Abstract
Background and aims
While nitrogen (N) derived from soil organic matter significantly sustains agricultural plants, the complexities of organic N utilization pathways remain poorly understood. Knowledge gaps persist regarding diverse organic N pools, the microbial processes in N mineralization, and how plants shape the N-mineralizing microbial community through root exudation.
Results
To address these gaps, we propose an integrated conceptual framework that explores the intricate interplay of soil, plant, and microbiome dynamics within the context of soil carbon (C) cycling. Emphasizing plant effects on gross depolymerization and deamination of organic N—a crucial yet often overlooked aspect—we aim to enhance our understanding of plant N utilization pathways. In this context, we suggest considering the linkages between root and hyphal exudation, followed by rhizosphere priming effects which in turn control N mobilization. Based on the relation between exudation and N turnover, we identify microbial necromass as a potentially important organic N source for plants. Furthermore, we propose applying root economic theory to gain insights into the diverse strategies employed by plants in accessing soil organic N. Stable isotope tracers and functional microbiome analytics provide tools to decipher the complex network of the pathways of organic N utilization.
Conclusions
The envisioned holistic framework for organic N utilization pathways, intricately connects plants, soil, and microorganisms. This lays the groundwork for sustainable agricultural practices, potentially reducing N losses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In agricultural systems, only about 50% of the nitrogen (N) taken up by annual crops is current-year fertilizer derived (Gardner and Drinkwater 2009; Yan et al. 2020), indicating that plants are supplied with N derived from soil organic matter (SOM) to a large extent. Up to now, the contribution of different SOM pools to N nutrition and the processes by which these compounds are made available to the plant are not well understood (Leinweber et al. 2013; Yan et al. 2020), although a greater uptake of organic N sources might improve the efficient use of N resources (Drinkwater et al. 2017) and mitigate the environmental impacts of agriculture.
Our incomplete knowledge of organic N utilization pathways, i.e. organic N conversion to plant-available N, can be explained in part by the historical focus on the inorganic N pool (Daly et al. 2021) but is certainly also caused by the complexity of the soil N cycle. This complexity arises because of the strong functional link between microorganisms, plants and the soil matrix which shape the N cycle and N bioavailability. Moreover, the conversion of N within the soil is closely linked to the quality of soil organic matter input. This is because the ratio of C to N in organic matter (C/N ratio) determines the balance between microbial net N mineralization and immobilization (Manzoni et al. 2012).
We see a strong need to determine plant-microbe interactive effects on SOM cycling. Hence, we propose an integrated conceptual framework that addresses the interplay of soil, plant and microbiome functioning, all within the context of soil C cycling. Such a holistic framework has the potential to significantly advance our understanding of the contribution of organic N utilization pathways to the N nutrition of plants. Specifically, we propose an increased focus on plant effects on depolymerization and deamination of organic N, a crucial yet often overlooked aspect in understanding plant N use efficiency. We will suggest potential organic N sources for plants and elucidate the processes through which plants can influence the availability of these organic N sources. Additionally, we advocate for the application of root economic theory to gain insights into the diverse strategies employed by plants in accessing and mobilizing soil organic N.
Soil N cycling and sources of organic N
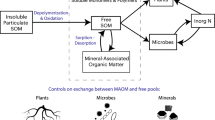
More than 90% of N in soil is present in organic form (Amelung 2001). Organic N primarily originates from plant litter decomposition, fire or animal residues and enters the soil from the surface as particulate organic matter (POM) or dissolved organic N (DON) (Knicker 2011). However, organic N is also introduced belowground in significant amounts through root litter and rhizodeposits (McNeill et al. 1997; Wichern et al. 2008; Arcand et al. 2013). Plant derived organic N is mostly present as particulate organic matter (POM) (Fig. 1) (Baldock and Skjemstad, 2000; Lavallee et al. 2020) and, unless protected within aggregates, susceptible to rapid decomposition by microbes (Cotrufo et al. 2019; Mueller and Koegel-Knabner, 2009; von Lützow et al. 2007). The products of microbial decomposition can contribute significantly (15–80%) to SOM (Liang et al. 2019; Angst et al. 2021; Camenzind et al. 2023). They have usually a lower C/N ratio than plant derived organic N (Khan et al. 2016; Wang et al. 2020b) and are to a large extent incorporated into mineral associated organic matter (MAOM) (Kopittke et al. 2018, 2020) which is more stable in soil compared to POM (Cotrufo et al. 2019).
Pools of nitrogen and pathways of N cycling between these pools. Considered as N pool are plant residues/POM, monomers as bioavailable N, mineral N as NH4+ and NO3- as well as microbial biomass N and microbial necromass N. Some of these pools are in exchange with MAOM via adsorption and desorption. DNRA = dissimilatory nitrate reduction to ammonium. Figure adapted from Schimel and Bennett (2004) and Daly et al. (2021)
About 30–60% of soil organic N consists of proteinaceous materials (e.g. proteins, peptides, and amino acids; plant or microbial derived), while amino sugars (e.g. muramic acid, glucosamine; microbial derived) make up 5–8% and heterocyclic N compounds (e.g. pyrroles, pyridines, pyrazoles; plant or microbial derived) account for 5–35%, although there are some uncertainties in quantification of heterocyclic N (Schulten and Schnitzer 1997; Nannipieri and Eldor 2009; Leinweber et al. 2013). Therefore, overall proteinaceous materials contribute significantly to soil organic N (Geisseler et al. 2010). Amino sugars also play an important role as they are the building blocks of microbial cell walls and they are used as biomarkers for microbial residues (i.e. microbial necromass). A major component of fungal cell walls is chitin, an unbranched polymer of N-acetylglucosamine (Rinaudo 2006). Bacterial cell walls are constructed of peptidoglycan consisting of glycan strands, repeating units of N-acetylglucosamine and N-acetylmuramic acid, crosslinked by short peptide stems (Steen et al. 2003; Vollmer et al. 2008).
To become plant available, complex organic N needs to be microbially depolymerized into its monomers (e.g. amino acids, amino sugars) before it is subsequently transformed into mineral forms such as NH4+ or NO3− (Schimel and Bennett 2004; Daly et al. 2021) (Fig. 1). Extracellular depolymerases are involved in the first step of soil organic N decomposition (Schimel and Bennett 2004). The most important extracellular depolymerases vary depending on the chemical composition of soil organic N. Proteases, chitinases, and peptidoglycan hydrolases are all prevalent among soil microorganisms, reflecting their widespread ability to degrade protein, chitin, and peptidoglycan (Geisseler et al. 2010 and references theirin). The small organic molecules released by extracellular depolymerases (e.g. free amino acids, free amino sugars) are available for microbial uptake, and their pool is therefore very small (less than 1% of the total pool) and highly dynamic (Wanek et al. 2010; Warren 2014; Hu et al. 2017). While there is evidence that crops can also take up dissolved organic N molecules (such as small peptides or amino acids), the ecological significance of this N acquisition pathway, particularly in agricultural systems remains controversial (Näsholm et al. 2009; Moreau et al. 2019). Specifically its relevance under field conditions and at realistic concentrations of organic N molecules in solution remains questionable (Jones et al. 2005). Therefore, NH4+ or NO3− can be considered the most important N forms for crop uptake (Schimel and Bennett 2004; Britto and Kronzucker 2013). NH4+ and NO3− are provided by the mineralization of N-containing monomers into its mineral forms (Fig. 1) and involves the deamination of amino acids and the hydrolysis of urea by ureases (Schimel and Bennett 2004; Daly et al. 2021). N mineralization is counteracted by N immobilization. This includes biotic N immobilization, the transformation of inorganic N by microorganisms and plants into organic N (Hart et al. 1994) and its subsequent incorporation into soil organic matter (Denk et al. 2017; Zhang et al. 2018) as well as plant N uptake (Van Groenigen et al. 2015). Additionally, N can be immobilized chemically by sorption to mineral surfaces (Bingham and Cotrufo 2016). This process is referred to as abiotic N immobilization.
