on microbial fermentation products. Free Radic Biol Med. 2004;36(2):212–25.
Braune A, Blaut M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes. 2016;7(3):216–34. https://doi.org/10.1080/19490976.2016.1158395.
Monagas M, Urpi-Sarda M, Sanchez-Patan F, Llorach R, Garrido I, Gomez-Cordoves C, et al. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010;1(3):233–53. https://doi.org/10.1039/c0fo00132e.
Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, et al. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem. 2008;283(14):9424–34. https://doi.org/10.1074/jbc.M706571200.
Bolca S, Van de Wiele T, Possemiers S. Gut metabotypes govern health effects of dietary polyphenols. Curr Opin Biotechnol. 2013;24(2):220–5. https://doi.org/10.1016/j.copbio.2012.09.009.
van Duynhoven J, Vaughan EE, Jacobs DM, Kemperman RA, van Velzen EJ, Gross G, et al. Metabolic fate of polyphenols in the human superorganism. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4531–8. https://doi.org/10.1073/pnas.1000098107.
Garcia-Villalba R, Vissenaekens H, Pitart J, Romo-Vaquero M, Espin JC, Grootaert C, et al. Gastrointestinal simulation model TWIN-SHIME shows differences between human Urolithin-Metabotypes in gut microbiota composition, pomegranate polyphenol metabolism, and transport along the intestinal tract. J Agric Food Chem. 2017;65(27):5480–93. https://doi.org/10.1021/acs.jafc.7b02049.
Tomas-Barberan FA, Selma MV, Espin JC. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr Opin Clin Nutr Metab Care. 2016;19(6):471–6. https://doi.org/10.1097/MCO.0000000000000314.
Chevalier C, Stojanovic O, Colin DJ, Suarez-Zamorano N, Tarallo V, Veyrat-Durebex C, et al. Gut microbiota orchestrates energy homeostasis during cold. Cell. 2015;163(6):1360–74. https://doi.org/10.1016/j.cell.2015.11.004.
Bortolin RC, Vargas AR, de Miranda RV, Gasparotto J, Chaves PR, Schnorr CE, et al. Guarana supplementation attenuated obesity, insulin resistance, and adipokines dysregulation induced by a standardized human Western diet via brown adipose tissue activation. Phytother Res. 2019;33(5):1394–403. https://doi.org/10.1002/ptr.6330.
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–17. https://doi.org/10.2337/db08-1637.
Li Z, Yi CX, Katiraei S, Kooijman S, Zhou E, Chung CK, et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67(7):1269–79. https://doi.org/10.1136/gutjnl-2017-314050.
Prasain JKG, C.; Barnes, S. Cranberry anti-cancer compounds and their uptake and metabolism: an updated review. Journal of Berry Research. 2020;10:1–10. https://doi.org/10.3233/JBR-180370.
May SM, G.; Marchesi, J.; Parry, L. Impact of black raspberries on the normal and malignant Apc deficient murine gut microbiome. Journal of Berry Research. 2018;10:61–76. https://doi.org/10.3233/JBR-180372.
Pan P, Oshima K, Huang YW, Yearsley M, Zhang J, Arnold M, et al. Gut bacteria are required for the benefits of black raspberries in Apc (min/+) mice. J Berry Res. 2018;8(4):239–49. https://doi.org/10.3233/JBR-180337.
Pierre JF, Martinez KB, Ye H, Nadimpalli A, Morton TC, Yang J, et al. Activation of bile acid signaling improves metabolic phenotypes in high-fat diet-induced obese mice. Am J Physiol Gastrointest Liver Physiol. 2016;311(2):G286–304. https://doi.org/10.1152/ajpgi.00202.2016.
Brandl K, Kumar V, Eckmann L. Gut-liver axis at the frontier of host-microbial interactions. Am J Physiol Gastrointest Liver Physiol. 2017;312(5):G413–9. https://doi.org/10.1152/ajpgi.00361.2016.
Fiorucci S, Mencarelli A, Palladino G, Cipriani S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci. 2009;30(11):570–80. https://doi.org/10.1016/j.tips.2009.08.001.
Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. 2018;68(4):1574–88. https://doi.org/10.1002/hep.29857.
Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9. https://doi.org/10.1038/nature04330.
Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77. https://doi.org/10.1016/j.cmet.2009.08.001.
Hui S, Liu Y, Huang L, Zheng L, Zhou M, Lang H, et al. Resveratrol enhances brown adipose tissue activity and white adipose tissue browning in part by regulating bile acid metabolism via gut microbiota remodeling. Int J Obes. 2020;44:1678–90. https://doi.org/10.1038/s41366-020-0566-y.
Wang P, Li D, Ke W, Liang D, Hu X, Chen F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int J Obes. 2020;44(1):213–25. https://doi.org/10.1038/s41366-019-0332-1.
Liao W, Yin X, Li Q, Zhang H, Liu Z, Zheng X, et al. Resveratrol-induced white adipose tissue Browning in obese mice by remodeling fecal microbiota. Molecules. 2018;23(12). https://doi.org/10.3390/molecules23123356.
Anhe FF, Nachbar RT, Varin TV, Trottier J, Dudonne S, Le Barz M, et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut. 2018;68:453–64. https://doi.org/10.1136/gutjnl-2017-315565.
Guo B, Liu B, Wei H, Cheng KW, Chen F. Extract of the microalga Nitzschia laevis prevents high-fat-diet-induced obesity in mice by modulating the composition of gut microbiota. Mol Nutr Food Res. 2019;63(3):e1800808. https://doi.org/10.1002/mnfr.201800808.
Han X, Guo J, Yin M, Liu Y, You Y, Zhan J, et al. Grape extract activates Brown adipose tissue through pathway involving the regulation of gut microbiota and bile acid. Mol Nutr Food Res. 2020;64(10):e2000149. https://doi.org/10.1002/mnfr.202000149.
Sheng Y, Liu J, Zheng S, Liang F, Luo Y, Huang K, et al. Mulberry leaves ameliorate obesity through enhancing brown adipose tissue activity and modulating gut microbiota. Food Funct. 2019;10(8):4771–81. https://doi.org/10.1039/c9fo00883g.
Gao X, Xie Q, Kong P, Liu L, Sun S, Xiong B, et al. Polyphenol- and caffeine-rich Postfermented Pu-erh tea improves diet-induced metabolic syndrome by remodeling intestinal homeostasis in mice. Infect Immun. 2018;86(1). https://doi.org/10.1128/IAI.00601-17.
Guo J, Han X, Tan H, Huang W, You Y, Zhan J. Blueberry extract improves obesity through regulation of the gut microbiota and bile acids via pathways involving FXR and TGR5. iScience. 2019;19:676–90. https://doi.org/10.1016/j.isci.2019.08.020.
Funding
The National Commission for Scientific and Technological Research (ANID, Chile) funded this work (grants FONDECYT #1171550 to D.F.G-D.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have nothing to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duarte, L., Gasaly, N., Poblete-Aro, C. et al. Polyphenols and their anti-obesity role mediated by the gut microbiota: a comprehensive review. Rev Endocr Metab Disord 22, 367–388 (2021). https://doi.org/10.1007/s11154-020-09622-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-020-09622-0