Abstract
Purpose
Plant microbial biostimulants, such as arbuscular mycorrhizal fungi (AMF), enhance nutrient concentration in fruits, including tomato. However, field studies on tomato AMF inoculation are scarce. AMF species belonging to Gigasporaceae and Glomeraceae families known to vary in life-history strategies may determine differential effects on plant nutrient benefits and residue decomposition. Despite this, the effect of different life-history strategies on nutrient acquisition of tomato fruits has not been investigated yet.
Methods
We studied the effect of inoculation of two tomato varieties with four AMF species belonging to Glomeraceae and Gigasporaceae. Fungal colonization, yield, fruit nutrient concentration, litter decomposition, and bacterial and fungal abundances in soil were assessed in the field under organic agriculture.
Results
Overall Gigasporaceae promoted the concentration of nutrients in tomato fruits compared to Glomeraceae. A variability in AM fungal colonization and fruit nutrient concentration was detected within Glomeraceae. Scutellospora pellucida increased the yield (+ 27%) of var. Rio Grande with respect to Gigaspora gigantea. In var. Rio Grande, inoculation with Funneliformis mosseae did not change litter decomposition as compared to non-inoculated controls, whereas it was lower than in Sclerocystis sinuosa and Gigasporaceae species, which showed the highest decomposition rates. AMF inoculation promoted soil total bacterial and fungal abundance and fungal:bacterial (F:B) ratio compared to controls, and members of Gigasporaceae had the highest F:B ratio.
Conclusion
These findings pointed at the inclusion of AM fungal life-history strategy within the selection criteria for the development of biofertilizers able to enhance the nutritional value of vegetables under organic farming systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato (Solanum lycopersicum L.) is the second most important vegetable in the world in terms of production, after potato (FAOSTAT database, average period 2011–2020; https://www.fao.org/faostat/en/#data/QCL), and one of the most studied fleshy fruits (Singh et al. 2021). Tomato fruits represent an important source of health-promoting compounds (Chaudhary et al. 2018; Dorais et al. 2008). Due to their importance for human health, the study of sustainable approaches, such as plant microbial biostimulants (González-González et al. 2020; Sharma et al. 2017), to improve the production and nutritional value of tomato is of great relevance to producers as well as consumers (Baulcombe et al. 2009). This is especially true in the recent scenario of “hidden hunger” due to shortage of micronutrients in crops that has been determined by several factors, among which plant breeding, agronomic management and the increase of atmospheric CO2 (Myers et al. 2014; Scharff et al. 2022). Indeed, beneficial microorganisms, such as arbuscular mycorrhizal fungi (AMF) could represent an important tool for enhancing tomato yield and mineral nutrient concentration (e.g., Coccina et al. 2019; Hart et al. 2015; Pellegrino et al. 2020).
Arbuscular mycorrhizal fungi (AMF, phylum Glomeromycota, Tedersoo et al. 2018) are ubiquitous in natural and agricultural ecosystems (Brundrett and Tedersoo 2018). They establish a symbiosis with the majority of plant species in terrestrial environments (ca. 67% of plant species) (Bueno et al. 2019; Maherali et al. 2016), supplying mineral nutrients to the plants in exchange for photosynthetically fixed carbon (C) (from 4 to 20% of the photosynthates) in the form of lipids as well as sugars (Bago et al. 2000; Jiang et al. 2017; Luginbuehl et al. 2017). Carbon-mineral exchanges between host and fungus occur through arbuscules, namely highly branched fungal structures present in cortical root cells (Parniske 2008). These fungi represent fundamental factors of plant productivity (Pellegrino et al. 2015; Zhang et al. 2019) since the extraradical mycelium can promote plant uptake and translocation of nutrients (e.g., P, N, K, Ca, Mg, Fe, Zn, Cu and Mn) (Lehmann et al. 2014; Lehmann and Rillig 2015; Watts-Williams and Cavagnaro 2014) and redistribute soil resources among plants linked by a common mycorrhizal network (Cardini et al. 2021; Javot et al. 2007; Jin et al. 2005). The effect of AM fungal inoculation on AM fungal root colonization, plant growth, and nutrient acquisition in different varieties of tomato has been well documented in microcosm (e.g., Al-Karaki 2000; Al-Karaki et al. 2001; Giovannetti et al. 2012; Hart et al. 2015). By contrast, few studies of AM fungal inoculation were carried out in open-field conditions (Bona et al. 2017; Bowles et al. 2016; Conversa et al. 2013), where agricultural practices (e.g., tillage and fertilization) might have negatively impacted native AMF-crop interactions (Gosling et al. 2010; Hamel et al. 1997; Piazza et al. 2019). In low fertile conditions, AM fungal inocula can successfully adapt and develop with native AMF (Pellegrino et al. 2012, 2022; Ryan and Graham 2018). Bowles et al. (2016), in a field experiment, comparing MYC + and the mutant nonmycorrhizal tomato genotype rmc, demonstrated the beneficial effect of AMF on