In general, N turnover rates are controlled by temperature, latitude, ecosystem type, soil clay content, soil microbial biomass, the soil C/N ratio and soil pH (Li et al. 2020). In particular, the soil C/N ratio has long been recognized as an important control over decomposition processes as it governs the nutrient demand and the structure of soil microbial communities, thereby affecting the production of extracellular enzymes and finally N turnover (Mooshammer et al. 2014). On the one hand, organic N compounds with relatively low C/N ratios are likely to be immobilized by sorption on minerals and within the mineral associated organic matter (MAOM) (Kopittke et al. 2018, 2020; Wang et al. 2020a; Buckeridge et al. 2022). This preferential sorption of N rich compounds can be explained by the onion layer model, according to which N-rich compounds sorb directly and more strongly on mineral surfaces compared to other organic matter compounds and form a stable organic layer (Kleber et al. 2007). On the other hand, compounds with low C/N ratios might be an attractive source of N for microbes. For example, narrow C/N legume residues were shown to be preferentially incorporated into microbial biomass and resulted in higher net N mineralization compared to wide C: N wheat residues (Luce et al. 2016). Also, under low N availability, microbial necromass was shown to be preferentially decomposed and metabolized by microorganisms compared to other SOM fractions, leading to a rapid turnover of microbial residues (Zeglin and Myrold 2013). The direct quantification of microbial necromass cycling rates in soil has just recently been established based on isotope pool dilution (Hu et al. 2018). The authors found that microbial cell wall residues rapidly turn over in soil and that products of this microbial cell wall decomposition (e.g. free amino sugars, amino acids) add significantly to the bioavailable soil organic N pool, indicating that necromass-derived N could significantly contribute to plant nutrition (Hu et al. 2018). Although the cycling of necromass N and its contribution to plant N nutrition has not been directly quantified in the presence of plants so far, two studies have attempted to indirectly assess its potential significance (Cui et al. 2020; Pausch et al. 2024).
First, Cui et al. (2020) conducted an incubation experiment with addition of high amounts of 13C-labeled glucose in order to study priming, i.e. the short-term change in the turnover of SOM caused by addition of easily available C (Kuzyakov 2002). Particularly the addition of excessive amounts of glucose resulted in distinctive peaks of glucose-derived CO2, deviating from the typical exponential decay pattern normally observed after glucose decomposition. These peaks were interpreted as indicative of the recycling and mineralization of 13C-enriched microbial necromass stimulated by glucose addition. Building on this observation, the authors hypothesized that N recycling from microbial necromass after root exudation could serve as a significant mechanism, mitigating microbial N deficiency and possibly enhancing plant N availability. Second, Pausch et al. (2024) observed an increase in 15N natural abundance in plants with increases in rhizosphere priming. Considering that microbial necromass is enriched in 15N compared to total soil N (Dijkstra et al. 2006; Craine et al. 2015) the authors concluded that rhizosphere priming likely promotes the recycling of necromass-N cycling and the acquisition of necromass-derived N by plants. Notably, as of our current knowledge, no other efforts have been made to quantify the importance of microbial necromass as an N source for plants, though, recent advancements in methodology offer promising tools to quantify turnover rates of microbial necromass (Hu et al. 2018; Warren 2021). Therefore, unraveling the significance of microbial necromass as a potential N source for plants is critical. We strongly advocate to explore its contribution to plant N nutrition in future studies.
Plant control on soil N cycling and mineralization
Although soil and rhizosphere microorganisms shape N cycling most strongly as they transfer N between different pools, plants also exert control over N mineralization and availability by the following processes: (1) Plants remove mineral N from the soil solution with their root N uptake and immobilize this N in their biomass, whereby they compete with microorganisms such as nitrifiers or denitrifies for N (Schimel and Bennett 2004; Kuzyakov and Xu 2013; Thion et al. 2016). (2) Part of the N that plants immobilize is returned as litter to the soil after plant death or organ senescence. The amount and quality of the plant litter has a distinct effect on its decomposition and mineralization by oligotrophs and copiotrophs in succession (Myrold and Bottomley 2008; Geisseler et al. 2010; Pascault et al. 2013). (3) Moreover, some of the C that is assimilated by plants is allocated to roots, mycorrhizal hyphae and finally released into soil via root and hyphal exudation (Dilkes et al. 2004; Jones et al. 2004). Some of those compounds can directly affect N turnover, such as biological nitrification inhibitors that reduce the microbial process of nitrification (Coskun et al. 2017; Nardi et al. 2020; Sun et al. 2016). Moreover, plants may release proteases from their roots into the soil, potentially elevating the concentration of free amino acids in the soil solution as a source of N (Godlewski and Adamczyk 2007; Adamczyk 2021). However, research aimed at comprehending the impact of plant-released proteases on increased proteolysis has yielded conflicting results and interpretations (Greenfield et al. 2020; Adamczyk 2021). The vast majority of C released by plants into soil is likely to affect N turnover rather indirectly, through elevation of microbial activity and turnover. Although it is not yet clear, to which extent plants can control the exudation of C into soil (Jones et al. 2009; Carvalhais et al. 2011) it is clear that this exudation, enhances microbial activity and shapes the rhizosphere microbial community (Huang et al. 2014; Zhalnina et al. 2018; Iannucci et al. 2021; Lewin et al. 2024). Soil microorganisms are largely C limited and easily available plant derived C (e.g. simple sugars such as glucose) shapes a rhizosphere microbial community, predominated by r strategists capable of resource competitive traits such as antibiotic production and biofilm formation. Competitive microbial communities profit from elevated C availability due to their high yield energy metabolism (i.e. ATP gain per substrate used) (Wood et al. 2023) accelerating N assimilation and possibly subsequent microbial N mineralization. However, the disparate range in metabolism of soil microbes hinders efficient classification into resource use strategies (Wood et al. 2023). Thus, up to now only a fragmented understanding of the effect of root and hyphal exudation on microbial N depolymerization of complex organic N and deamination of organic N monomers such as amino acids along the root soil continuum exists. A multitude of functional genetic traits of microbes needs to be assessed to better understand how changes in belowground plant activity shape microbial community assembly and affects microbial community members capable to perform N mineralization or denitrification (Legay et al. 2020). To better understand the overall process of gross N mineralization (GNM) and the involved functional microbial groups, we propose using ‘omic’ approaches, such as metagenomics and -transciptomics, to decipher active microbial N processes in the root-associated microbial community. This would follow the recently suggested approach of holo-omics (Xu et al. 2021; Rai et al. 2022) which is considered as a simultaneous assessment of the plant metabolism including root exudate synthesis and metabolic activity based on specific metabolic pathways of plant-root associated microbial communities. If such approaches are also combined with compound occurrence by metabolomics, this would allow for mapping the connections between root exudates and the microbial communities responsible for key steps like dissimilation of inorganic N, depolymerization of complex organic N, and deamination of monomers such as amino acids.
