Abstract
Soil acidification is an issue for agriculture that requires effective management, typically in the form of lime (calcium carbonate, CaCO3), application. Mid infrared (MIR) spectroscopy methods offer an alternative to conventional laboratory methods, that may enable cost-effective and improved measurement of soil acidity and responses to liming, including detection of small–scale heterogeneity through the profile. Properties of an acidic soil following lime application were measured using both MIR spectroscopy with Partial Least Squares Regression (MIR-PLSR) and laboratory measurements to (a) compare the ability of each method to detect lime treatment effects on acidic soil, and (b) assess effects of the different treatments on selected soil properties. Soil properties including soil pH (in H2O and CaCl2), Aluminium (Al, exchangeable and extractable), cation exchange capacity (CEC) and organic carbon (OC) were measured at a single field trial receiving lime treatments differing in rate, source and incorporation. Model performance of MIR-PLSR prediction of the soil properties ranged from R2 = 0.582, RMSE = 2.023, RPIQ = 2.921 for Al (extractable) to R2 = 0.881, RMSE = 0.192, RPIQ = 5.729 for OC. MIR-PLSR predictions for pH (in H2O and CaCl2) were R2 = 0.739, RMSE = 0.287, RPIQ = 2.230 and R2 = 0.788, RMSE = 0.311, RPIQ = 1.897 respectively, and could detect a similar treatment effect compared to laboratory measurements. Treatment effects were not detected for MIR-PLSR-predicted values of CEC and both exchangeable and extractable Al. Findings support MIR-PLSR as a method of measuring soil pH to monitor effects of liming treatments on acidic soil to help inform precision agricultural management strategies, but suggests that some nuance and important information about treatment effects of lime on CEC and Al may be lost. Improvements to prediction model performance should be made to realise the full potential of this approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil acidity is a globally-significant issue for agriculture and may reduce crop production if not managed (Sumner et al., 2003). Acidification increases H+ and Al3+ ion concentrations in the soil while reducing other exchangeable cations (Ca2+, Mg2+, K+, and Na+) and cation exchange capacity ( Xu et al., 2002). This can lead to various soil nutrient imbalances, deficiencies and toxicities which may result in reduced crop growth (Condon et al., 2021). Soil pH can vary vertically and horizontal through the soil profile, and so targeted measurement and management of acidity is important. Acidification of soils in the subsurface, typically between 50 and 150 mm below the soil surface, is increasing in farming systems and, similarly to surface acidity, is detrimental to crop productivity (Mclay et al., 1994; Paul et al., 2003; Whitten et al., 2000). Acid layers in the subsurface have adverse impacts on root growth, nodule numbers and efficacy of nitrogen (N2)-fixing rhizobia, and overall plant vigour (Rengel et al., 2000a; Tang, 2004).
The most common and effective amelioration technique for soil acidity is the addition of neutralising agents, typically lime (calcium carbonate, CaCO3), to raise the soil pH to suitable levels (typically pH 6–7) (Goulding, 2016; Li et al., 2019). Studies (Chimdi et al., 2012; Paradelo et al., 2015; Saarsalmi et al., 2011) have demonstrated that the liming can influence the soil chemical properties. The change in pH is currently the most commonly measured soil property in acid remediation. However, there are other metrics by which acid remediation are assessed, including Aluminium concentration (Anderson et al., 2021; Chimdi et al., 2012), Cation Exchange Capacity (Chimdi et al., 2012; Saarsalmi et al., 2011) and Organic Carbon content (Grover et al., 2017; Paradelo et al., 2015).
Conventional laboratory soil testing methods are often time-, energy- and cost-intensive when high throughput sampling is required. Infrared spectroscopy (IR) offers an alternative to laboratory methods that is fast, accurate for various soil properties, and relatively low cost (Lelago & Bibiso, 2022; Soriano-Disla et al., 2014; Stenberg et al., 2010). Recent advances in precision agriculture and the need for high throughput assessment of soil properties have led to development of spectroscopy as a valuable tool in this space (Gebbers & Adamchuk, 2010). IR analyses are characterised by the spectral region of radiation involved, with near IR (NIR; wavelength 700–2 500 nm, wave number 14,000–4 000 cm−1) and Mid-IR (MIR; wavelength 2 500–25,000 nm, wave number 4 000–400 cm−1) being two commonly used spectral regions for soil analysis (Bellon-Maurel and McBratney, 2011; Soriano-Disla et al., 2019). Studies have demonstrated that MIR tends to offer more accurate predictions of soil OC, pH and CEC than NIR (McCarty et al., 2002; Soriano-Disla et al., 2014; Vohland et al., 2014).
For soil acidification, the use of MIR has been tested as an alternative method to determine liming requirement. Metzger et al. (2020) achieved a R2 of 0.76 and RMSE of 1.68 in their investigation of MIR-PLSR LR predictions for tilled soils in Ireland. Leenen et al. (2019) evaluated LR derivation based on MIR-PLSR predictions of OC, clay content, and pH, as well as direct LR prediction with MIR-PLSR and found satisfactory performance for both approaches (R2 = 0.54–0.82; RMSE = 857–1414 and R2 = 0.52–0.77; RMSE = 811–1420 respectively). To our knowledge, the potential use of MIR for the purpose of monitoring acidic soil responses to liming has not yet been demonstrated.