tomato yield (+ 25%) and leaf and fruit N and P uptake (+ 22% and + 26%, respectively). Similarly, plants of tomato (var. PKM-1 and var. TC 2000) inoculated at nursery with Rhizophagus intraradices or a mix of AM fungal species and transplanted in the field showed a higher AM fungal colonization and arbuscule frequency at harvest, as well as enhanced plant growth, yield attributes, and N and P uptake in shoot and roots (Bona et al. 2017; Subramanian et al. 2006). By contrast, tomato var. Rio Grande, Roma and Perfect Peel, pre-inoculated at nursery with Funneliformis mosseae IMA1, Rhizophagus intraradices IMA6 and a mix of the two species, and transplanted in the field, although variably colonized by AMF, did not show yield enhancement compared with mock-inoculated controls (Njeru et al. 2017).
Some studies have shown that AM fungal species belonging to different families, such as Gigasporaceae and Glomeraceae, may vary in their life-history characteristics, such as sporulation, extraradical mycelium development and root colonization strategies, with important consequences on mycorrhizal functioning (e.g., Brundrett et al. 1999; Hart and Reader 2002, 2004; Klironomos and Hart 2002). Members of the Glomeraceae family are known to have a highly infective extra-radical mycelium (Morton 1993; Tommerup and Abbott 1981), to contact roots quickly (fast colonizer) and to allocate a large fraction of fungal biomass inside the roots, forming vesicles for storage of lipids (Hart and Reader 2002; Morton and Benny 1990). On the other hand, members of the Gigasporaceae family regenerate most frequently from spores (Morton 1993; Tommerup and Abbott 1981), and usually extensively colonize the soil, exhibiting a slow and limited colonization of the roots (Hart and Reader 2002, 2004; Maherali and Klironomos 2012). Moreover, Glomeraceae have very delicate hyphae characterized by a shosrt longevity, creating a smaller extraradical mycelium, whereas Gigasporaceae have robust and densely aggregated hyphae characterized by a slower turn-over, creating a larger extraradical mycelium (Jakobsen et al. 1992; Smith et al. 2000). Indeed, a high ratio of soil to root colonization may provide the crop with the greatest benefit in term of plant growth and P uptake (Hart and Reader 2002). Conversely, a little net nutritional benefit may occur when the ratio of soil to root colonization is low, since a small extension of the extraradical mycelium would limit the nutrient transfer to the crop (Hart and Reader 2002). Meanwhile, a larger AM fungal root colonization can be positively related to a better protection of the host against root phatogens (Maherali and Klironomos 2007; Chagnon et al. 2013), putately associated to high plant biomass.
Arbuscular mycorrhizal fungi, although known to have no saprotrophic capacity, represent an important group of microorganisms involved in litter decomposition (Cheng et al. 2012; Gui et al. 2017; Hodge and Storer 2015), a crucial process affecting the release of nutrients and the stabilization of soil organic carbon (SOC). Herman et al. (2012) and Hodge et al. (2001) proved that AMF enhance litter decomposition, increase N capture from organic patches and alter the C flow through changes in soil microbial communities. Arbuscular mycorrhizal fungi might impact litter decomposition by affecting soil saprotrophic microorganisms (Nuccio et al. 2013) and/or soil structure (Pellegrino et al. 2021, 2022; Rillig and Mummey 2006). Recently, multipartite synergies between AMF and soil microbial communities were demonstrated to substantially enhance plant and fungal N acquisition from organic matter (Hestrin et al. 2019). These synergies resulted in a greater relative acquisition from organic versus mineral nutrient stocks, but without loss of soil organic matter (SOM) due to the stimulation of the primary productivity and the SOM formation by root and mycorrhizal C inputs. However, we are still far from fully understanding the mechanisms by which AMF are able to influence litter decomposition in field conditions, where inoculated members of the families Glomeraceae and Gigasporaceae may differently affect fungal to bacterial ratio and organic residue decomposition due to their peculiar colonization strategies (Mei et al. 2022). Fungal:bacterial ratio is a suitable proxy for of litter degradation efficiency, ranging from low to high values in intensive and extensive agricultural systems, respectively (Delgado-Baquerizo et al. 2015; García-Palacios et al 2013). Fungi have long generation times, are able to decompose recalcitrant substrates and have a high organic substrate degradation efficiency. Bacteria show instead a rapid growth response in soils containing readily decomposable substrate and have a low substrate degradation efficiency (Joergensen and Wichern 2008). Indeed, bacteria have a biomass with a C/N ratio lower than fungi and immobilize more N per unit of C assimilated (Austin et al. 2004). At soil C/N higher than 10 and with organic residues having high C/N and high proportion of recalcitrant substrates, a fungal-dominated community is expected to increase residue decomposition (García-Palacios et al. 2013; Delgado et al. 2015).
