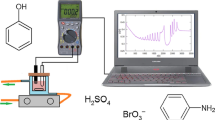
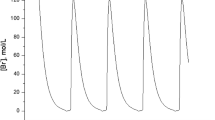
Abstract
This review summarizes the highlights of the history of oscillating reactions since the discovery of Bray in 1917 through the discovery of Belousov, the exponential growth of the number of works in the field that followed it and chemical chaos. It focuses on the work of Professor Slobodan Anić and the Belgrade group.
Similar content being viewed by others
References
Anić S, Mitić D, Kolar-Anić LJ (1985) The Bray–Liebhafsky reaction. I. Controlled development of oscillations. J Serb Chem Soc 50:53
Anić S, Mitić D, Veselinović D, Kolar-Anić LJ (1985) The Bray–Liebhafsky reaction. II. Potentiometric and pH-metric tracing. J Serb Chem Soc 50:529
Anić S, Lj Kolar-Anić (1986) Some new details in the kinetic considerations of the oscillatory decomposition of hydrogen peroxide. Ber Bunsenges Phys Chem 90:539
Anić S, Lj Kolar-Anić (1986) The oscillatory decomposition of hydrogen peroxide monitored by the potentiometric method with platinum and silver(+)/sulfur(2−) indicator electrode. Ber Bunsenges Phys Chem 90:1084
Anić S, Lj Kolar-Anić (1987) The influence of potassium iodate on hydrogen peroxide decomposition in Bray–Liebhafsky reaction. Ber Bunsenges Phys Chem 91:1010
Anić S, Mitić D, Curćija M (1987) The Bray–Liebhafsky reaction. III. Oscillatory decomposition of hydrogen peroxide in the presence of comparatively high acidity. J Serb Chem Soc 52:575
Bray WC (1921) A periodic reaction in homogeneous solution and its relation to catalysis. J Am Chem Soc 43:1262
Liebhafsky HA (1931) Reactions involving hydrogen peroxide, iodine and iodate ion. IV. The oxidation of iodine to iodate ion by hydrogen peroxide. J Am Chem Soc 53:2074
Liebhafsky HA, Wu LS (1974) Reactions involving hydrogen peroxide, iodine, and iodate ion. V. Introduction to the oscillatory decomposition of hydrogen peroxide. J Am Chem Soc 96:7180
Belousov B P (1958) Periodically acting reaction and its mechanism. Sb Ref Radiatsion Med 145 (in Russian)
Zhabotinsky AM (1964) Periodic process of the oxidation of malonic acid in solution (study of kinetics of Belousov’s reaction). Biofizika 9:306 (in Russian)
Belousov BP (1985) A periodic reaction and its mechanism. In: Field RJ, Burger M (eds) Oscillations and traveling waves in chemical system. Wiley, New York, p 605
Zaikin A, Zhabotinsky AM (1970) Concentration wave propagation in two-dimensional liquid-phase self-oscillating system. Nature 225:535
Zhabotinsky AM (1991) A history of chemical oscillations and waves. Chaos 1:379
Epstein IR, Pojman J (1998) Introduction to nonlinear chemical dynamics. Oscillations, waves, patterns and Chaos. Oxford University Press, New York
Körös E, Orban M (1978) Uncatalyzed oscillatory chemical reactions. Nature 273:371
Briggs TS, Rauscher WC (1973) An oscillating iodine clock. J Chem Edu 50:496
Furrow SD (1985) Chemical oscillators based on iodate ion and hydrogen peroxide. In: Field RJ, Burger M (eds) Oscillations and traveling waves in chemical system. Wiley, New York, p 171
Furrow SD, Cervellati R, Amadori G (2002) New substrates for the oscillating briggs-rauscher reaction. J Phys Chem A 106:5841
Furrow SD, Aurentz DJ (2010) Reactions of iodomalonic acid, diiodomalonic acid and other organics in the briggs–rauscher oscillating system. J Phys Chem 114:2526
Furrow SD, Cervellati R, Greco E (2012) A study of the cerium-catalyzed briggs–rauscher oscillating reaction. Z Naturforsch B 67:89
Schmitz G, Furrow SD (2015) Bray–Liebhafsky and non-catalysed briggs–rauscher oscillating reactions. Russ J Phys Chem A 89:114