Plant strategies to access organic N
Plant species differentially influence the N cycle (Wang et al. 2020c), primarily through variations in their rates of C exudation and N uptake (Moreau et al. 2015). These variations reflect phylogenetically conserved plant resource utilization strategies. Plant ecologists have described these strategies to explain plant trait diversity from a resource acquisition point of view (root economic theory) (Wright et al. 2004; Freschet et al. 2010; Reich 2014; Díaz et al. 2016). Different strategies thereby evolved to serve the same function in different ways while resulting in a continuous functional trade-off between the opposing strategies with consequences for plant fitness under specific conditions (Laughlin et al. 2021). Resource acquisitive plant species are characterized by a fast strategy with short root longevity, low root tissue density (RTD) and narrow C/N ratios while resource conservative plants exhibit opposite root traits (Eissenstat 1992; Ostonen et al. 2007; Freschet et al. 2010; Luke McCormack et al. 2012; Reich 2014). These traits distribute along the fast-slow resource conservation gradient. Recently, this framework of resource acquisition was expanded for the collaboration gradient to include the symbiosis with mycorrhiza (Bergmann et al. 2020) and later on for root exudation (Wen et al. 2021). The “outsourcing” strategy within the collaboration gradient is characterized by high root diameter (D), low specific root length (SRL) and strong mycorrhizal symbiosis. In contrast, plants following the “do it yourself” strategy exhibit a fine-root system with high SRL and have lower mycorrhizal colonization rates (Bergmann et al. 2020). Root exudation, particularly concerning root exudates associated with the mobilization of soil N is typically linked with the fast side of the resource conservation gradient (Wen et al. 2021). As such, it is expected to be independent of the collaboration gradient (Wen et al. 2021) (Fig. 2). As both, root exudation and mycorrhizal collaboration, require C investment for the plant, a tradeoff between root exudation and investment into mycorrhizal symbiosis can be expected (Jones et al. 2004; Kaiser et al. 2015). The diverse root economic strategies illustrated in Fig. 2 embody the aforementioned processes, through which plants regulate soil N cycling and N availability. This regulation involves: (a) the uptake of N and its uptake rates, (b) the quality (chemical composition, structure, C/N ratio) and quantity of litter input, (c) the C input to the soil via roots and hyphae. Consequently, we propose that a thorough examination of these diverse root economic strategies will substantially enhance our comprehension of the organic N utilization pathways employed by annual crop plants. As the majority of annual crops, such as cereals, mostly align with the fast side of the resource conservation gradient (Roumet et al. 2006; Cornwell and Cornelissen 2013; García-Palacios et al. 2013) our focus in this context predominantly centers on the fast side of the resource conservation gradient.
Root economic strategies that are expected for plants based on the work of Bergmann et al. (2020) and Wen et al. (2021). While the “Conservation gradient”, refers to nutrient acquisition, the “Collaboration gradient” refers to collaboration with mycorrhizae. Root exudation, particularly concerning root exudates associated with the mobilization of soil N is typically linked with the fast side of the resource conservation gradient. As such, it is expected to be independent of the collaboration gradient (Wen et al. 2021). Root hair length is suggested to be negatively associated to mycorrhization as proposed in Kothari et al. (1990)
Strategies related to root exudation
Based on the root economic strategies summarized in Fig. 2, fast type plants that focus on root exudation are expected to grow a fine-root system with a narrow C/N ratio and a high exudation rate. Their high-quality litter (i.e. narrow C/N ratio) together with high exudation rates and fast fine-root turnover probably favors a high activity and abundance of microorganisms and fast N turnover in the soil.
High root exudation rates of ‘fast’ type plants can induce rhizosphere priming, i.e. a short term increase in soil organic matter (SOM) decomposition caused by addition of easily available C from the root to the soil (Kuzyakov 2002). Rhizosphere priming is positively related to gross N mineralization (Holz et al. 2023; Fig. 3) and is therefore likely an important mechanism for plant N nutrition (Henneron et al. 2020). However, the quantification of priming-derived N to plant N as well as the underlying mechanisms remain largely unknown and warrant further investigation. We suggest that root exudation initiates a cascade of processes along the developing root that finally results in increased plant access to SOM derived N (Fig. 4 left). In principle these processes will also occur in ‘slow’ type plants that invest in root exudates, but to a less intense degree.
Summarized literature data for the relationship between soil priming (a short term increase in SOM decomposition caused by addition of easily available C to the soil) and gross N mineralization in excess of the control treatment. The correlation was significant at R²=0.21 (Holz et al. 2023)
Hypothesized organic N utilization pathways for strategies focusing on root exudates (left) and those in combination with mycorrhiza (right). Both scenarios are not exclusive and that a specific plant likely relies on both mechanisms but to different degrees. Though both strategies described rely on exudation, we suggest that they may exhibit certain differences as outlined in the section “Differences in root exudate and AM fungi induced N cycling”. Although, plants might take up N in the form of either NH4+ or, if nitrified, as NO3- but for simplicity, only NH4+, as the direct product of N mineralization is indicated here. Note that apart from the processes outlined here, plants also take up N directly from the soil solution. CUE: carbon use efficiency
High exudation from the root tip and root hair zone (Dennis et al. 2010; Holz et al. 2017) will result in short lived regions of high C availability. In this area a high abundance and activity of bacterial dominated copiotrophs characterized by high N uptake is expected (Folman et al. 2001; Kuzyakov and Xu 2013; de Vries and Bardgett 2016). The high C input through root exudates will likely cause microbial N limitation and result in organic N mining through exo-enzymes (Dijkstra et al. 2013). These two factors, N limitation and enzyme activity can then cause increased rhizosphere priming, gross N mineralization and immobilization into the microbial biomass (Geisseler et al. 2010; Farrell et al. 2014).
In the region behind the root tip root exudation decreases and easily available C becomes exhausted. This depletion may lead to a shift in microbial communities towards low C use efficiency (oligotrophic/stress tolerant microorganisms) (Bernard et al. 2022). This resource constrained microbial community may recycle nutrients from “old” communities, i.e. from microbial necromass (Kaiser et al. 2014; Cui et al. 2020; Pausch et al. 2024). Due to the narrow C/N ratio of the necromass, net N mineralization will occur, enhancing plant N availability (Eshel and Beeckman 2013). Additionally, microbial grazers (nematodes, protists, phages) will be attracted by the high microbial abundance. If microbial grazers consume microorganisms, mineral N is released into the soil caused by small differences in C/N ratios between predators and prey and a low assimilation efficiency of predators (microbial loop) (Bonkowski 2004; Kuzyakov and Mason-Jones 2018). The mineral N released via the microbial loop is then potentially available to the plant (Fig. 4). Based on the sequence of processes described, we expect a tight link between exudation, fast microbial build up, priming and subsequent N mineralization from the microbial necromass. These processes are likely triggered by rapid changes in root exudation and microbial activity and composition along the root.
In comparison to ‘fast’ economic crops, ‘slow’ type plants that invest in root exudates produce long living dense fine roots. These roots have a wider C/N ratios and higher chemical recalcitrance which means that they exhibit an elemental composition, presence of functional groups, and molecular conformation that restrict their microbial decomposition (Freschet et al. 2010; Reich 2014) resulting in a low microbial activity and abundance and slower N cycling compared to ‘fast” type plants (Chapman et al. 2006; Henneron et al. 2020). Due to the wide litter C/N ratio and the slower N cycling, a larger share of organic N is likely to be present as POM compared to MAOM (Averill and Waring 2018). In these plant-soil systems, slow root turnover, together with lower exudation (Fig. 2) will likely result in low priming effects. In light of the higher occurrence of POM-N in comparison to ‘fast’ type plants, we propose that POM-N serves as a significant N source for these plants, leading to a reduction in microbial necromass recycling compared to the scenario with ‘fast’ type plants.