The aims of this study were to measure and monitor acidic soil properties following lime application to (a) compare the ability of MIR spectroscopy with conventional laboratory methods to detect treatment effects of lime on selected properties of an acidic soil, and (b) assess the effects of different amelioration treatments on selected soil properties. Several key soil properties including soil pH (in H2O and CaCl2), Aluminium (Al, both exchangeable and extractable), cation exchange capacity (CEC) and Organic carbon (OC) were measured at a single, replicated field trial in a dryland agricultural cropping region of South Australia, receiving different lime treatments. The statistical analyses of MIR- PLSR predicted properties were compared with statistical analyses of laboratory measurements of the same properties. We hypothesized that (a) MIR-based measurements would have a similar ability to detect a lime treatment effect as laboratory measurements and (b) selected soil properties would be most affected by treatments involving high lime rates and incorporation.
Methods
Sample site and sample collection
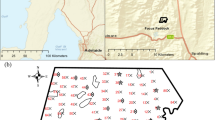
Soils were collected from one field trial site at Sandilands, located on the Yorke Peninsula in South Australia (34°33'15.4"S 137°42'09.6"E) in 2021. The trial was established in 2019 on an acidic soil (pH 4.29 +/− 0.24 in a 1:5 soil:0.01M CaCl2 extract) that is used for dryland agricultural cropping, and comprised 4 replicate blocks (20 m × 40 m) containing different treatments and lime sources, rates and incorporation methods (Table 1). All Lime sources used were from deposits located in South Australia. The most commonly used lime source (LS1) was Angaston Penlime, from Penrice Quarries. The Agricola lime source (LS2) was sourced from Agricola Mining, and Kulpara lime (LS3) was a dolomitic lime sourced from Hallett Resources. The Warooka lime source (LS4) was a calcareous sand product collected from a calcareous sand hill on the Yorke Peninsula, South Australia. The soil at this location is a sandy loam (16% clay to 250 mm) over medium clay (Chromosol in the Australian Soil Classification (Isbell 2016), Luvisol in World Reference Base for Soil Resources (Food and Agriculture Organization of the United Nations, 2006)) and was limed 26 months prior to sampling. The treatments selected for sampling were limed at three rates (2, 4 or 6 t/ha) (surface application or tilled with a tyned cultivator to a depth of 75mm) with lime comprising both calcium carbonate (CaCO3) and dolomite (CaMg(CO3)2), and one treatment including a surface application of gypsum at 5t/ha (Table 1). Across each of the four replicate blocks, ten cores (0–200 mm) were collected within each treatment, and sub-sampled at 0–50 mm, 50–100 mm and 100–200 mm with pseudoreplicate samples (n = 10) of the same depth increment combined within each replicate block for laboratory analysis. This set of soils (Set A) was used in all statistical analysis presented in this work. An additional set of soils (Set B) from the same trial site across selected treatments and at a depth interval of 25 mm, was also collected. Set B soils did not undergo laboratory analysis except for MIR spectra measurements. All soils were oven dried at 40 °C for 12 h and sieved to < 2mm prior to laboratory analysis, then further ground using a pestle and mortar to < 0.1 mm for MIR analysis.
Soil property analysis
Soil (Set A only) property data were determined using methods outlined in Rayment and Lyons (2011). Briefly, pH was measured in both a 1:5 soil:water extract (pH (H2O)) and 1:5 soil:0.01M calcium chloride extract (pH (CaCl2)), organic carbon was measured following the Walkley and Black method, and CEC measured using an ammonium acetate extract at pH 7. Exchangeable Al was determined via an ammonium acetate extract (Rayment & Lyons 2011) while extractable Al was determined using an extractant of 0.01M CaCl2 in a 1:10 soil to extract ratio. Aluminium in all soil extracts was measured via ICPAES (Smith et al. 1994)—Perkin Elmer model 8300DV at a wavelength of 396.153 nm with a multi-component spectral fitting (MSF) correction for Ca.
Infrared spectral measurements
Four sub-samples (approx. 1 g) of each finely ground dry soil sample from both Set A (n = 120) and Set B (n = 96) were placed into a 20 mm diameter stainless steel auto-sampler cup and the surface levelled in preparation for MIR spectral acquisition. Spectra were acquired using a Thermo Nicolet 6700 Fourier transform infrared spectrometer (fitted with a diffuse reflectance attachment) (Thermo Fisher Scientific Inc., MA, USA). Each sample was scanned 25 times with a KBr beam-splitter and a deuterated triglycine sulphate (DTGS) detector, with a spectral range of 7800−400 cm−1 at a 4 cm−1 resolution. Spectra are expressed in absorbance (A) units where A = 1/(log reflectance). An initial background reference scan was made prior to each sample run using an easiDiff Aluminium alignment mirror disc. The four separate spectra of each sample were analysed for uniformity, and outliers and non-uniform spectra were removed, before being averaged to reduce signal-to-noise ratio, with the averaged spectra used for pre-processing and analysis as described below.
Partial least squares modelling: data preparation, preprocessing and transformation
In order to translate highly multivariate spectral data into meaningful values for soil monitoring, spectral processing and multivariate analysis was required. Chemometric methods are often used for calibration and validation analysis (e.g. using Partial Least Squares Regression, PLSR) from reference laboratory chemistry data and corresponding spectra (Nocita et al., 2015; Soriano-Disla et al., 2014; Wijewardane et al., 2018). Predictions rely on the presence of characteristic features (e.g. peaks indicative of clay or carbonate functional groups) within the IR absorbance spectra that are directly due to, or highly correlated with, the property being measured (McCarty et al., 2002; Janik et al., 2007; Soriano-Disla et al., 2014).