Field RJ, Körös E, Noyes RM (1972) Oscillations in chemical systems, 2. Thorough analysis of temporal oscillations in the bromate-cerium-malonic acid system. J Am Chem Soc 94:8649
Sharma KR, Noyes RM (1976) Oscillations in chemical systems, 13. A detailed molecular mechanism for the Bray–Liebhafsky reaction of iodate and hydrogen peroxide. J Am Chem Soc 98:4345
Schmitz G (1974) Oscillations entretenues dans un système chimique homogène. J Chim Phys 71:689
Schmitz G (1987) Cinétique de la réaction de Bray. J Chim Phys 84:957
Matsuzaki I, Nakajima T, Liebhafsky HA (1975) Mechanism of the oscillatory decomposition of hydrogen peroxide in the presence of iodate ion and iodine. Faraday Symp Chem Soc 9:55
Liebhafsky HA, Furuichi R, Roe G (1981) Reactions involving hydrogen peroxide, iodine, and iodate ion. 7. The smooth catalytic decomposition of hydrogen peroxide, mainly at 50 °C. J Am Chem Soc 103:51
Schmitz G (1999) Effects of oxygen on the Bray–Liebhafsky reaction. Phys Chem Chem Phys 1:4605
L Kolar-Anić, Čupić Ž, Anić S, Schmitz G (1997) Pseudo-steady states in the model of the Bray–Liebhafsky oscillatory reaction. J Chem Soc Faraday Trans 93:2147
L Kolar-Anić, Misljenović D, Stanisavljev D, Anić S (1990) Applicability of Schmitz’s model to dilution-reinitiated oscillations in the Bray–Liebhafsky reaction. J Phys Chem 94:8144
Lj Kolar-Anić, Vukelić N, Dj Mišljenović, Anić S (1995) On the instability domains of some models for Bray–Liebhafsky oscillatory reaction. J Serb Chem Soc 60:1005
Anić S, Stanisavljev D, Čupić Ž, Radenković M, Vukojević V, Lj Kolar-Anić (1998) The selforganization phenomena during catalytic decomposition of hydrogen peroxide. Sci Sinter 30:49
Schmitz G, L Kolar-Anić, Anić S, Čupić Ž (2000) The illustration of multistability. J Chem Edu 77:1502
Anić S, L Kolar-Anić (1988) Kinetic aspects of the Bray–Liebhafsky oscillatory reaction. J Chem Soc, Faraday Trans I 84:3413
Anić S, Mitić D (1988) The Bray–Liebhafsky reaction.IV. New results in the studies of hydrogen peroxide oscillatory decomposition at high acidity. J Serb Chem Soc 53:371
Anić S, Stanisavljev D, Krnajski Belovljev G, LJ Kolar-Anić (1989) Examination of the temperature variations on the Bray–Liebhafsky oscillatory reaction. Ber Bunsenges Phys Chem 93:488
Anić S, Lj Kolar-Anić, Stanisavljev D, Begović N, Mitić D (1991) Dilution reinitiated oscillations in the Bray–Liebhafsky system. Reac Kin Catal Lett 43:155
Kragović M, Anić S (2008) Bray–Liebhafsky oscillatory reaction generated in batch reactor. Influence of pulse involving iodide at the end of preoscillatory period. In: Antic-Jovanović A (ed) Physical chemistry, vol I. Society of Physical Chemists of Serbia, Belgrade, p. 238
Anić S, Stanisavljev D (1996) Bray–Liebhafsky oscillatory reaction new kinetic data on low-acidity reaction systems. J Serb Chem Soc 61:125
Anić S, Kolar-Anić LJ (1996) The Bray–Liebhafsky reaction kinetics in iodide oscillations. J Serb Chem Soc 61:885
Anić S (1997) Relation between the number of oscillations and the activation energy of an oscillatory process. J Serb Chem Soc 62:65
Anić S, Lj Kolar-Anić, Körös E (1997) Methods to determine activation energies for the two kinetic states of the oscillatory Bray–Liebhafsky reaction. React Kinet Catal Lett 61:111
Vukojević V, Pejić N, Stanisavljev D, Anić S, Lj Kolar-Anić (1999) Determination of Cl−, Br−, I-, Mn2+, malonic acid and quercetin by perturbation of a non-equilibrium stationary state in the Bray–Liebhafsky reaction. Anal (Camb) 124:147
Cirić J, Anić S, Čupić Ž, Kolar-Anić LJ (2000) The Bray–Liebhafsky oscillatory reaction. kinetic investigations in reduction and oxidation pathways based on hydrogen peroxide concentration monitoring. Sci Sinter 32:187