Strategies in combination with arbuscular mycorrhizal (AM) fungal colonization
We propose that fast plants that invest in collaboration with mycorrhiza grow a fine-root system with each root having a comparably short life span, a narrow C/N ratio and a high C investment in AM fungi, the mycorrhizal form most relevant for annual agricultural crops.
AM fungi possess a very weak exo-enzymatic repertoire (Tisserant et al. 2013) and it is therefore unlikely that they directly acquire mineral N from organic matter (Hodge and Storer 2014; Jansa et al. 2019). Therefore AM fungi must rely on mineralization by either saprotrophic or hypersymbiotic microbes, i.e. microbes that rely on mycorrhiza derived C as an energy source (Quilliam et al. 2010; Jansa et al. 2013). We propose that the symbiotic relationship with mycorrhizal fungi and the exudation of readily available C through their hyphae initiate a series of processes, ultimately leading to enhanced availability of N derived from organic matter for the plant. These processes are summarized in Fig. 4 (right) and described below. In principle these processes are also expected for slow mycorrhizal plants, but to a less intense degree.
The AM fungi hyphal exudation of labile C is likely to stimulate the activity and abundance of saprotrophic or hypersymbiotic microbes (Toljander et al. 2007; Herman et al. 2012) and induce priming of SOM (Talbot et al. 2008; Paterson et al. 2016). Moreover, the mycorrhizal fungi’s effective assimilation of NH4+ from the soil solution significantly diminishes soil NH4+ concentrations. This intensifies microbial N limitation, extracellular enzyme production by saprotrophic or hypersymbiotic microbes and rhizosphere priming (Hodge et al. 2001; Atul-Nayyar et al. 2009). As described above, the high C availability likely results in microbial N immobilization (Geisseler et al. 2010; Farrell et al. 2014). At the same time, the high microbial abundance is expected to attract microbial grazers, such as nematodes or protists and phages resulting in the ‘microbial loop’ with a net release of mineral N into soil (Bonkowski 2004; Kuzyakov and Mason-Jones 2018) and finally an increased N availability for the plant. It has been shown that in the presence of AM fungi a large fraction organic N supplied as chitin is relatively fast transferred plants (Bukovská et al. 2018; Jansa et al. 2019). This transfer is probably governed by prokaryotes and fungi specialized on chitin mineralization, while the N will likely be made available to plant uptake via the soil microbial loop (Jansa et al. 2019). There is good evidence that AM fungi hyphal development is positively correlated to soil protist abundance (Amora-Lazcano et al. 1998; Bukovská et al. 2016). Hence, we suggest that the ‘microbial loop’ plays an important role in AMF-induced N cycling. Additionally, the very efficient uptake of NH4+ by AM fungi (Herdler et al. 2008; Koller et al. 2013) reduces plant-denitrifier competition while it increases competition with nitrifiers (Legay et al. 2020) and results in a high immobilization rate of NH4+ into AM fungi biomass.
However, as of now, there is no established direct correlation between AM fungi induced priming, which triggers NH4+ release via the ‘microbial loop,’ and the subsequent NH4+ uptake by AM fungi. Additionally, it remains unclear, whether a portion of this N taken up by hyphae is allocated to the plant. Hence, we recommend that future research endeavors focus on elucidating the relationship between AM fungi-induced priming and the uptake of priming-derived N.
‘Slow’ type plants that invest in collaboration with mycorrhiza produce denser plant tissue with wide C/N ratios and thus reduced decomposability to soil microorganisms as compared to plants following fast strategies. The low-quality litter (i.e. wider C/N ratio) input together with the slow root growth results in slow N cycling in the surrounding soil as compared to plants following fast strategies. Additionally, the transfer of N derived from SOM from the fungus to the plant, as well as the mutualistic benefits of the mycorrhizal symbiosis, will be reduced compared to fast-type plants (Ingraffia et al. 2020).
Differences in root exudate and AM fungi induced N cycling
Although both strategies described rely on exudation, we suggest that they may exhibit certain differences. While root exudation is strongest at the root tip and root elongation zone (Dennis et al. 2010; Holz et al. 2017) the symbiosis with AM fungi is present along the whole root axis (Guo et al. 2008; Long et al. 2013) and consequently, hyphal exudation might therefore differ in its spatial distrbution from root exudation. Therefore, priming induced N cycling is expected in different soil locations for plants with contrasting belowground strategies. Additionally, the very efficient uptake of NH4+ by AM fungi (Herdler et al. 2008; Koller et al. 2013) and the fact that their small diameter hyphae can reach soil pores that plants cannot reach, likely leads to a very efficient N uptake by mycorrhiza. This could lead to a high immobilization rate of NH4+ into AM fungi which has also been shown to reduce N losses from soil (Asghari and Cavagnaro 2012; Storer et al. 2018; Veresoglou et al. 2019). Finally there are some indications that AM fungi might react more plasticly to reduced soil N availability compared to root exudation. Low N availability increased AM fungal abundance (Treseder 2004; van Diepen et al. 2010; Zhang et al. 2020) and the percentage of plant derived N from fertilizers (Azcón et al. 2008). Therefore, plants focusing on AM fungi might be particularly successful in low N conditions.
Conclusions and a perspective for future research
In order to gain conclusive insights into the influence of plants with diverse root economic strategies on their organic N utilization, we advocate for a comprehensive research approach that integrates various scientific disciplines.
Screening for root strategies
Major information can be gained by investigation of plant root and rhizosphere traits. Conducting comparative screenings of root traits and root exudation pattern of plant species spanning a spectrum of resource acquisition strategies will serve as a critical foundation for subsequent studies. In the context of root exudation, sampling technique and the chosen analysis of root exudates is relevant. In terms of sampling technique soil-based sampling approaches should be chosen for example by the hydroponic-hybrid approach (Oburger and Jones 2018; Santangeli et al. 2024) in order to account for the effect that soil conditions have on exudate composition and quantity (Oburger and Schmidt 2015). With regard to the analysis, it is important to quantify the total C released by plant roots and to combine this for example with non-targeted metabolomic fingerprinting of exudates (Fuhrer and Zamboni 2015; Oburger and Jones 2018); which provides information on the exudate compound composition in its entire complexity that controls microbial community composition and activity (van Dam and Bouwmeester 2016).
Linking root strategy to C exudation and N turnover
Understanding how plant species with different root economic strategies modulate their organic N utilization pathways requires linking rhizosphere priming with gross N transformation rates, for instance, through15N tracing (Rütting et al. 2011; Holz et al. 2016). Innovative methods now enable quantification of turnover rates of microbial necromass (Hu et al. 2018; Warren 2021) which could potentially be coupled with measurement of rhizosphere priming. Additionally, analyzing total N and 15N isotopes (natural abundance) of different N pools, particularly mineral N and microbial biomass, along with amino sugars as indicators of microbial necromass can link rhizosphere priming to the turnover of specifc organic N pools (Pausch et al. 2024).
Quantification of accessible organic N
While linking rhizosphere priming to N turnover rates aids in understanding the extent to which root strategies influence N cycling and availability, it is crucial to quantify the amount of organic N accessible to plants. This involves understanding which organic N pools contribute to plant nutrition, including fresh root and shoot litter, microbial necromass or MAOM and POM. Incorporating 15N-labelled organic N pools, such as necromass (Schmitt et al. 2022), and quantifying their turover and plant uptake presents an opportunity to assess the contribution of organic N pools to plant nutrition.
