Abstract
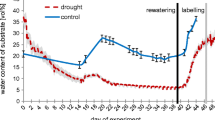
Some plant species use different strategies to acquire phosphorus (P) dependent on environmental conditions, but studies investigating the relative significance of P-acquisition strategies with changing P availability are rare. We combined a natural P availability gradient and a glasshouse study with 10 levels of P supplies to investigate the roles of rhizosphere carboxylates and transpiration-driven mass flow in P acquisition by Agonis flexuosa. Leaf P concentrations of A. flexuosa decreased and leaf manganese (Mn) concentrations increased with decreasing soil P concentration along a dune chronosequence. In the glasshouse, in response to decreasing P supply, shoot growth and root length decreased, leaf P and Mn concentrations decreased, rhizosphere carboxylates decreased, transpiration rate and transpiration ratio increased and the percentage of root length colonized by arbuscular mycorrhizal fungi was unchanged. Although it was proved leaf Mn concentration was a good proxy for rhizosphere carboxylate amounts in the glasshouse study, the enhanced plant P acquisition at low P supply was related to transpiration-induced mass flow rather than carboxylates. We deduced that the higher leaf Mn concentrations in low soil P availability of the field were likely a result of increased mass flow. In summary, as soil P availability declined, A. flexuosa can shift its P-acquisition strategy away from a mycorrhizal mode towards one involving increased mass flow.









Similar content being viewed by others
References
Albornoz FE, Burgess TI, Lambers H, Etchells H, Laliberté E (2016) Native soil-borne pathogens equalize differences in competitive ability between plants of contrasting nutrient-acquisition strategies. J Ecol 105:549–557
Arines J, Vilariño A, Sainz M (1989) Effect of different inocula of vesicular-arbuscular mycorrhizal fungi on manganese content and concentration in red clover (Trifolium pratense L.) plants. New Phytol 112:215–219
Barber S (1995) Soil nutrient bioavailability: a mechanistic approach, 2nd edn. Wiley, New York
Bougher NL, Grove TS, Malajczuk N (1990) Growth and phosphorus acquisition of Karri (Eucalyptus diversicolor F. Muell.) seedlings inoculated with ectomycorrhizal fungi in relation to phosphorus supply. New Phytol 114:77–85
Brouwer R (1962) Nutritive influences on the distribution of dry matter in the plant. Neth J Agric Sci 10:399–408
Brundrett MC, Abbott LK (1991) Roots of jarrah forest plants. I. Mycorrhizal associations of shrubs and herbaceous plants. Aust J Bot 39:445–457
Cawthray GR (2003) An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J Chromatogr A 1011:233–240
Cernusak LA, Winter K, Aranda J, Turner BL, Marshall JD (2007) Transpiration efficiency of a tropical pioneer tree (Ficus insipida) in relation to soil fertility. J Exp Bot 58:3549–3566
Cernusak LA, Winter K, Turner BL (2011) Transpiration modulates phosphorus acquisition in tropical tree seedlings. Tree Physiol 31:878–885
Cramer MD, Hoffmann V, Verboom GA (2008) Nutrient availability moderates transpiration in Ehrharta calycina. New Phytol 179:1048–1057
Cramer MD, Hawkins HJ, Verboom GA (2009) The importance of nutritional regulation of plant water flux. Oecologia 161:15–24
de Campos MCR, Pearse SJ, Oliveira RS, Lambers H (2013) Viminaria juncea does not vary its shoot phosphorus concentration and only marginally decreases its mycorrhizal colonization and cluster-root dry weight under a wide range of phosphorus supplies. Ann Bot 111:801–809
DPaW (2016) Descriptions and mapping by the Western Australian Herbarium. Department of Environment and Conservation, Perth
Fox J (2003) Effect displays in R for generalised linear models. J Stat Softw 8:1–27
Gerdemann JW (1968) Vesicular-arbuscular mycorrhiza and plant growth. Annu Rev Phytopathol 6:397–418
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrbizal infection in roots. New Phytol 84:489–500
Grigg AM, Veneklaas EJ, Lambers H (2008) Water relations and mineral nutrition of closely related woody plant species on desert dunes and interdunes. Am J Bot 56:27–43