The aim of this work was to evaluate in field conditions under organic agriculture the effect of nursery inoculation of tomato (Solanum lycopersicum L.) with four single AM fungal isolates belonging to Glomeraceae and Gigasporaceae on AM fungal root colonization, yield, litter decomposition, and bacterial and fungal abundance in soil, as proxy for nutrient cycling and C storage. Two varieties of tomato, characterized by different growth habits, were studied: var. Pisanello (Berni et al. 2018) with an indeterminate growth habit and var. Rio Grande with a determinate growth habit (Fig. S1a). We hypothesized similar responses in AM fungal root colonization, yield and nutrient concentration in fruits of both varieties to inoculation with isolates belonging to Gigasporaceae and Glomeraceae. Although in field conditions competition with other microorganisms occurs, members of Gigasporaceae, better exploring the soil by hyphae and less colonizing the roots, would have a stronger effect on nutrient uptake, plant growth and traslocation of nutrients to fruits, whereas members of Glomeraceae, extensively colonizing the root system, would enhance root pathogen protection thus increasing plant growth and fruit yield (Fig. S1b). We also hypothesized differences at family level in the effect of AM fungal inoculation on organic residue decomposition and soil fungal and bacterial abundance. After AM fungal inoculation, we expected a shift from bacterial- to fungal-dominated communities with a general increase of residue decomposition. However, given the life-history strategies of Gigasporaceae, we expected that members of this family would increase fungal abundance in soil more than members of Glomeraceae. This would imply a higher fungal:bacterial ratio and a higher residue decomposition under inoculation with Gigasporaceae members (Fig. S1b).
Materials and methods
Fungal material
Arbuscular mycorrhizal fungi (AMF) used were: Gigaspora gigantea PA125 and Scutellospora pellucida MN408A belonging to Gigasporaceae, and Funneliformis mosseae MD118 and Sclerocystis sinuosa MD126 belonging to Glomeraceae. Inocula were obtained from pot cultures maintained in the collection of the Crop Science Research Center of the Scuola Superiore Sant’Anna, Italy. The inocula of the single AM fungal isolates were produced in 15 L pots (four pots for each isolate). Pots were filled with sandy soil and Terragreen (calcinated clay, OILDRI, Chicago, IL, USA) (1:1 by volume) and with 1.5 L per pot of starting crude inoculum (10% by volume). The substrate was previously steam-sterilized (121 °C for 25 min, on two consecutive days) to kill native AMF. All the pots received 3 L of a filtrate, obtained by sieving a mixture of the four AM fungal inocula through a sieve with pore diameter of 50 μm, to ensure a common prokaryotic community for all treatments. The host plant was Sorghum vulgare L., following Morton et al. (1993). Plants were grown in a climatic chamber (27 °C day and 21 °C night temperature; 18:6 h light:dark cycle, 420 μmol m−2 s−1), supplied with tap water as needed and with a weekly fertilization of half-strength Hoagland’s solution (250 ml per pot). Three months after inoculation, plant shoots were harvested and soil and roots used as inocula (i.e., crude inoculum: mycorrhizal roots and soil containing spores and extraradical mycelium). Details about AM fungal isolate geographical origin, collector and original supplier are given in Table S1.