Resolve microbial functionality in rhizosphere N cycling
Unraveling the dynamics of microbial-mediated organic N depolymerization and microbial assimilation of N is crucial for understanding plant-microbiota interactions and synergism for N nutrition (Sieradzki et al. 2023a, b). The use of stable isotope probing, in combination with metagenomic and metatranscriptomic approaches, allows for linking the quantification of N transformations to microbial community structure and changes in microbial gene expression. By combining the analysis of plant metabolism including root exudate synthesis and metabolic activity with stable isotope probing, in combination with metagenomic and metatranscriptomic approaches (holo-omics; Rai et al. 2022; Xu et al. 2021) would allow for mapping the connections between root exudates and the microbial communities responsible for key steps in organic N mobilization while also quantifying organic N mobilization. However, further bioinformatic developments are needed to assess gene expression related to the degradation of macromolecular N (Sieradzki et al. 2023a). Additionally, a future challenge lies in predicting extracellular protease activities based on gene expression.
In summary, an interdisciplinary approach, combining methods and concepts from plant functional ecology, metagenomics in combination with isotope approaches holds significant potential to unravel the complex interactions between plants with varying root strategies and soil microbiota in regard to organic N utilization pathways.
Data availability
The manuscript does not include data.
References
Adamczyk B (2021) Root-derived proteases as a plant tool to access soil organic nitrogen; current stage of knowledge and controversies. Plants 10:731
Amelung W (2001) Methods using amino sugars as markers for microbial residues in soils. In: Lal R, Kimble JM, Follet RF, Stewart BA (eds) Assessment methods for Soil Carbon. Lewis Publishers Boca Raton
Amora-Lazcano E, Vázquez MM, Azcón R (1998) Response of nitrogen-transforming microorganisms to arbuscular mycorrhizal fungi. Biol Fertil Soils 27:65–70. https://doi.org/10.1007/s003740050401
Angst G, Mueller KE, Nierop KGJ, Simpson MJ (2021) Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biol Biochem 156:108189. https://doi.org/10.1016/j.soilbio.2021.108189
Arcand MM, Knight JD, Farrell RE (2013) Estimating belowground nitrogen inputs of pea and canola and their contribution to soil inorganic N pools using 15 N labeling. Plant Soil 371:67–80
Asghari HR, Cavagnaro TR (2012) Arbuscular mycorrhizas reduce nitrogen loss via leaching. PLoS ONE 7:e29825
Atul-Nayyar A, Hamel C, Hanson K, Germida J (2009) The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza 19:239–246. https://doi.org/10.1007/s00572-008-0215-0
Averill C, Waring B (2018) Nitrogen limitation of decomposition and decay: how can it occur? Glob Chang Biol 24:1417–1427. https://doi.org/10.1111/gcb.13980
Azcón R, Rodríguez R, Amora-Lazcano E, Ambrosano E (2008) Uptake and metabolism of nitrate in mycorrhizal plants as affected by water availability and N concentration in soil. Eur J Soil Sci 59:131–138
Baldock JA, Skjemstad JO (2000) Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org Geochem 31:697–710. https://doi.org/10.1016/S0146-6380(00)00049-8
Bergmann J, Weigelt A, Van Der Plas F et al (2020) The fungal collaboration gradient dominates the root economics space in plants. Sci Adv 6:1–10. https://doi.org/10.1126/sciadv.aba3756
Bernard L, Basile-Doelsch I, Derrien D et al (2022) Advancing the mechanistic understanding of the priming effect on soil organic matter mineralisation. Funct Ecol 36:1355–1377. https://doi.org/10.1111/1365-2435.14038
Bingham AH, Cotrufo MF (2016) Organic nitrogen storage in mineral soil: implications for policy and management. Sci Total Environ 551–552:116–126. https://doi.org/10.1016/j.scitotenv.2016.02.020
Bonkowski M (2004) Protozoa and plant growth: the microbial loop in soil revisited. New Phytol 162:617–631. https://doi.org/10.1111/j.1469-8137.2004.01066.x
Britto DT, Kronzucker HJ (2013) Ecological significance and complexity of N-source preference in plants. Ann Bot 112:957–963
Buckeridge KM, Mason KE, Ostle N et al (2022) Microbial necromass carbon and nitrogen persistence are decoupled in agricultural grassland soils. Commun Earth Environ 3:1–10. https://doi.org/10.1038/s43247-022-00439-0
Bukovská P, Gryndler M, Gryndlerová H et al (2016) Organic nitrogen-driven stimulation of arbuscular mycorrhizal fungal hyphae correlates with abundance of ammonia oxidizers. Front Microbiol 7:1–15. https://doi.org/10.3389/fmicb.2016.00711
Bukovská P, Bonkowski M, Konvalinková T et al (2018) Utilization of organic nitrogen by arbuscular mycorrhizal fungi—is there a specific role for protists and ammonia oxidizers? Mycorrhiza 28:269–283. https://doi.org/10.1007/s00572-018-0825-0
Camenzind T, Mason-Jones K, Mansour I et al (2023) Formation of necromass-derived soil organic carbon determined by microbial death pathways. Nat Geosci 16:115–122. https://doi.org/10.1038/s41561-022-01100-3
Carvalhais LC, Dennis PG, Fedoseyenko D et al (2011) Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J Plant Nutr Soil Sci 174:3–11. https://doi.org/10.1002/jpln.201000085
Chapman SK, Langley JA, Hart SC, Koch GW (2006) Plants actively control nitrogen cycling: uncorking the microbial bottleneck. New Phytol 169:27–34. https://doi.org/10.1111/j.1469-8137.2005.01571.x
Cornwell WK, Cornelissen JHC (2013) A broader perspective on plant domestication and nutrient and carbon cycling. New Phytol 198:331–333. https://doi.org/10.1111/nph.12219
Coskun D, Britto DT, Shi W, Kronzucker HJ (2017) How plant root exudates shape the nitrogen cycle. Trends Plant Sci 22:661–673. https://doi.org/10.1016/j.tplants.2017.05.004
Cotrufo MF, Ranalli MG, Haddix ML et al (2019) Soil carbon storage informed by particulate and mineral-associated organic matter. Nat Geosci 12:989–994. https://doi.org/10.1038/s41561-019-0484-6
Craine JM, Brookshire ENJ, Cramer MD et al (2015) Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 396:1–26. https://doi.org/10.1007/s11104-015-2542-1
Cui J, Zhu Z, Xu X et al (2020) Carbon and nitrogen recycling from microbial necromass to cope with C:N stoichiometric imbalance by priming. Soil Biol Biochem 142:107720. https://doi.org/10.1016/j.soilbio.2020.107720
Daly AB, Jilling A, Bowles TM et al (2021) A holistic framework integrating plant-microbe-mineral regulation of soil bioavailable nitrogen. Biogeochemistry 154:211–229. https://doi.org/10.1007/s10533-021-00793-9
de Vries FT, Bardgett RD (2016) Plant community controls on short-term ecosystem nitrogen retention. New Phytol 210:861–874. https://doi.org/10.1111/nph.13832
Denk TRA, Mohn J, Decock C et al (2017) The nitrogen cycle: a review of isotope effects and isotope modeling approaches. Soil Biol Biochem 105:121–137. https://doi.org/10.1016/j.soilbio.2016.11.015
Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol 72:313–327. https://doi.org/10.1111/j.1574-6941.2010.00860.x
Díaz S, Kattge J, Cornelissen JHC et al (2016) The global spectrum of plant form and function. Nature 529:167–171. https://doi.org/10.1038/nature16489
Dijkstra P, Ishizu A, Doucett R et al (2006) 13 C and 15 N natural abundance of the soil microbial biomass. Soil Biol Biochem 38:3257–3266
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA (2013) Rhizosphere priming: a nutrient perspective. Front Microbiol 4:1–8. https://doi.org/10.3389/fmicb.2013.00216
Dilkes N, Jones D, Farrar J (2004) Temporal dynamics of carbon partitioning and rhizodeposition in wheat. Plant Physiol 134:706–715. https://doi.org/10.1104/pp.103.032045.cesses
Drinkwater LE, Schipanski M, Snapp S, Jackson LE (2017) Ecologically based nutrient management for development. In: Agricultural Systems: Agroecology and Rural Innovation, Academic Press, pp 203–257. https://doi.org/10.1016/B978-0-12-802070-8.00007
Eissenstat DM (1992) Costs and benefits of constructing roots of small diameter. J Plant Nutr 15:763–782. https://doi.org/10.1080/01904169209364361
Eshel A, Beeckman T (2013) Plant roots: the hidden half. CRC Press
Farrell M, Prendergast-Miller M, Jones DL et al (2014) Soil microbial organic nitrogen uptake is regulated by carbon availability. Soil Biol Biochem 77:261–267. https://doi.org/10.1016/j.soilbio.2014.07.003
Folman LB, Postma J, Veen JA (2001) Ecophysiological characterization of Rhizosphere BacterialCommunities at different Root locations and Plant Developmental stages ofCucumber grown on Rockwool. Microb Ecol 42:586–597. https://doi.org/10.1007/s00248-001-0032-x
Freschet GT, Cornelissen JHC, Van Logtestijn RSP, Aerts R (2010) Evidence of the ‘plant economics spectrum’ in a subarctic flora. J Ecol 98:362–373. https://doi.org/10.1111/j.1365-2745.2009.01615.x
Fuhrer T, Zamboni N (2015) High-throughput discovery metabolomics. Curr Opin Biotechnol 31:73–78
García-Palacios P, Milla R, Delgado-Baquerizo M et al (2013) Side-effects of plant domestication: ecosystem impacts of changes in litter quality. New Phytol 198:504–513. https://doi.org/10.1111/nph.12127
Gardner JB, Drinkwater LE (2009) The fate of nitrogen in grain cropping systems: a meta-analysis of 15 N field experiments. Ecol Appl 19:2167–2184. https://doi.org/10.1890/08-1122.1
Geisseler D, Horwath WR, Joergensen RG, Ludwig B (2010) Pathways of nitrogen utilization by soil microorganisms - a review. Soil Biol Biochem 42:2058–2067. https://doi.org/10.1016/j.soilbio.2010.08.021
Godlewski M, Adamczyk B (2007) The ability of plants to secrete proteases by roots. Plant Physiol Biochem 45:657–664
Greenfield LM, Hill PW, Paterson E et al (2020) Do plants use root-derived proteases to promote the uptake of soil organic nitrogen? Plant Soil 456:355–367
Guo D, Xia M, Wei X et al (2008) Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol 180:673–683
Hart SC, Stark JM, Davidson EA, Firestone MK (1994) Nitrogen mineralization, immobilization, and nitrification. In: Weaver RW, Angle JS, Bottomley PJ (eds) Methods of Soil Analysis: part 2 - microbiological and biochemical properties. Soil Science Society of America, Madison, Wisconsin, pp 985–1018
Henneron L, Kardol P, Wardle DA et al (2020) Rhizosphere control of soil nitrogen cycling: a key component of plant economic strategies. New Phytol 228:1269–1282. https://doi.org/10.1111/nph.16760
Herdler S, Kreuzer K, Scheu S, Bonkowski M (2008) Interactions between arbuscular mycorrhizal fungi (Glomus intraradices, Glomeromycota) and amoebae (Acanthamoeba castellanii, Protozoa) in the rhizosphere of rice (Oryza sativa). Soil Biol Biochem 40:660–668. https://doi.org/10.1016/j.soilbio.2007.09.026
Herman DJ, Firestone MK, Nuccio E, Hodge A (2012) Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol Ecol 80:236–247. https://doi.org/10.1111/j.1574-6941.2011.01292.x
Hodge A, Storer K (2014) Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386:1–19. https://doi.org/10.1007/s11104-014-2162-1
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299. https://doi.org/10.1038/35095041
Holz M, Aurangojeb M, Kasimir Å et al (2016) Gross Nitrogen Dynamics in the Mycorrhizosphere of an Organic Forest Soil. Ecosystems 19:284–295. https://doi.org/10.1007/s10021-015-9931-4
Holz M, Zarebanadkouki M, Kuzyakov Y et al (2017) Root hairs increase rhizosphere extension and carbon input to soil. Ann Bot 121:61–69. https://doi.org/10.1093/aob/mcx127
Holz M, Paterson E, Pausch J (2023) Rhizosphere carbon priming: a plant mechanism to enhance soil nitrogen accessibility? Plant Soil 488:175–185. https://doi.org/10.1007/s11104-023-05979-8
Hu Y, Zheng Q, Wanek W (2017) Flux analysis of free amino sugars and amino acids in soils by isotope tracing with a novel liquid chromatography/high resolution mass spectrometry platform. Anal Chem 89:9192–9200
Hu Y, Zheng Q, Zhang S et al (2018) Significant release and microbial utilization of amino sugars and D-amino acid enantiomers from microbial cell wall decomposition in soils. Soil Biol Biochem 123:115–125
Huang X-F, Chaparro JM, Reardon KF et al (2014) Rhizosphere interactions: root exudates, microbes, and microbial communities. Botany 92:267–275
Iannucci A, Canfora L, Nigro F et al (2021) Relationships between root morphology, root exudate compounds and rhizosphere microbial community in durum wheat. Appl Soil Ecol 158:103781
Ingraffia R, Amato G, Sosa-Hernández MA et al (2020) Nitrogen type and availability drive Mycorrhizal effects on Wheat Performance, Nitrogen Uptake and Recovery, and production sustainability. Front Plant Sci 11:760. https://doi.org/10.3389/fpls.2020.00760
Jansa J, Bukovská P, Gryndler M (2013) Mycorrhizal hyphae as ecological niche for highly specialized hypersymbionts – or just soil free-riders? Front Plant Sci. https://doi.org/10.3389/fpls.2013.00134
Jansa J, Forczek ST, Rozmoš M et al (2019) Arbuscular mycorrhiza and soil organic nitrogen: network of players and interactions. Chem Biol Technol Agric 6:1–10. https://doi.org/10.1186/s40538-019-0147-2
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–480. https://doi.org/10.1111/j.1469-8137.2004.01130.x
Jones DL, Healey JR, Willett VB et al (2005) Dissolved organic nitrogen uptake by plants—an important N uptake pathway? Soil Biol Biochem 37:413–423
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33. https://doi.org/10.1007/s11104-009-9925-0
Kaiser C, Franklin O, Dieckmann U, Richter A (2014) Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecol Lett 17:680–690. https://doi.org/10.1111/ele.12269
Kaiser C, Kilburn MR, Clode PL et al (2015) Exploring the transfer of recent plant photosynthates to soil microbes: mycorrhizal pathway vs direct root exudation. New Phytol 205(4):1537–1551. https://doi.org/10.1111/nph.13138
Khan N, Seshadri B, Bolan N et al (2016) Root Iron Plaque on Wetland plants as a Dynamic Pool of nutrients and contaminants. Adv Agron 138:1–96. https://doi.org/10.1016/bs.agron.2016.04.002