Hayes P, Turner BL, Lambers H, Laliberté E (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102:396–410
Hopper SD, Gioia P (2004) The Southwest Australian Floristic Region: evolution and conservation of a global hotspot of biodiversity. Annu Rev Ecol Evol Syst 35:623–650
Howard K, Dell B, Hardy GE (2000) Phosphite and mycorrhizal formation in seedlings of three Australian Myrtaceae. Am J Bot 48:725–729
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol 120:371–379
Jeffery RP, Simpson RJ, Lambers H, Kidd DR, Ryan MH (2017) Root morphology acclimation to phosphorus supply by six cultivars of Trifolium subterraneum L. Plant Soil 412:21–34
Koide RT (1991) Nutrient supply, nutrient demand and plant response to mycorrhizal infection. New Phytol 117:365–386
Kooyman RM, Laffan SW, Westoby M (2017) The incidence of low phosphorus soils in Australia. Plant Soil 412:143–150
Krüger M, Teste FP, Laliberté E, Lambers H, Coghlan M, Zemunik G, Bunce M (2015) The rise and fall of arbuscular mycorrhizal fungal diversity during ecosystem retrogression. Mol Ecol 24:4912–4930
Laliberté E, Turner BL, Costes T, Pearse SJ, Wyrwoll KH, Zemunik G, Lambers H (2012) Experimental assessment of nutrient limitation along a 2-million year dune chronosequence in the south-western Australia biodiversity hotspot. J Ecol 100:631–642
Laliberté E, Zemunik G, Turner BL (2014) Environmental filtering explains variation in plant diversity along resource gradients. Science 345:1602–1605
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713
Lambers H, Brundrett MC, Raven JA, Hopper SD (2010) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 334:11–31
Lambers H, Shane MW, Laliberté E, Swarts ND, Teste FP, Zemunik G (2014) Plant mineral nutrition. In: Lambers H (ed) Plant life on the sandplains in Southwest Australia, a global biodiversity hotspot. UWA, Crawley, pp 101–127
Lambers H, Hayes PE, Laliberté E, Oliveira RS, Turner BL (2015) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90
Lehmann A, Rillig MC (2015) Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops—a meta-analysis. Soil Biol Biochem 81:147–158
Marx DH (1972) Ectomycorrhizae as biological deterrents to pathogenic root infections. Annu Rev Phytopathol 10:429–454
Matimati I, Verboom GA, Cramer MD (2014) Nitrogen regulation of transpiration controls mass-flow acquisition of nutrients. J Exp Bot 65:159–168
McArthur WM (1991) Reference soils of south-western Australia. Department of Agriculture Western Australia, Australian Society of Soil Science, Perth
Motomizu S, Wakimoto T, Toei K (1983) Spectrophotometric determination of phosphate in river waters with molybdate blue and malachite green. Analyst 108:361–367
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Nazeri NK, Lambers H, Tibbett M, Ryan MH (2014) Moderating mycorrhizas: arbuscular mycorrhizas modify rhizosphere chemistry and maintain plant phosphorus status within narrow boundaries. Plant Cell Environ 37:911–921
Pang JY, Yang JY, Lambers H, Tibbett M, Siddique KHM, Ryan MH (2015) Physiological and morphological adaptations of herbaceous perennial legumes allow differential access to sources of varyingly soluble phosphate. Physiol Plant 154:511–525
Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H (2006) Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant Soil 288:127–139
Pearse SJ, Veneklaas EJ, Cawthray G, Bolland MDA, Lambers H (2007) Carboxylate composition of root exudates does not relate consistently to a crop species’ ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol 173:181–190
Pinheiro JC, Bates DM (2000) Mixed effects models in S and S-PLUS. Springer, New York
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2016) nlme: Linear and nonlinear mixed effects models. R package version 3.1-128, http://CRAN.R-project.org/package=nlme
Playford PE, Cockbain AE, Low GH (1976) Geology of the Perth Basin. Geological Survey, Perth
Reddell P, Yun Y, Shipton WA (1997) Cluster roots and mycorrhizae in Casuarina cunninghamiana: their occurrence and formation in relation to phosphorus supply. Aust J Bot 45:41–51