Experimental field site and climatic data
The experiment was carried out in 2019 and 2020 in two distinct fields at the organic farm “Fattoria Le Prata” Pisa, Italy (43°44’ N, 10°24’E; 2 m above sea level and 0.0% slope). The soil in 2019 was a silty-clay loam (8.0% sand, 54.1% silt and 37.9% clay) with 35.9 g kg−1 soil organic carbon (SOC) (Walkley–Black; Nelson and Sommers 1982), 7.9 pH (deionized water 1:2.5 w/v; McLean 1982), 2.2 g kg−1 total N (Kjeldahl; Bremner and Mulvaney 1982) (dotation suitable for plant growth) and 1.89 g kg−1 P, 22.00 mg kg−1 available P (Olsen) (Olsen and Sommers 1982) (very low availability). The soil in 2020 was a silty-clay loam (10.2% sand, 50.2% silt and 39.6% clay) with 30.5 g kg−1 SOC (Walkley–Black), 8.0 pH(H2O), 1.83 g kg−1 total N (Kjeldahl) (dotation for plant growth), 1.72 g kg−1 P and 17.0 mg kg−1 available P (Olsen) (very low availability). Climate of the site is cold, humid Mediterranean (Csa) according to the Köppen-Geiger climate classification (Kottek et al. 2006). Averaged over 1990–2020, mean annual maximum and minimum air temperatures were 20.4 and 9.9 °C, respectively, and annual precipitation was 1084 mm. During the field experiment (May–September), maximum and minimum temperatures were 27.4 °C and 16.0 °C, respectively, in 2019, and 27.4 °C and 15.5 °C in 2020, while total precipitation was 236 mm and 270 mm in 2019 and 2020, respectively. The preceding crop of tomato grown in 2019 and 2020 was organically-fertilized bread wheat. Details about temperature (mean, minimum and maximum daily temperature) and rainfall for the tomato growth cycles are given in Fig. S2.
Experimental set-up
A first experiment was set-up in 2019 with tomato (Solanum lycopersicum L.) var. Pisanello, an old Tuscan variety described and conserved in the regional genetic bank for the conservation of endangered (or threatened) varieties (http://germoplasma.regione.toscana.it/; Berni et al. 2018) and a second experiment was set-up in 2020 with tomato var. Rio Grande, a modern and widely used variety. Inoculation by AMF was performed in greenhouse at sowing, before plantlet transplanting to the field. In detail, 160 seeds of S. lycopersicum were placed in a propagation tray with hole dimension of 26.4 ml (total volume: 4.22 L), containing as substrate a mixture of peat, soil, coarse silica sand and heat-expanded clay (1:1:2:2 by volume). The soil utilized in the mixture was a sandy loam collected at the “Centro di Ricerche Agro-Ambientali Enrico Avanzi”, University of Pisa, Italy. Chemical and physical characteristics of the soil used were as follows: 8.0 pH (deionized water 1:2.5 w/v; McLean 1982), 15.3% clay, 30.1% silt, 54.6% sand, 2.2% SOM (Walkley–Black; Nelson and Sommers 1982), 1.3 g kg−1 total N (Kjeldahl; Bremner and Mulvaney 1982), 469.5 mg kg−1 total P (Olsen), 17.6 mg kg−1 extractable P (Olsen) (Olsen and Sommers 1982) and 149.6 mg kg−1 extractable K (Thomas 1982). The mixture was steam-sterilized (121 °C for 25 min, on two consecutive days) to kill naturally occurring AMF. The tray (one for each AM fungal isolate and one for the control) were inoculated either with 850 mL of crude inoculum of one out of the four AM fungal isolates or with 850 mL of a sterilized mixture of them (non-mycorrhizal control). The non-mycorrhizal control was steam-sterilized (121 °C for 25 min, on two consecutive days). Potential differences in AM fungal colonization ability of the four isolates were balanced using such high amounts of inoculum (10% by volume). Each tray received 565 mL of a filtrate, obtained by sieving (pore diameter of 43 μm) a mixture of the four crude inocula and of a sample of the agricultural soil used for the preparation of the substrate, to ensure an initial common prokaryotic community to all treatments. Plantlets were grown in the climatic chamber from the start of April to the end of May (24 °C day and 18 °C night temperature; 14:10 h light:dark cycle, 420 μmol m−2 s−1) on both years of cultivation (2019 and 2020) for about 60 days. Plantlets were supplied with tap water as needed and with a weekly fertilization of half-strength Hoagland’s solution (70 mL per tray). Plants were transplanted into the field in May (mean length of seedlings: 18 cm) (var. Pisanello: 16th May 2019; var. Rio Grande: 23rd May 2020). For both tomato varieties, the experiment layout was a completely randomized design with four fungal treatments (G. gigantea, G.giga; S.pellucida, S.pellu; F. mosseae, F.mos; S. sinuosa, S.sin) and the mock-inoculated control (-M), and three replicate plots. Each replicate plot of the experiment with var. Pisanello had a size of 9.6 m length × 1.6 m width (15.4 m2) and was composed by two rows with 30 plants for each row. Each replicate plot of the experiment with var. Rio Grande had a size of 2.5 m length × 1.25 m width (3 m2) and was composed by four rows with eight plants for each row. Plants of var. Pisanello were spaced 30 cm within the row, 1 m between rows and 160 cm between twin rows and plants of var. Rio Grande were spaced 25 cm within and between rows. In both years, the plots were separated by uninoculated plants of the same varieties. Schematic overview of the experimental design in 2019 and 2020 are presented in Fig. S3a and Fig. S3a. The tomato plants were daily watered through drip irrigation with pressure regulation, allowing similar water flow for each plot. No mineral fertilization neither chemical or mechanical weed control were applied.