Spectral pre-processing and chemometric modelling were undertaken in this study using Unscrambler 10.2 software (CAMO Software AS, Oslo, Norway) and OPUS software. The MIR spectra and corresponding chemical data were imported into the software programs prior to analysis. Spectra were truncated to 4000–700 cm–1 before Principal Component Analysis (PCA) was performed to identify and remove any outliers, and to assess the uniformity of each data set. PLSR modelling was used for property prediction, and this method was optimised via the pre-treatment of raw spectra. The aim of this was to retain sufficient information from the spectra and reduce the influence of random noise and scattering. Common pre-treatment measures were used prior to PLSR including: smoothing of the spectra, using first or second derivatives of the spectra (Savitzky et al., 1964), standard normal variate (SNV) (Barnes et al., 1989), multiplicative scatter correction (MSC) (Geladi et al., 1985) and baseline correction. Pre-treatment methods were applied to maximise the variance explained by the model (R2) and to minimise the prediction error (root mean square error, RMSE) with an optimal number of latent variables or factors. Spectra were analysed in the OPUS software program, using the ‘Optimise’ function in order to determine the appropriate spectral preprocessing selections to achieve optimal model performance for the MIR-PLSR prediction of pH, Al, CEC and OC. This function runs multiple PLSR models with varying combinations of spectral pre-treatments and spectral regions, to find the combination of results in the highest R2 and RMSE. The top three results of the Optimise test were then implemented in the Unscrambler software, and all further analysis was undertaken here. For each of the four prediction models, baseline offset correction with Standard Normalised Variance was the highest performing pre-processing option, and use of the 4000–700 cm−1 MIR region gave the results in both the OPUS and Unscrambler software. Initially, models were built using the full data set and a leave-2 or leave-3 out cross-validation to establish model performance. The data were then separated into a cross validation calibration (n = 60, 50%) and predictive validation (n = 60, 50%) data set for property prediction. A Kennard Stone (K-S) algorithm was used to select samples from the cross-validation calibration set for predictive validation (Kennard and Stone 1969) as K-S has performed best when compared with other algorithms for a soil IR property predictions, including for CEC and pH (Ng et al. 2018). Samples with Al (exchangeable and extractable) < LOD were treated as having no Al present, and included in the regression models. Model performance was evaluated by means of the coefficient of determination (R2), RMSE, and by ratio of performance to the interquartile range (RPIQ). Once MIR-PLSR property prediction models were established, full predictions were run on Set B soils using the prediction models generated from Set A soils. The purpose of this was to explore the ability of MIR to produce soil information at a finer scale than traditional laboratory analyses, and avoid the extra cost and time required to undertake traditional laboratory analyses. Calibration and validation was not performed on Set B soils as Set B acted a test set for independent prediction. Model performance was assumed to be the same as the Set A soils as all soils were collected from the same profile and depth range.
Statistical analysis
Initially, a paired t test was used to determine whether the mean difference between paired observations (laboratory-measured and MIR-PLSR measured) on Set A soils differed significantly from zero. A significance level of P = 0.05 was selected, to support or reject the null hypothesis that there was no significant difference between the means of pairs of data.
The effects of liming on soil pH (in H2O and CaCl2), Al (exchangeable and extractable), CEC and OC at each of the three depth intervals and across different treatments were analysed using a two-way analysis of variance (ANOVA). Significant (P ≤ 0.05) differences between means were identified using the Least Significant Difference (LSD) test. Tukey’s HSD test was used in post hoc pairwise multiple comparisons. In all statistical tests the level of significance was set at P ≤ 0.05. All analyses were computed using the R software (R Core Team, 2022).
Results and discussion
Control (unlimed) soil properties
Descriptive statistics for the laboratory-measured pH (in H2O and CaCl2), Al (exchangeable and extractable), CEC and OC of the control soil (untreated) are presented in Table 2. pH (H2O) values ranged from 4.51 to 5.29 and pH (CaCl2) values ranged from 4.03 to 4.6 across all depths. At pH (H2O) of less than 4.5, Al3+ concentrations increase in soil and may reduce plant root growth depending on the tolerance of the plant species (Kidd & Proctor, 2000). The levels of Al exchangeable (3.5–28 cmolc/kg) and of Al extractable (1.5–14 mg/kg) in the control soil were both above recommended limits in Australia (Gazey and Azam 2018), which indicates remediation via liming is required at both the surface and subsurface. CEC values ranged from 3.92 to 8.9 cmolc/kg with a mean of 5.93 +/− 1.43 cmolc/kg. These low CEC values fall within the typical range of a soil with a sandy texture (Rayment and Lyons 2011). OC content ranged from 0.43 to 2.25% and had a mean value of 1.29 +/− 0.57%, typical of Australian cropping soils (Viscarra Rossel et al., 2014). All laboratory-measured average values are presented in Fig. 1.
Laboratory-measured soil properties
Figure 1 depicts the soil properties of interest at the three depths as measured by conventional laboratory methods. Data presented are averaged values for each depth (0–50 mm, 50–100 mm and 100–200 mm) from all four replicate blocks that were sampled, and indicate how each of the liming treatments differ from the control (in red).