Radenković M, Schmitz G, Kolar-Anić LJ, Anić S (1996) Dependence of the rate of iodine oxidation by hydrogen peroxide on the acidity of the Bray–Liebhafsky system. In: Ribnikar S, Anić S (eds) Physical chemistry ‘96. Society of Physical Chemists of Serbia, Belgrade, p 137
Radenković M, Schmitz G, Lj Kolar-Anić (1997) Simulation of iodine oxidation by hydrogen peroxide in acid media, on the basis of the model of Bray–Liebhafsky reaction. J Serb Chem Soc 62:367
Schmitz G (2010) Iodine oxidation by hydrogen peroxide in acidic solutions, Bray–Liebhafsky reaction and other related reactions. Phys Chem Chem Phys 12:6605
Schmitz G (2011) Iodine oxidation by hydrogen peroxide and Bray–Liebhafsky oscillating reaction: effect of the temperature. Phys Chem Chem Phys 13:7102
Szabo E, Ševčik P (2013) Reexamination of gas production in the Bray–Liebhafsky reaction: what happened to O2 pulses? J Phys Chem A 117:10604
Blagojević S, Pejić N, Anić S, Kolar-Anić LJ (2000) Belousov–Zhabotinsky oscillatory reaction. Kinetics of malonic acid decomposition. J Serb Chem Soc 65:709
Lj Kolar-Anić, Blagojević S, Pejić N, Begović N, Blagojević St N, Anić S (2006) New evidence of transient complex oscillations in a closed, well-stirred Belousov–Zhabotinsky system. J Serb Chem Soc 71:605
Blagojević SM, Anić S, Čupić Ž, Pejić N, LJ Kolar-Anić (2008) Malonic acid concentration as a control parameter in the kinetic analysis of the Belousov–Zhabotinsky reaction under batch conditions. Phys Chem Chem Phys 10:6658
Blagojević SM, Anić S, Čupić Ž, Pejić N, Lj Kolar-Anić (2009) Temperature influence on the malonic acid decomposition in the Belousov–Zhabotinsky reaction. Russ J Phys Chem A 83:1496
Blagojević SM, Anić S, Čupić Ž (2011) Influence of most important radicals on the numerically simulated Belousov–Zhabotinsky oscillatory reaction under batch conditions. Russ J Phys Chem A 85:2274
Blagojević SM, Anić S, Čupić Ž, Blagojević SN, Lj Kolar-Anić (2013) Numerical evidence of complex nonlinear phenomena of the Belousov–Zhabotinsky oscillatory reaction under batch conditions. Russ J Phys Chem A 87:2140
Gleick J (2008) Chaos: making a new science. Penguin Books, New York
Lorenz EN (1963) Deterministic nonperiodic flow. J Atmos Sci 20:130
Gyorgyi L, Field RJ (1992) A three-variable model of deterministic chaos in the Belousov–Zhabotinsky reaction. Nature 355:808
Petrov V, Gáspár V, Masere J, Showalter K (1993) Controlling chaos in the Belousov–Zhabotinsky reaction. Nature 361:240
Vukojević V, Anić S, Kolar-Anić LJ (2000) Investigation of dynamic behavior of the Bray–Liebhafsky reaction in the CSTR. Determination of bifurcation points. J Phys Chem A 104:10731
Vukojević V, Anić S, Kolar-Anić LJ (2002) Investigation of dynamic behavior of the Bray–Liebhafsky reaction in the CSTR properties of the system examined by pulsed perturbations with I−. Phys Chem Chem Phys 4:1276
Kolar-Anić LJ, Vukojević V, Pejić N, Grozdić T, Anić S (2004) Deterministic chaos in open well-stirred Bray–Liebhafsky reaction system. In: Boccaletti S et al (ed) AIP Conf, (experimental chaos), vol. 742. American Institute of Physics, New York, p 3
Pejić N, Maksimović J, Ribić D, Lj Kolar-Anić (2009) Dynamic states of the Bray–Liebhafsky reaction when sulphuric acid is the control parameter. Russ J Phys Chem A 83:1490
Pejić N, Vukojević V, Maksimović J, Ivanović AZ, Anić S, Čupić Ž, Lj Kolar-Anić (2011) Dynamic behavior of the Bray–Liebhafsky oscillatory reaction controlled by sulfuric acid and temperature. Russ J Phys Chem A 85:2310
Nuša-Bubanja I, Anić S, Kolar-Anić LJ (2014) Intermittent oscillation in Bray–Liebhafsky reaction system. In: Čupić Ž, Anić S (eds) Physical chemistry, vol I. Society of Physical Chemists of Serbia, Belgrade, p 336