Mycorrhizal infection potential of the AM fungal inocula and of the experimental soil
Infectivity of the experimental field soil and of the AM fungal inocula was evaluated using a modified mycorrhizal infection potential (MIP) test in a growth chamber with (24 °C day and 18 °C night temperature; 12:12 h light:dark cycle, 420 μmol m−2 s−1) (Pellegrino et al. 2011). Three S. vulgare seeds were sown in 50 mL sterile plastic tubes filled with 25 mL of the four AM fungal inocula and of the experimental field soil, and 25 mL of sterile quartz grit. The experimental field soil was obtained by taking five soil samples in 2019 and 2020 before tomato transplanting. Soil samples were collected by using a soil corer (8 cm diameter) at a depth of 30 cm and then they were air dried. Three replicate plastic tubes were used for each AM fungal inoculum (n = 12) and for each soil samples (five soil replicates per year; n = 30). After emergence, plants were thinned to one per tube. Plants were harvested after two-week growth and root systems were cleared and stained, using lactic acid instead of phenol (Phillips and Hayman 1970). Then, the roots were mounted on microscope slides, examined under an optical microscope (Leitz Laborlux S, Wetzlar, Germany), and AM fungal root colonization parameters (% of arbuscules, vesicles and AM fungal root colonization) were assessed (McGonigle et al. 1990).
Effect of AM fungal inoculation on tomato root colonization
Arbuscular mycorrhizal fungal root colonization of var. Pisanello was assessed at the BBCH 62 growth stage (2nd inflorescence with first flower open), whereas the AM fungal root colonization of var. Rio Grande was assessed at BBCH 22 (2nd primary apical side shoot visible), BBCH 62 and BBCH 89 (fully ripe: all fruits have typical fully ripe color) (Meier 2001). On 22nd July 2019 (BBCH 62) two plants of var. Pisanello from each replicate plot were sampled, and AM fungal root colonization parameters (percentage of AM fungal root colonization and of root length containing arbuscules and vesicle) were assessed under an optical microscope (Leitz Laborlux S, Wetzlar, Germany), after clearing and staining, using lactic acid instead of phenol (Phillips and Hayman 1970), following the gridline intersect method (McGonigle et al. 1990). On 23rd May, 7th July and 27th August 2020 (BBCH 22, BBCH 62 and BBCH 89, respectively) two plants of var. Rio Grande from each replicate plot were sampled and AM fungal root colonization parameters were assessed, as previously described.
Effect of AM fungal inoculation on tomato yield and fruit nutrient concentrations
Plants of var. Pisanello were harvested 11 times, from 22nd July to 4th October 2019, while plants of var. Rio Grande were harvested six times, from 3rd August to 21th September 2020. For both varieties, the harvests were performed on 10 plants in the central area of each plot and fruit fresh weight per plant and number of fruits per plant were measured. Fruits of ripeness stage 6 (red) (USDA 1975) were collected at each harvest. This allowed to calculate the fruit fresh weight per plant at each harvest (Yield) and the production for the whole harvesting period per plant, namely the total fresh weight per plant (Total yield). In addition, the shoot dry weight (SWD) of tomato var. Rio Grande was determined at BBCH 22, after oven drying at 65 °C up to constant weight. For total yield and SDW we also calculated the host benefit as (fresh or dry weight inoculated plant – fresh/dry weight not inoculated plant)/fresh or dry weight not inoculated plant) × 100.