MIR-PLSR prediction of soil properties
MIR-PLSR model performance of all soil samples in Set A is shown in Table 3 and Fig. 2 (see Table S1 for statistical summary of data), with predicted values for the various treatments presented in supplementary material (Figure S1). The prediction of pH in both H2O and CaCl2 with MIR-PLSR (RMSE = 0.287, R2 = 0.739 RPIQ = 2.230 and RMSE = 0.311, R2 = 0.788, RPIQ = 1.897 respectively) are similar to those observed in other MIR studies. For example, Pirie et al. (2005) predicted pH with R2 of 0.71, while Viscarra Rossel et al. (2006) obtained similar accuracy for the prediction of pH in water (R2 = 0.69) and CaCl2 (R2 = 0.7). Janik et al. (2009) achieved R2 of 0.75 and 0.69 for the prediction of pH in water and CaCl2 suspension, respectively. Although pH does not have a direct spectral response, it is related to other factors such as organic acids, exchangeable cations and carbonates (Reeves, 2010; Sarathjith et al., 2014, Minasny et al., 2009). This has been termed ‘surrogate’ or ‘secondary’ correlation. It is also noted the results in the current study have higher predictive capability than previous research using NIR to measure the pH of acidic soils for precision agricultural management (Sleep et al., 2021).
Exchangeable Al was also well-predicted in the MIR-PLSR model (RMSE = 3.308, R2 = 0.714, RPIQ = 4.917) which is consistent with findings in some previous studies ( R2 of 0.81–0.98, Minasny et al., 2009; Ng et al., 2022). Jones (1984) showed Al saturation on exchange sites can be predicted from other (non-acidic) exchangeable cation saturation, organic carbon content, and pH. This suggests exchangeable Al may also be predicted via a secondary correlation to soil properties that are spectrally active. Extractable Al was less well-predicted in the present study, and had the poorest model performance of all the prediction models (RMSE = 2.023, R2 = 0.582, RPIQ = 2.921). Ng et al. (2022), in their recent assessment of the ability of MIR to accurately measure numerous soil properties, found that, although many extractable elements were poorly predicted, the prediction of extractable Al performed well (R2 = 0.95, RMSE = 3.34). This may be due partly to the larger number of soil types (e.g. higher clay content) used in their study, enabling a broader range of Al extractable values.
The relatively strong regression between predicted versus laboratory CEC values (R2 = 0.760, RMSE = 1.479, RPIQ = 0.994) plotted in Fig. 2E, is likely due to the high sensitivity of the MIR for clay type, clay content, sand content and organic matter, which are all strong determinants of soil CEC. A review by Soriano-Disla et al (2014) found inconsistent but generally well-predicted results from 13 studies that tested MIR for CEC prediction, with R2 values ranging from 0.34 to 0.98 (median = 0.85). Possible explanations given for this variability were the wide range of laboratory methods used to determine CEC, and low R2 values were attributed to insufficient samples. A number of studies included in the review achieved R2 values of higher than 0.9, and the review concluded that MIR is useful for the accurate prediction of CEC.
The prediction model for soil OC had an RMSE = 0.192, R2 = 0.881 and RPIQ = 5.729, and this result supports an increasing body of literature highlighting the successes of IR in measuring soil OC (Baldock et al. 2014; Calderón et al. 2011; Janik et al. 2007). This success is likely due to the chemical groups comprising soil organic matter being nearly all infrared active (Leifeld 2006).
Fine scale prediction of soil properties using MIR-PLSR models
Predicted soil properties for Set B soils based on MIR-PLSR prediction models from Set A soils are shown in Fig. 3. Using these higher resolution (i.e. 25 mm increments) MIR scans, the main zone of influence of the liming appears to extend from the surface to 100 mm depth in the soil profile, which was not clear in the coarser resolution sampling (Figs. 2 and 3). This serves as a demonstration of the potential of MIR to provide higher throughput soil information where a finer scale of detail is beneficial. Given the spatial heterogeneity of soil pH and associated properties down a soil profile, and potential for different lime incorporation and movement patterns, this approach may prove useful to inform targeted and effective management.
Comparison of laboratory measured and MIR-PLSR predicted soil properties
To assess difference between laboratory and MIR-PLSR results, a paired sample t-test was run with a confidence interval of 95% and the results are presented in Table 4. Results showed a p value of > 0.05 for all variables, indicating that the null hypothesis (mean difference between all laboratory-measured and PLSR-MIR predicted soil properties (pH, OC, CEC and Al) is zero) was supported. This indicates that, on average, there was no significant difference between means of paired laboratory measured and MIR-PLSR predicted soil properties.
Effects of lime treatment on soil chemical properties
Two-way ANOVA was also undertaken on both sets of data to determine if the significance of either lime treatment, depth or the interaction of both, differed depending on the data set used (either laboratory or MIR) in the statistical analysis. The results on the laboratory data indicated that the interaction effect of treatment x depth on the selected soil properties was significant for soil pH (H2O and CaCl2) and CEC. Treatment and depth effects were significant for Al (extractable and exchangeable) individually, but the interaction of the two was not significant. Soil OC only differed significantly with depth. ANOVA data for the MIR predicted properties indicate that interaction effect of treatment × depth on the selected soil properties was significant for soil pH (H2O and CaCl2) but with a lower level of significance than that of the laboratory measured properties. For soil Al (extractable and exchangeable), CEC and OC, only the effect of depth was significant (Table 5).
Effects of lime on soil pH
For soil pH in H2O values predicted via MIR-PLSRindicate that, at 0–50 mm, three treatments (LS1 4T + gypsum, LS1 4T + till and LS1 6T + till) differed significantly from the untreated control soil. Treatment LS1 6T was not significant whereas treatment LS 4T + gypsum was significant when MIR-PLSR-predicted pH(H2O) values were used instead of laboratroy measured values. MIR_PLSR predictions based on pH measured in CaCl2 indicate that only two treatments (LS1 4T + till and LS1 6T + till) were significanlty different from the control. This shows a decrease in the detection of significant treatement effects when compared with laboratory based measurements of pH in CaCl2, which showed significant differences between lime treatment and the control in all but two treatments (LS1 2T and LS3 4T) (Table 1).