Ryan MH, Small DR, Ash JE (2000) Phosphorus controls the level of colonisation by arbuscular mycorrhizal fungi in conventional and biodynamic irrigated dairy pastures. Aust J Exp Agric 40:663–670
Ryan MH, Tibbett M, Edmonds-Tibbett T, Suriyagoda LDB, Lambers H, Cawthray GR, Pang J (2012) Carbon trading for phosphorus gain: the balance between rhizosphere carboxylates and arbuscular mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ 35:2170–2180
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, London
Smith S, Smith F, Jakobsen I (2003) Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol 133:16–20
Strobel N, Sinclair W (1991) Role of flavanolic wall infusions in the resistance induced by Laccaria bicolor to Fusarium oxysporum in primary roots of Douglas-fir. Pathology 81:420–425
Suriyagoda LDB, Lambers H, Renton M, Ryan MH (2012) Growth, carboxylate exudates and nutrient dynamics in three herbaceous perennial plant species under low, moderate and high phosphorus supply. Plant Soil 358:100–112
Suriyagoda LBD, Tibbett M, Edmonds-Tibbett T, Cawthray GR, Ryan MH (2016) Poor regulation of phosphorus uptake and rhizosphere carboxylates in three phosphorus-hyper accumulating species of Ptilotus. Plant Soil 402:145–158
Teste FP, Laliberté E, Lambers H, Auer Y, Kramer S, Kandeler E (2016) Mycorrhizal fungal biomass and scavenging declines in phosphorus-impoverished soils during ecosystem retrogression. Soil Biol Biochem 92:119–132
Turner BL, Condron LM (2013) Pedogenesis, nutrient dynamics, and ecosystem development: the legacy of TW Walker and JK Syers. Plant Soil 367:1–10
Turner BL, Condron LM, France CAM, Lehmann J, Solomon D, Peltzer DA, Richardson SJ (2016) Sulfur dynamics during long-term ecosystem development. Biogeochemistry 128:281–305
Turner BL, Laliberté E, Hayes PE (2017) A climosequence of chronosequences in southwestern Australia. bioRxiv. doi:10.1101/113308
Valentine AJ, Osborne BA, Mitchell DT (2001) Interactions between phosphorus supply and total nutrient availability on mycorrhizal colonization, growth and photosynthesis of cucumber. Sci Hortic 88:177–189
Veneklaas EJ, Stevens J, Cawthray GR, Turner S, Grigg AM, Lambers H (2003) Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248:187–197
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Viscarra RA, Bui EN (2016) A new detailed map of total phosphorus stocks in Australian soil. Sci Total Environ 542:1040–1049
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Wouterlood M, Cawthray GR, Scanlon TT, Lambers H, Veneklaas EJ (2004) Carboxylate concentrations in the rhizosphere of lateral roots of chickpea (Cicer arietinum) increase during plant development, but are not correlated with phosphorus status of soil or plants. New Phytol 162:745–753
Zemunik G, Turner BL, Lambers H, Laliberté E (2015) Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nat Plants 1:15050
Acknowledgements
This research was supported by the Chinese National Natural Scientific Foundation (31570455) and Youth Innovation Promotion Association, CAS (2016381). We are grateful to Rob Creasy and Bill Piasini at the School of Plant Biology’s Plant Growth Facility for their great help with the glasshouse experiment, to Greg Cawthray for help with HPLC, to Evonne Walker for help with mycorrhizal check experiments, to Elizabeth Halladin for providing leaf area meters and to Wenli Ding for helpful advice on the experiments. Megan Ryan was funded by an ARC Future Fellowship (FT140100103).
Author information
Authors and Affiliations
Contributions
HL and GH conceived and designed the experiments. GH and PH performed the experiments. GH and PH analyzed the data. GH and PH wrote the manuscript; other authors provided editorial advice.
Corresponding author
Additional information
Communicated by Kouki Hikosaka.
Rights and permissions
About this article
Cite this article
Huang, G., Hayes, P.E., Ryan, M.H. et al. Peppermint trees shift their phosphorus-acquisition strategy along a strong gradient of plant-available phosphorus by increasing their transpiration at very low phosphorus availability. Oecologia 185, 387–400 (2017). https://doi.org/10.1007/s00442-017-3961-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3961-x