The fruits of var. Pisanello sampled at first and second harvest (22nd of July and 1st August 2019, respectively) and the fruits of var. Rio Grande sampled at the second harvest (10th August 2020) were washed and immediately stored at -80 °C. Fruit samples were lyophilized and ground to fine powder prior to the analysis of mineral nutrients. Approximately 0.2 g of lyophilized tomato were digested using the COOLPEX Smart Microwave Reaction System (Yiyao Instrument Technology Development Co., Ltd., Shanghai, China) after the addition of 8.0 mL of nitric acid (65%). The solution was diluted with Milli-Q water and analyzed. The concentration of P, K, Ca, Mg, Zn, Fe, Cu and Mn was determined using a Microwave Plasma Atomic Emission Spectroscopy instrument (4210 MP-AES, Agilent Technologies, Santa Clara, CA, USA) (Liberato et al. 2020), while N concentration was determined using the Kjedahl method (Jones et al. 1991). Increases of minerals (e.g., P, K, Mn, Cu and Zn) in fruits are known to be of relevance not only for tomato nutrition, productivity and health, but also for fruit quality (e.g., synthesis of phytonutrients such as ascorbic acid and β-complex vitamins) (Dorais et al. 2008). Moreover, phytonutrients in tomato are strongly affected by intensity, duration, and quality of light. For example, vitamin C, lycopene, β-carotene and phenols in tomato fruits increase per se with the increase of light intensity and duration. Thus, we sampled in the first harvests so that the measurement of the effect of AM fungal inoculation would remain within the linear growth of phytonutrient accumulation in fruits.
Effect of AM fungal inoculation on residue decomposition and bacterial and fungal abundance in soil
In each replicate plot of var. Rio Grande, four litter bags filled with a standard organic substrate (hay) were inserted vertically into the soil at 10 cm soil depth near to the tomato roots. Five grams of hay composed by Dactylis glomerata L. (45%), Phleum pratense L. (50%) and Urtica dioica L. (5%) (“Vita Verde Small Alpine Hay”, Vitakraft pet care GmbH & Co. KG, Bremen, Germany) were inserted into 10 cm × 10 cm polypropylene net litter bags (1.5-mm mesh) (Tenax-ortoclima plus, Tenax s.p.a., Lecco, Italy). The hay was ground in a 3 mm grid forage mill (Retsch GmbH, Haan, DE). Main hay characteristics on dry basis at the beginning of the experiment were: 32.4% C, 0.82% N, 18.8 MJ kg−1 energy, 2.9% protein, 3.7% lipid, 12.2% lignin (determined as acid detergent lignin, ADL; Van Soest et al. 1991), 35.5% cellulose and 5.7% hemicellulose (Baldi et al. 2021). The litter bags were sealed using staples and hot glue. A plastic label was added to the litter bags for identification in the field and for easiness of finding purposes. Four litter bags per plot were positioned at plant transplanting (23th May, BBCH 22) (defined as litter deposition time). Then, one litter bag from each plot was collected after 40 days (2nd July 2020), 52 (14th July 2020), 67 (29th July 2020) and 86 (10th August 2020) days from litter deposition. After sampling, litter bags were opened, gently cleaned from soil and spontaneous plants, dried at 40 °C and weighted. The percentage of remaining mass compared to the initial one was calculated. Litter bag decomposition coefficient (k) was also calculated from mass loss and expressed as a first-order decay constant. The k for each sample was calculated as follows:
where k is the first-order rate constant (day−1); Co is the initial weight of the sample; Ct is the litter bags dry weight at each sampling data (t).