At both 50–100 mm and 100–200 mm, there was no significant difference between lime treatments and the control and this finding agrees with the laboratory-measured results. This indicates that, 2 years following liming, and even where lime has been incorporated into subsurface layers, the effects of lime on soil pH was only significant in the surface soil where the lime was applied. The two tillage treatments were significantly different from the control treatment in both MIR-PLSR predicted pH in both H2O and CaCl2 and the pH of these two treatments were highest in the 0–50 mm layer for both laboratory meausred and MIR-PLSR predicted results (Fig. 4). Thus, hypothesis (a), that MIR-based measurements would have a similar ability to detect a lime treatment effect as conventional laboratory-based measurements was partially supported, but nuance and potentially important information was lost about other treatment effects. This, however, may be partially offset by potential of MIR-PLSR to obtain higher resolution information as shown in the Set B results (Fig. 3). Hypothesis (b) was supported as the treatments involving high lime rates and incorporation significantly affected soil pH in all instances in the 0–50 mm surface layer. No significant differences in pH were detected amongst treatments in the 50–100 or 100–200 mm layers, even when incorporation of lime was used (Figs. 4, 5, 6).
Results of Tukey’s HSD test for treatment × depth interaction responses to lime treatments based on MIR-PLSR predicted soil pH. graph headings specify liming treatment as described in Table 1. Different letters within each treatment indicate significant differences among depths at 5%
Results of Tukey’s HSD test for treatment × depth interaction responses to lime treatments based on MIR-PLSR predicted soil pH (1:5 in CaCl2). Graph Headings specify liming treatment as described in Table 1. Different letters within each treatment indicate significant differences among depths at 5%
Effects of lime on soil Al (exchangeable and extractable)
The two-way ANOVA output of the interaction effects of lime Treatment × Depth, indicated that laboratory-measured exchangeable and extractable Al was affected significantly by liming treatment and depth individually, whereas no treatment effects were detected in the MIR-PLSR-predicted Al concentrations in the soil samples. The significant treatment effects on exchangeable Al observed for LS1 6T, LS1 6T + till and LS14T + gypsum (Figure S4), and significant treatments effects on extractable Al observed for all treatments except LS2 4T (Figure S6) were all lost with MIR-PLSR values. In each of these Al measurements, the inability of MIR-PLSR to detect significant differences between treatments is a disadvantage, as Al3+ toxicity is widely regarded as the most detrimental consequence of low pH soils to plant growth.
Effects of lime on soil CEC
The comparison of laboratory measurements with MIR-PLSR measurements of CEC to assess effects of lime gave similar results to the Al analyses: treatment effects of lime on soil CEC that were present in the laboratory results were lost when MIR-PLSR was used. Post hoc analysis of laboratory-measured CEC shows that, at 0–50 mm, 4 (LS 1 4T, LS1 4T + gypsum, L1 6T and LS16T + till) of the 9 lime treatments differed significantly from the control soil (Fig. 6). At 50–100 mm and at 100–200 mm, there was no significant difference in CEC between treatments and the control. Lime source 1 significantly affected CEC in most instances at 0–50 mm except at the low 2 t/ha lime appliation rate,. Tukey’s HSD results for MIR-PLSR-predicted CEC indicate that only CEC in the top 0–50 mm differed significantly from the CEC at both 50–100 mm and 100–200 mm (Figure S6) and did not show the significant treatment effects that were evident when lab data were used. It may be because the RMSE was too high in the predicted CEC values to reflect differences between treatments and, as a result, detail and important information is lost from the use of MIR-PLSR-predicted properties in this instance.
Results of Tukey’s HSD test for Depth responses to lime treatments based on Laboratory- measured soil CEC. Graph Headings specify liming treatment as described in Table 1. Different letters within each treatment indicate significant differences among depths at 5%
Effects of lime on soil OC
Results from two-way ANOVA indicated that the treatments did not differ in total SOC (Table 5). Differences in SOC were observed at different depths, and the post hoc results indicate similar groupings between laboratory-measured and MIR-PLSR predicted results (Figure S7). OC was highest (1.8% and 1.71%) in the 0–50 mm layer, and decreased with depth. The effects of lime on OC levels in soil have been investigated and results vary, as liming has been shown to increase, decrease and have no effect on soil OC mineralisation, depending on the primary process involved. (Haynes and Naidu, 1998; Paradelo et al. 2015). Furthermore, soil OC comprises various forms of organic matter that differ in decomposability, chemical stability, and reactivity with the soil matrix and lime additions (Wang et al, 2016). The net effects of liming on OC due to combined influenced of soil processes and forms of OC are not yet fully understood, and require further investigation (Paradelo et al. 2015). This study did not explore the various forms of OC in soil, but does indicate that MIR-PLSR predicted OC is an accurate alternative to laboratory measurements in a liming context.