Kolar-Anić LJ, Grozdić T, Vukojević V, Schmitz G, Anić S (2004) Simulations of complex oscillations based on a model of the Bray–Liebhafsky reaction. In: Anić S, Čupić Ž, Kolar-Anić LJ (eds) Selforganization in nonequilibrium systems. Society of Physical Chemists of Serbia, Belgrade, p 115
Schmitz G, Lj Kolar-Anić, Anić S, Grozdić T, Vukojević V (2006) Complex and chaotic oscillations in a model for the catalytic hydrogen peroxide decomposition under open reactor conditions. J Phys Chem A 110:10361
Schmitz G, Kolar-Anić LJ, Anić S, Čupić Ž (2008) Stoichiometric network analysis and associated dimensionless kinetic equations application to a model of the Bray–Liebhafsky reaction. J Chem Phys A 112:13452
Ivanović AZ, Čupić Ž, Janković M, Lj Kolar-Anić, Anić S (2008) The chaotic sequences in the Bray–Liebhafsky reaction in an open reactor. Phys Chem Chem Phys 10:5848
Ivanović AZ, Čupić Ž, LJ Kolar-Anić, Janković M, Anić S (2009) Large deviation spectra of chaotic time series from Bray–Liebhafsky reaction. Russ J Phys Chem A 83:1526
Ivanović AZ, Čupić Ž, Marinović SR, Schmitz G, Kolar-Anić LJ (2010) Critical manifold of the model for Bray–Liebhafsky oscillatory reaction. In: Anić S, Čupić Ž (eds) Physical chemistry, vol I. Society of Physical Chemists of Serbia, Belgrade, p 236
Ivanović AZ, Marković V, Anić S, Lj Kolar-Anić, Čupić Ž (2011) Structures of chaos in open reaction systems. Phys Chem Chem Phys 13:20162
Čupić Ž, Ivanović-Šašić A, Anić S, Stanković B, Maksimović J, Lj Kolar-Anić, Schmitz G (2013) Tourbillion in the phase space of the Bray–Liebhafsky nonlinear oscillatory reaction and related multiple-time- scale model. MATCH Commun Math Comput Chem 69:805
Čupić Ž, Blagojević SN, Blagojević S M, Anić S, Ivanović-Šašić A (2014) Ghost of the lost fixed point in a model of the Bray–Liebhafsky reaction. In Čupić Ž, Anić S (ed) Physical chemistry, vol I. Society of Physical Chemists of Serbia, Belgrade, p. 236, 360
Čupić Ž, Lj Kolar-Anić, Anić S, Macešić S, Maksimović J, Pavlović M, Milenković M, Bubanja I, Greco E, Furrow SD, Cervellati R (2014) Regularity of intermittent bursts in briggs–rauscher oscillating systems with phenol. Helv Chim Acta 97:321
Showalter K, Epstein IR (2015) From chemical systems to systems chemistry: patterns in space and time. CHAOS 25:097613
Clarke B (1980) Stability of complex reaction networks. In: Prigogine I, Rice S (eds) Advances in chemical physics. Wiley, New York, pp 1–215
Clarke B (1988) Stoichiometric network analysis. Cell Biophys 12:237
Clarke B, Jiang W (1993) Method for deriving Hopf and saddle-node bifurcation hypersurfaces and application to a model of the Belousov–Zhabotinskii system. J Chem Phys 99:4464
Schmitz G (1991) Etude du Braylator par la méthode de Clarke. J Chim Phys 88:15
Lj Kolar-Anić, Čupic Ž, Schmitz G, Anić S (2010) Improvement of the stoichiometric network analysis for determination of instability conditions of complex nonlinear reaction systems. Chem Eng Sci 65:3718
Maćešić S, Čupić Ž, Anić S, Lj Kolar-Anić (2015) Autocatalator as the source of instability in the complex non-linear neuroendocrine model. Int J Nonlinear Mech 73:25
Maćešić S, Čupić Ž, Blagojević S, Pejić N, Anić S, Lj Kolar-Anić (2015) Current rates and reaction rates in the stoichiometric network analysis (SNA). Open Chem 13:591
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmitz, G. Historical overview of the oscillating reactions. Contribution of Professor Slobodan Anić. Reac Kinet Mech Cat 118, 5–13 (2016). https://doi.org/10.1007/s11144-015-0968-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-015-0968-3