Kleber M, Sollins P, Sutton R (2007) A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry 85:9–24. https://doi.org/10.1007/s10533-007-9103-5
Knicker H (2011) Soil organic N-An under-rated player for C sequestration in soils? Soil Biol Biochem 43:1118–1129. https://doi.org/10.1016/j.soilbio.2011.02.020
Koller R, Rodriguez A, Robin C et al (2013) Protozoa enhance foraging efficiency of arbuscular mycorrhizal fungi for mineral nitrogen from organic matter in soil to the benefit of host plants. New Phytol 199:203–211. https://doi.org/10.1111/nph.12249
Kopittke PM, Hernandez-Soriano MC, Dalal RC et al (2018) Nitrogen-rich microbial products provide new organo-mineral associations for the stabilization of soil organic matter. Glob Chang Biol 24:1762–1770. https://doi.org/10.1111/gcb.14009
Kopittke PM, Dalal RC, Hoeschen C et al (2020) Soil organic matter is stabilized by organo-mineral associations through two key processes: the role of the carbon to nitrogen ratio. Geoderma 357:113974
Kothari SK, Marschner H, George E (1990) Effect of VA mycorrhizal fungi and rhizosphere microorganisms on root and shoot morphology, growth and water relations in maize. New Phytol 116:303–311
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci Fur Pflanzenernahrung Und Bodenkd 165:382–396. https://doi.org/10.1002/1522-2624(200208)165:4<382::AID-JPLN382>3.0.CO;2-#
Kuzyakov Y, Mason-Jones K (2018) Viruses in soil: Nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biol Biochem 127:305–317. https://doi.org/10.1016/j.soilbio.2018.09.032
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656–669
Laughlin DC, Mommer L, Sabatini FM et al (2021) Root traits explain plant species distributions along climatic gradients yet challenge the nature of ecological trade-offs. Nat Ecol Evol 5:1123–1134
Legay N, Clément JC, Grassein F et al (2020) Plant growth drives soil nitrogen cycling and N-related microbial activity through changing root traits. Fungal Ecol 44:100910. https://doi.org/10.1016/j.funeco.2019.100910
Leinweber P, Kruse J, Baum C et al (2013) Chapter two - advances in understanding organic nitrogen chemistry in soils using state-of-the-art analytical techniques. Adv Agron 119:83–151. https://doi.org/10.1016/B978-0-12-407247-3.00002
Lewin S, Wende S, Wehrhan M et al (2024) Cereals rhizosphere microbiome undergoes host selection of nitrogen cycle guilds correlated to crop productivity. Sci Total Environ 911:168794
Li Z, Zeng Z, Tian D et al (2020) Global variations and controlling factors of soil nitrogen turnover rate. Earth Sci Rev 207:103250. https://doi.org/10.1016/j.earscirev.2020.103250
Liang C, Amelung W, Lehmann J, Kästner M (2019) Quantitative assessment of microbial necromass contribution to soil organic matter. Glob Chang Biol 25:3578–3590. https://doi.org/10.1111/gcb.14781
Long Y, Kong D, Chen Z, Zeng H (2013) Variation of the linkage of root function with root branch order. PLoS ONE 8:e57153
Luce MS, Whalen JK, Ziadi N, Zebarth BJ (2016) Net nitrogen mineralization enhanced with the addition of nitrogen-rich particulate organic matter. Geoderma 262:112–118
Luke McCormack M, Adams TS, Smithwick EAH, Eissenstat DM (2012) Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol 195:823–831. https://doi.org/10.1111/j.1469-8137.2012.04198.x
Manzoni S, Taylor P, Richter A et al (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196:79–91. https://doi.org/10.1111/j.1469-8137.2012.04225.x
McNeill AM, Zhu C, Fillery IRP (1997) Use of in situ 15 N-labelling to estimate the total below-ground nitrogen of pasture legumes in intact soil–plant systems. Aust J Agric Res 48:295–304
Mooshammer M, Wanek W, Hämmerle I et al (2014) Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil nitrogen cycling. Nat Commun 5:3694. https://doi.org/10.1038/ncomms4694
Moreau D, Pivato B, Bru D et al (2015) Plant traits related to nitrogen uptake influence plant-microbe competition. Ecology 96:2300–2310. https://doi.org/10.1890/14-1761.1
Moreau D, Bardgett RD, Finlay RD et al (2019) A plant perspective on nitrogen cycling in the rhizosphere. Funct Ecol 33:540–552. https://doi.org/10.1111/1365-2435.13303
Myrold DD, Bottomley PJ (2008) Nitrogen mineralization and immobilization. Nitrogen Agric Syst 49:157–172. https://doi.org/10.2134/agronmonogr49.c5
Nannipieri P, Eldor P (2009) The chemical and functional characterization of soil N and its biotic components. Soil Biol Biochem 41:2357–2369. https://doi.org/10.1016/j.soilbio.2009.07.013
Nardi P, Laanbroek HJ, Nicol GW et al (2020) Biological nitrification inhibition in the rhizosphere: determining interactions and impact on microbially mediated processes and potential applications. FEMS Microbiol Rev 44:874–908. https://doi.org/10.1093/femsre/fuaa037
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytol 182:31–48. https://doi.org/10.1111/j.1469-8137.2008.02751.x
Oburger E, Jones DL (2018) Rhizosphere Sampling root exudates – Mission impossible ? Rhizosphere 6:116–133. https://doi.org/10.1016/j.rhisph.2018.06.004
Oburger E, Schmidt H (2015) New methods to unravel Rhizosphere processes. Trends Plant Sci xx:1–13. https://doi.org/10.1016/j.tplants.2015.12.005
Ostonen I, Püttsepp Ü, Biel C et al (2007) Specific root length as an indicator of environmental change. Plant Biosyst - Int J Deal all Asp Plant Biol 141:426–442. https://doi.org/10.1080/11263500701626069
Pascault N, Ranjard L, Kaisermann A et al (2013) Stimulation of different functional groups of Bacteria by Various Plant Residues as a driver of Soil Priming Effect. Ecosystems 16:810–822. https://doi.org/10.1007/s10021-013-9650-7
Paterson E, Sim A, Davidson J, Daniell TJ (2016) Arbuscular mycorrhizal hyphae promote priming of native soil organic matter mineralisation. Plant Soil 408:243–254. https://doi.org/10.1007/s11104-016-2928-8
Pausch J, Holz M, Zhu B, Cheng W (2024) Rhizosphere priming promotes plant nitrogen acquisition by microbial necromass recycling. Plant Cell Environ 47(6):1987–1996. https://doi.org/10.1111/pce.14858
Quilliam RS, Hodge A, Jones DL (2010) Sporulation of arbuscular mycorrhizal fungi in organic-rich patches following host excision. Appl Soil Ecol 46:247–250. https://doi.org/10.1016/j.apsoil.2010.08.005
Rai S, Omar AF, Rehan M et al (2022) Crop microbiome: their role and advances in molecular and omic techniques for the sustenance of agriculture. Planta 257:27. https://doi.org/10.1007/s00425-022-04052-5
Reich PB (2014) The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J Ecol 102:275–301. https://doi.org/10.1111/1365-2745.12211
Rinaudo M (2006) Chitin and chitosan: Properties and applications. Prog Polym Sci 31:603–632
Roumet C, Urcelay C, Díaz S (2006) Suites of root traits differ between annual and perennial species growing in the field. New Phytol 170:357–368. https://doi.org/10.1111/j.1469-8137.2006.01667.x