Conclusions
This study assessed the use of MIR-PLSR prediction as a tool to measure and monitor soil properties pre- and post-lime application compared to laboratory-measurements. Results indicate that MIR-PLSR performed well for predictions of soil pH (H2O and CaCl2) and was able to detect a similar treatment effect to that of laboratory measurements for however, some treatment effects were lost. Performance was less effective for CEC and Al as all treatment effects were not detected when MIR-PLSR-predicted values were used. While this study demonstrates the success of MIR-PLSR to predict select soil properties within a combined data set, results indicate that nuance may be missed with this method in terms of some treatment effects. When larger differences were present between treatments, MIR-PLSR was able to detect these, most importantly for soil pH, which is the most commonly used metric of acid soil remediation success. Organic Carbon was predicted well, although treatment effects were not present in either laboratory based or MIR-PLSR predicted results. When soil property responses to lime were relatively small (i.e. for Al and CEC), the error of MIR-PLSR prediction was likely too high to detect these differences, and so important statistically significant treatment effects were not detected when MIR-PLSR was used. It should be noted that this study was conducted at one site with a relatively limited number of samples. Increasing the sample number to may improve the performance of prediction models in the future. Additionally, this method could be tested in a range of soil types and in situations where treatment effects may be more significant, to explore if the limitations of MIR-PLSR are applicable in other contexts. Overall, this study supports the ability of MIR-PLSR to cost effectively and rapidly provide information on some properties of limed soils, but prediction models should be improved in order to access information about the treatment effects of lime and enhance the potential of this method for management of soil acidification with precision agriculture. MIR-PLSR has potential, once a suitable calibration is established, to reduce the cost associated with testing for soil acidity and provide information at a higher spatial resolution, when compared to traditional laboratory chemistry analyses.
References
Anderson, G. C., Pathan, S., Hall, D. J. M., Sharma, R., & Easton, J. (2021). Short- and long-term effects of lime and gypsum applications on acid soils in a water-limited environment: 3. soil solution chemistry. Agronomy, 11(5), 826. https://doi.org/10.3390/agronomy11050826
Baldock, J. A., Hawke, B., Sanderman, J., & Macdonald, L. M. (2014). Predicting contents of carbon and its component fractions in Australian soils from diffuse reflectance mid-infrared spectra. Soil Research, 51(8), 577–595. https://doi.org/10.1071/SR13077
Barnes, R. J., Dhanoa, M. S., & Lister, S. J. (1989). Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Applied Spectroscopy, 43, 772–777. https://doi.org/10.1366/0003702894202201
Bellon-Maurel, V., & McBratney, A. (2011). Near-infrared (NIR) and mid-infrared (MIR) spectroscopic techniques for assessing the amount of carbon stock in soils–critical review and research perspectives. Soil Biology and Biochemistry, 43(7), 1398–1410. https://doi.org/10.1016/j.soilbio.2011.02.019
Calderón, F. J., Reeves, J. B., Collins, H. P., & Paul, E. A. (2011). Chemical differences in soil organic matter fractions determined by diffuse-reflectance mid-infrared spectroscopy. Soil Science Society of America Journal, 75(2), 568–579. https://doi.org/10.2136/sssaj2009.0375
Chimdi, A., Gebrekidan, H., Kibret, K., & Tadesse, A. (2012). Effects of liming on acidity-related chemical properties of soils of different land use systems in Western Oromia. Ethiopia. https://doi.org/10.5829/idosi.wjas.2012.8.6.1686
Condon, J., Burns, H., & Li, G. (2021). The extent, significance and amelioration of subsurface acidity in southern New South Wales, Australia. Soil Research, 59(1), 1. https://doi.org/10.1071/SR20079
Food and Agriculture Organization of the United Nations. (2006). World reference base for soil resources, 2006: A framework for international classification, correlation, and communication. Food and Agriculture Organization of the United Nations.
Gazey, C., & Azam, G. (2018). Effects of soil acidity. Text. Retrieved June 17, 2022 from https://www.agric.wa.gov.au/soil-acidity/effects-soil-acidity?nopaging=1
Gebbers, R., & Adamchuk, V. I. (2010). Precision agriculture and food security. Science. https://doi.org/10.1126/science.1183899
Geladi, P., MacDougall, D., & Martens, H. (1985). Linearization and scatter-correction for near-infrared reflectance spectra of meat. Applied Spectroscopy, 39, 491–500. https://doi.org/10.1366/0003702854248656
Goulding, K. W. T. (2016). Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use and Management, 32(3), 390–399. https://doi.org/10.1111/sum.12270
Grover, S. P., Butterly, C. R., Wang, X., & Tang, C. (2017). The short-term effects of liming on organic carbon mineralisation in two acidic soils as affected by different rates and application depths of lime. Biology and Fertility of Soils, 53(4), 431–443. https://doi.org/10.1007/s00374-017-1196-y
Harding, A., & Hughes, B. (2018). Current and potential lime sources in South Australia. Government of South Australia.
Isbell, R. (2016). The Australian soil classification. CSIRO Publishing.