On the tomato roots (a pool of the two root systems excavated from the soil of each replicate plot and used for assessing AM fungal root colonization), we employed a combination of washing and ultrasound treatments to separate the soil rhizospheric fraction (Bulgarelli et al. 2015). To assess the bacterial and fungal abundance, DNA from the rhizospheric soil was extracted (0.25 g dry soil per sample) using the Dneasy PowerSoil Kit (QIAGEN, Venlo, Netherlands), following the manufacturer’s instructions. Soil DNA extraction was performed at stage BBCH 89 (var. Pisanello 30th August 2019, var. Rio Grande 27th August 2020). To minimize DNA extraction bias, soil samples were extracted in triplicate and DNA was pooled prior to the analysis. Fungal and bacterial relative abundance in each sample were quantified using a modification of the technique described by Fierer et al. (2005). Quantitative PCR (qPCR) analysis was conducted using a CFX Connect Real-Time System thermal cycler (Biorad, Hercules, California) with a program of 50 °C for 10 min, 95 °C for 15 min, 40 cycles of 95 °C for 1 min, and 53 °C for 30 s, followed by melting curve analysis. qPCR reactions consisted of 10 μL of a qPCR Master Mix (KAPA SYBR® FAST, Kapa Biosystems, USA), 0.5 μL each of forward and reverse primers, 7 μL sterile water, and 15 ng DNA quantified by a Qubit 4 fluorometer using the Qubit 1 × dsDNA HS Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The 16S rRNA gene region was used as bacterial target, whereas ITS1 region was used to estimate fungal abundance. The bacterial 16S rRNA gene was amplified using the pair of primers Eub338/Eub518 (Eub338: 5′-ACT CCT ACG GGA GGC AGC AG-3′; Eub518: 5′-ATT ACC GCG GCT GCT GG-3′), while the fungal internal transcribed spacer (ITS) region was amplified using the pair of primers ITS1f/5.8 s (ITS1f: 5′-TCC GTA GGT GAA CCT GCG G-3′; 5.8 s: 5′-CGC TGC GTT CTT CAT CG-3′) (Fierer et al. 2005). The efficiency of the two pair of primers was similar (99.8%). Each 96-well plate also contained reactions with ten-fold serial dilutions of pure bacterial and fungal DNA (Bacillus subtilis and Amanita rubescens) in order to verify the linearity of the relationship between threshold cycle (Ct) and DNA concentration. Standard curves were generated using triplicate of plasmids, from 103 to 109 copies of the template. Sample Ct values were divided by the mean slope of the standard curves across all runs, to ensure that increases in fungal and bacterial abundance were equally weighted. Each sample was run in triplicate, and the mean Ct value was used for analysis. The gene copies were referred to g of dry soil. The fungal:bacteria ratio was determined as the ratio of the ITS1 Ct to the 16S Ct. The fungal:bacteria ratio (calculated as fungal gene copy number divided by bacterial gene copy number; F:B ratio) was used as a predictor of organic residue decomposition and thus nutrient cycling (García-Palacios et al. 2013; Delgado et al. 2015).
Statistical analysis
One-way analysis of variance (ANOVA) was performed on AM fungal root colonization, total yield, fruit nutrient concentrations and contents, soil bacterial and fungal abundance and fungal:bacterial ratio, after the necessary transformations (e.g., log10, arcsen). Orthogonal contrasts were used to test differences between + M (all four isolates) vs -M (mock inoculated controls) (1st comparison); between Gigasporaceae and Glomeraceae (2nd comparison: inter-family diversity); between the two isolates of Gigasporaceae (3rd comparison: intra-family diversity) and between the two isolates of Glomeraceae (4th comparison: intra-family diversity). Moreover, at each harvest, fruit yield was analyzed using one-way ANOVA with AM fungal inoculation [five levels: control (-M), G. gigaspora, S. pellucida, F. mosseae and S. sinuosa] as fixed factor. Mycorrhizal infection potential (MIP) data of the AM fungal inocula were analyzed by one-way ANOVA with AM fungal inoculation (G. gigaspora, S. pellucida, F. mosseae, S. sinuosa) as fixed factor, whereas MIP data of the field soil were analyzed by one-way ANOVA with year (2019 and 2020) as fixed factor. Litter bag data of var. Rio Grande were analyzed by two-way ANOVA with AM fungal inoculation (five level) and time of field exposure (four treatments: 40, 52, 67, 79 days) as fixed factors. All data were ln- or arcsine-transformed when needed to fulfil the assumptions of ANOVA. Post-hoc Tukey-B significant difference test was used for comparison among treatments. Means and standard errors given in tables and figures are for untransformed data. All analyses were performed using the software package on SPSS 21.0 (SPSS Inc., Chicago, IL, USA).