Rütting T, Huygens D, Staelens JM et al (2011) Advances in 15 N tracing experiments: new labelling and dataanalysis approaches. Biochem Soc Trans 39:279–283
Santangeli M, Steininger-Mairinger T, Vetterlein D et al (2024) Maize (Zea mays L.) root exudation profiles change in quality and quantity during plant development – a field study. Plant Sci 338:111896. https://doi.org/10.1016/j.plantsci.2023.111896
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602. https://doi.org/10.1890/03-8002
Schmitt M, Jarosch KA, Hertel R et al (2022) Manufacturing triple-isotopically labeled microbial necromass to track C, N and P cycles in terrestrial ecosystems. Appl Soil Ecol 171:104322. https://doi.org/10.1016/j.apsoil.2021.104322
Schulten H-R, Schnitzer M (1997) The chemistry of soil organic nitrogen: a review. Biol Fertil Soils 26:1–15. https://doi.org/10.1007/s003740050335
Sieradzki ET, Nuccio EE, Pett-ridge J (2023a) Expression of macromolecular organic nitrogen degrading enzymes identi fi es potential mediators of soil organic N availability to an annual grass. ISME J 17:967–975. https://doi.org/10.1038/s41396-023-01402-3
Sieradzki ET, Nuccio EE, Pett-ridge J, Firestone MK (2023b) Rhizosphere and detritusphere habitats modulate expression of soil N-cycling genes during plant development. Environ Microbiol 8
Steen A, Buist G, Leenhouts KJ et al (2003) Cell wall attachment of a widely distributed peptidoglycan binding domain is hindered by cell wall constituents. J Biol Chem 278:23874–23881
Storer K, Coggan A, Ineson P, Hodge A (2018) Arbuscular mycorrhizal fungi reduce nitrous oxide emissions from N2O hotspots. New Phytol 220:1285–1295. https://doi.org/10.1111/nph.14931
Sun L, Lu Y, Yu F et al (2016) Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol 212:646–656. https://doi.org/10.1111/nph.14057
Talbot JM, Allison SD, Treseder KK (2008) Decomposers in disguise: mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct Ecol 22:955–963. https://doi.org/10.1111/j.1365-2435.2008.01402.x
Thion CE, Poirel JD, Cornulier T et al (2016) Plant nitrogen-use strategy as a driver of rhizosphere archaeal and bacterial ammonia oxidiser abundance. FEMS Microbiol Ecol 92:fiw091. https://doi.org/10.1093/femsec/fiw091
Tisserant E, Malbreil M, Kuo A et al (2013) Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc Natl Acad Sci 110:20117–20122. https://doi.org/10.1073/pnas.1313452110
Toljander JF, Lindahl BD, Paul LR et al (2007) Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. FEMS Microbiol Ecol 61:295–304. https://doi.org/10.1111/j.1574-6941.2007.00337.x
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
van Dam NM, Bouwmeester HJ (2016) Metabolomics in the rhizosphere: tapping into belowground chemical communication. Trends Plant Sci 21:256–265
van Diepen LTA, Lilleskov EA, Pregitzer KS, Miller RM (2010) Simulated nitrogen deposition causes a decline of intra-and extraradical abundance of arbuscular mycorrhizal fungi and changes in microbial community structure in northern hardwood forests. Ecosystems 13:683–695
Van Groenigen JW, Huygens D, Boeckx P et al (2015) The soil N cycle: new insights and key challenges. Soil 1:235–256. https://doi.org/10.5194/soil-1-235-2015
Veresoglou SD, Verbruggen E, Makarova O et al (2019) Arbuscular mycorrhizal fungi alter the community structure of ammonia oxidizers at high fertility via competition for soil NH 4+. Microb Ecol 78:147–158
Vollmer W, Blanot D, De Pedro MA (2008) Peptidoglycan structure and architecture. FEMS Microbiol Rev 32:149–167. https://doi.org/10.1111/j.1574-6976.2007.00094.x
von Lützow M, Kögel-Knabner I, Ekschmitt K et al (2007) SOM fractionation methods: relevance to functional pools and to stabilization mechanisms. Soil Biol Biochem 39:2183–2207. https://doi.org/10.1016/j.soilbio.2007.03.007
Wanek W, Mooshammer M, Blöchl A et al (2010) Determination of gross rates of amino acid production and immobilization in decomposing leaf litter by a novel 15 N isotope pool dilution technique. Soil Biol Biochem 42:1293–1302. https://doi.org/10.1016/j.soilbio.2010.04.001
Wang C, Wang X, Pei G et al (2020a) Stabilization of microbial residues in soil organic matter after two years of decomposition. Soil Biol Biochem 141:107687. https://doi.org/10.1016/j.soilbio.2019.107687
Wang L, Gan Y, Bainard LD et al (2020b) Expression of N-cycling genes of root microbiomes provides insights for sustaining oilseed crop production. Environ Microbiol 22:4545–4556. https://doi.org/10.1111/1462-2920.15161
Wang X, Wang C, Cotrufo MF et al (2020c) Elevated temperature increases the accumulation of microbial necromass nitrogen in soil via increasing microbial turnover. Glob Chang Biol 26:5277–5289. https://doi.org/10.1111/gcb.15206
Warren CR (2014) Organic N molecules in the soil solution: what is known, what is unknown and the path forwards. Plant Soil 375:1–19
Warren C (2021) What are the products of enzymatic cleavage of organic N? Soil Biol Biochem 154:108152. https://doi.org/10.1016/j.soilbio.2021.108152
Wen Z, White PJ, Shen J, Lambers H (2021) Linking root exudation to belowground economic traits for resource acquisition. New Phytol 233:1620–1635. https://doi.org/10.1111/nph.17854
Wichern F, Eberhardt E, Mayer J et al (2008) Nitrogen rhizodeposition in agricultural crops: methods, estimates and future prospects. Soil Biol Biochem 40:30–48
Wood JL, Malik AA, Greening C et al (2023) Rethinking CSR theory to incorporate microbial metabolic diversity and foraging traits. ISME J. https://doi.org/10.1038/s41396-023-01486-x
Wright IJ, Reich PB, Westoby M et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Xu L, Pierroz G, Wipf HM-L et al (2021) Holo-omics for deciphering plant-microbiome interactions. Microbiome 9:1–11
Yan M, Pan G, Lavallee JM, Conant RT (2020) Rethinking sources of nitrogen to cereal crops. 191–199. https://doi.org/10.1111/gcb.14908
Zeglin LH, Myrold DD (2013) Fate of decomposed fungal cell wall material in organic horizons of old-growth Douglas‐fir forest soils. Soil Sci Soc Am J 77:489–500
Zhalnina K, Louie KB, Hao Z et al (2018) Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol 3:470–480
Zhang J, Cai Z, Müller C (2018) Terrestrial N cycling associated with climate and plant-specific N preferences: a review. Eur J Soil Sci 69:488–501. https://doi.org/10.1111/ejss.12533
Zhang H, Wang X, Gao Y (2020) Short-term N transfer from alfalfa to maize is dependent more on arbuscular mycorrhizal fungi than root exudates in N deficient soil. 23–41
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the reviews conception and design. The first draft of the manuscript was written by Maire Holz and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Jeff R. Powell.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Holz, M., Lewin, S., Kolb, S. et al. How to get to the N – a call for interdisciplinary research on organic N utilization pathways by plants. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06839-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06839-9