Janik, L. J., Skjemstad, J. O., Shepherd, K. D., & Spouncer, L. R. (2007). The prediction of soil carbon fractions using mid-infrared-partial least square analysis. Soil Research, 45(2), 73. https://doi.org/10.1071/SR06083
Janik, L. J., Forrester, S. T., & Rawson, A. (2009). The prediction of soil chemical and physical properties from mid-infrared spectroscopy and combined partial least-squares regression and neural networks (PLS-NN) analysis. Chemometrics and Intelligent Laboratory Systems, 97, 179–188. https://doi.org/10.1016/j.chemolab.2009.04.005
Jones, C. A. (1984). Estimation of percent aluminum saturation from soil chemical data. Communications in Soil Science and Plant Analysis, 15(3), 327–335. https://doi.org/10.1080/00103628409367478
Kennard, R. W., & Stone, L. A. (1969). Computer aided design of experiments. Technometrics, 11(1), 137–148. https://doi.org/10.1080/00401706.1969.10490666
Kidd, P. S., & Proctor, J. (2000). Effects of aluminium on the growth and mineral composition of Betula pendula Roth. Journal of Experimental Boonyt, 51, 1057–1066. https://doi.org/10.1093/jexbot/51.347.1057
Leenen, M., Welp, G., Gebbers, R., & Pdtzold, S. (2019). Rapid determination of lime requirement by mid-infrared spectroscopy: A promising approach for precision agriculture. Journal of Plant Nutrition and Soil Science. https://doi.org/10.1002/jpln.201800670
Leifeld, J. (2006). Application of diffuse reflectance FT-IR spectroscopy and partial least-squares regression to predict NMR properties of soil organic matter. European Journal of Soil Science, 57(6), 846–857. https://doi.org/10.1111/j.1365-2389.2005.00776.x
Lelago, A., & Bibiso, M. (2022). Performance of mid infrared spectroscopy to predict nutrients for agricultural soils in selected areas of Ethiopia. Heliyon, 8(3), e09050. https://doi.org/10.1016/j.heliyon.2022.e09050
Li, G., Hayes, R., Condon, J., Moroni, S., Tavakkoli, E., Burns, H. (2019). Addressing subsoil acidity in the field with deep liming and organic amendments: Research update for a long-term experiment. In Cells to satellites (pp. 1–4). Presented at the 19th Australian Agronomy Conference, Australian Society for Agronomy. Retrieved June 23, 2020 from https://researchoutput.csu.edu.au/en/publications/addressing-subsoil-acidity-in-the-field-with-deep-liming-and-orga
Metzger, K., Zhang, C., Ward, M., & Daly, K. (2020). Mid-infrared spectroscopy as an alternative to laboratory extraction for the determination of lime requirement in tillage soils. Geoderma. https://doi.org/10.1016/j.geoderma.2020.114171
McCarty, G. W., Reeves, J. B., Reeves, V. B., Follett, R. F., & Kimble, J. M. (2002). Mid-infrared and near-infrared diffuse reflectance spectroscopy for soil carbon measurement. Soil Science Society of America Journal, 66(2), 640–646. https://doi.org/10.2136/sssaj2002.6400a
Mclay, C. D. A., Ritchie, G. S. P., & Porter, W. M. (1994). Amelioration of subsurface acidity in sandy soils in low rainfall regions.1. Responses of wheat and lupins to surface-applied gypsum and lime. Soil Research, 32(4), 835–846. https://doi.org/10.1071/sr9940835
Minasny, B., Tranter, G., McBratney, A. B., Brough, D. M., & Murphy, B. W. (2009). Regional transferability of mid-infrared diffuse reflectance spectroscopic prediction for soil chemical properties. Geoderma, 153(1), 155–162. https://doi.org/10.1016/j.geoderma.2009.07.021
Ng, W., Minasny, B., Jeon, S. H., & McBratney, A. (2022). Mid-infrared spectroscopy for accurate measurement of an extensive set of soil properties for assessing soil functions. Soil Security, 6, 100043. https://doi.org/10.1016/j.soisec.2022.100043
Ng, W., Minasny, B., Malone, B., & Filippi, P. (2018). In search of an optimum sampling algorithm for prediction of soil properties from infrared spectra. PeerJ, 6, e5722. https://doi.org/10.7717/peerj.5722
Nocita, M., Stevens, A., van Wesemael, B., Aitkenhead, M., Bachmann, M., Barthhs, B., Ben Dor, E., Brown, D. J., Clairotte, M., Csorba, A., Dardenne, P., Demattj, J. A. M., Genot, V., Guerrero, C., Knadel, M., Montanarella, L., Noon, C., Ramirez-Lopez, L., Robertson, J., … Wetterlind, J. (2015). Soil Spectroscopy: An Alternative to wet chemistry for soil monitoring. Advances in Agronomy. https://doi.org/10.1016/bs.agron.2015.02.002
Paradelo, R., Virto, I., & Chenu, C. (2015). Net effect of liming on soil organic carbon stocks: a review. Agriculture Ecosystems & Environment, 202, 98–107. https://doi.org/10.1016/j.agee.2015.01.005
Paul, K. I., Black, S., A., & Conyers, M. K. (2003). Development of acidic subsurface layers of soil under various management systems. In Advances in Agronomy (pp. 187–214). https://doi.org/10.1016/S0065-2113(02)78005-X
Pirie, A., Singh, B., & Islam, K. (2005). Ultra-violet, visible, near-infrared, and mid-infrared diffuse reflectance spectroscopic techniques to predict several soil properties. Soil Research, 43, 713–721. https://doi.org/10.1071/SR04182
R Core Team (2022). R software. Vienna, Austria. https://www.R-project.org/
Rayment, G. E., & Lyons, D. J. (2011). Soil chemical methods: Australasia. CSIRO Publishing.
Reeves, J. B. (2010). Near- versus mid-infrared diffuse reflectance spectroscopy for soil analysis emphasizing carbon and laboratory versus on-site analysis: Where are we and what needs to be done? Geoderma, 158, 3–14. https://doi.org/10.1016/j.geoderma.2009.04.005
Rengel, Z., Tang, C., Raphael, C., & Bowden, J. W. (2000a). Understanding subsoil acidification: Effect of nitrogen transformation and nitrate leaching. Soil Research, 38(4), 837. https://doi.org/10.1071/SR99109
Saarsalmi, A., Tamminen, P., Kukkola, M., & Levula, T. (2011). Effects of liming on chemical properties of soil, needle nutrients and growth of Scots pine transplants. Forest Ecology and Management, 262(2), 278–285. https://doi.org/10.1016/j.foreco.2011.03.033
Sarathjith, M. C., Das, B. S., Wani, S., & Sahrawat, K. L. (2014). Dependency measures for assessing the covariation of spectrally active and inactive soil properties in diffuse reflectance spectroscopy. Soil Science Society of America Journal, 78, 1522–1530. https://doi.org/10.2136/sssaj2014.04.0173
Savitzky, Abraham, & Golay, M. J. E. (1964). Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Analytical Chemistry, 36, 1627–1639. https://doi.org/10.1021/ac60214a047
Sleep, B., Mason, S., Janik, L., & Mosley, L. (2021). Application of visible near-infrared absorbance spectroscopy for the determination of soil pH and liming requirements for broad-acre agriculture. Precision Agriculture. https://doi.org/10.1007/s11119-021-09834-7
Smith, C., Peoples, M., Keerthisinghe, G., James, T., Garden, D., & Tuomi, S. (1994). Effect of surface applications of lime, gypsum and phosphogypsum on the alleviating of surface and subsurface acidity in a soil under pasture. Soil Research, 32(5), 995. https://doi.org/10.1071/SR9940995
Soriano-Disla, J. M., Janik, L. J., Forrester, S. T., Grocke, S. F., Fitzpatrick, R. W., & McLaughlin, M. J. (2019). The use of mid-infrared diffuse reflectance spectroscopy for acid sulfate soil analysis. Science of the Total Environment, 646, 1489–1502. https://doi.org/10.1016/j.scitotenv.2018.07.383
Soriano-Disla, J. M., Janik, L. J., Rossel, R. A. V., MacDonald, L. M., & McLaughlin, M. J. (2014). The performance of visible, near-, and mid-infrared reflectance spectroscopy for prediction of soil physical, chemical, and biological properties. Applied Spectroscopy Reviews, 49(2), 139–186.
Stenberg, B., Viscarra Rossel, R. A., Mouazen, A. M., & Wetterlind, J. (2010). Chapter five-visible and near infrared spectroscopy in soil science. In D. L. Sparks (Ed.), Advances in agronomy. Academic Press.
Sumner, M., Noble, D., & Rengel, Z. (2003). Soil acidification: The world story. Handbook of soil acidity (pp. 1–28). Marcel Dekker.
Tang, C. (2004). Causes and management of subsoil acidity. SuperSoil 2004. Presented at the 3rd Australian New Zealand Soils Conference, University of Sydney, Australia: www.regional.org.au/au/asssi/
Viscarra Rossel, R. A., Walvoort, D. J. J., McBratney, A. B., Janik, L. J., & Skjemstad, J. O. (2006). Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma, 131(1), 59–75. https://doi.org/10.1016/j.geoderma.2005.03.007
Viscarra Rossel, R. A., Webster, R., Bui, E. N., & Baldock, J. A. (2014). Baseline map of organic carbon in Australian soil to support national carbon accounting and monitoring under climate change. Global Change Biology, 20(9), 2953–2970. https://doi.org/10.1111/gcb.12569
Vohland, M., Ludwig, M., Thiele-Bruhn, S., & Ludwig, B. (2014). Determination of soil properties with visible to near- and mid-infrared spectroscopy: effects of spectral variable selection. Geoderma. https://doi.org/10.1016/j.geoderma.2014.01.013
Wang, X., Tang, C., Baldock, J. A., Butterly, C. R., & Gazey, C. (2016). Long-term effect of lime application on the chemical composition of soil organic carbon in acid soils varying in texture and liming history. Biology and Fertility of Soils, 52(3), 295–306. https://doi.org/10.1007/s00374-015-1076-2
Whitten, M. G., Wong, M. T. F., & Rate, A. W. (2000). Amelioration of subsurface acidity in the south-west of Western Australia: downward movement and mass balance of surface-incorporated lime after 2–15 years. Soil Research, 38(3), 711. https://doi.org/10.1071/SR99054
Wijewardane, N. K., Ge, Y., Wills, S., & Libohova, Z. (2018). Predicting physical and chemical properties of US soils with a mid-infrared reflectance spectral library. Soil Science Society of America Journal, 82, 722–731. https://doi.org/10.2136/sssaj2017.10.0361
Xu, R. K., Coventry, D. R., Farhoodi, A., & Schultz, J. E. (2002). Soil acidification as influenced by crop rotations, stubble management, and application of nitrogenous fertiliser, Tarlee, South Australia. Australian Journal of Soil Research, 40(3), 483–496. https://doi.org/10.1071/SR00104
Acknowledgements
This research was funded by the Grains Research and Development Corporation (GRDC) to DAS1905-01 led by the Department of Primary industries and Regions (PIRSA). RH acknowledges the support of a GRDC PhD scholarship provided by DAS1905-01. The authors are grateful for the help of the Les Janik (University of Adelaide) for advice with spectral analysis, Brian Hughes and Andrew Harding (PIRSA) for project support and advice.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hume, R., Marschner, P., Mason, S. et al. Using mid-infrared spectroscopy as a tool to monitor responses of acidic soil properties to liming: case study from a dryland agricultural soil trial site in South Australia. Precision Agric 25, 1340–1359 (2024). https://doi.org/10.1007/s11119-024-10114-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11119-024-10114-3