The permutational analysis of variance (PERMANOVA) was used to test the overall effect of AM fungal inoculation (Inoc) (five levels: -M, G.giga, S.pellu, F.mos, S.sin) on total fruit yield and fruit concentration of nutrients and AM fungal root traits (% of arbuscules, vesicles, AMF root colonization) of tomato varieties Pisanello and Rio Grande. Response data were fourth-root transformed and normalized, and the resemblance matrix was calculated using the Euclidean distance between samples. P values were calculated using the Monte-Carlo test (Anderson and Braak 2003). Since PERMANOVA is sensitive to differences in multivariate location (average community composition of a group) and dispersion (within-group variability), the analysis of homogeneity of multivariate dispersion (PERMDISP; Anderson 2006) was performed to check the homogeneity of dispersion among groups (beta-diversity) (Anderson et al. 2006). When PERMANOVA indicated a significant effect, the principal coordinate analysis (PCO) was performed to visualize the most relevant patterns in the data. The circle in each plot, whose diameter is 1.0, allows the reader to understand the scale of the vectors in the vector plot. PERMANOVA pairwise comparisons between all pairs of the Inoc levels was also performed. Moreover, for the var. Rio Grande, to test the significance of the relationship between the matrix of percentages of remaining mass of litter bags at the four sampling times and the matrix of soil fungal and bacterial abundance, a RELATE analysis, based on Spearman rank and 999 permutations, was performed (ρ = 1 perfect relationship) (Clarke and Warwick 2001). All the analyses were carried out using PRIMER 7 and PERMANOVA + software (Anderson et al. 2008; Clarke and Gorley 2015).
Results
Mycorrhizal infection potential of the AM fungal inocula and of the experimental soil
The mycorrhizal infection potential (MIP) used to measure the infectivity of the experimental soil was not significantly different between the two years. Thus, averaging over years, the percentages of root length containing arbuscules and vesicles and AM fungal root colonization of S. vulgare (used as host plant in the test tube experiment) were 2.2% ± 1.2, 0.03% ± 0.03 and 9.1% ± 2.4, respectively (mean ± SE of five replicates per each field; data not shown). The MIP was also similar among the AM fungal inocula (Table S1), with percentages of root length containing arbuscules and vesicles and AMF root colonization of 8.6% ± 1.4, 0.2% ± 0.09 and 23.8% ± 2.1, respectively (mean ± SE of three replicates per each inoculum; data not shown).
Effect of AM fungal inoculation on tomato root colonization
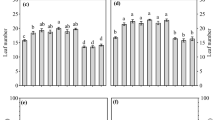
In 2019, at stage BBCH 62, the presence of arbuscules, vesicles and AMF root colonization in roots of inoculated plants of tomato (S. lycopersicum L.) var. Pisanello (+ M: all AM fungal isolates) was significantly higher than in the mock-inoculated treatment (-M) (Fig. 1a-c; Table S2). Moreover, there was a significant intra-family variability (Glomeraceae): S. sinuosa showed a higher AM fungal root colonization than F. mosseae (Fig. 1d).
Presence of arbuscules (a) and vesicles (b), and AMF root colonization (c) of Solanum lycopersicum L. var. Pisanello: -M (mock inoculation, control) vs + M (AMF inoculation) (n = 3 and n = 12, respectively). AMF root colonization: Funnelliformis mosseae (F.mos) vs Sclerocystis sinuosa (S.sin) (d) (n = 3). Figure reports only the significant results among all the tested linear orthogonal contrasts (Table S2). Sampling was performed at BBCH 62
In 2020, at BBCH 22, AM fungal inoculation significantly increased the occurrence of arbuscules and AM fungal colonization in roots of tomato var. Rio Grande, whereas did not affect the presence of vesicles (Fig. 2a, c; Table S3). Moreover, the inoculation with isolates belonging to Gigasporaceae significantly increased the occurrence of arbuscules in the roots of tomato compared with the isolates belonging to the family Glomeraceae (Fig. 2b). At BBCH 62, the percentage of root length containing arbuscules and AM fungal root colonization was significantly increased by inoculation (Fig. 2d, eb). At BBCH 89, inoculation with F. mosseae significantly increased percentage of AM fungal root colonization and of root length containing vesicles compared with S. sinuosa (Fig. 2h, ic). Moreover, the occurrence of vesicles in the roots of tomato was significantly increased by AM fungal inoculation (Fig. 2f). Finally, an inter-family variability was detected: the occurrence of vesicles was promoted in the roots of tomato inoculated with isolates belonging to the family Gigasporaceae compared with the ones inoculated with the isolates belonging to the family Glomeraceae (Fig. 2g).
Presence of arbuscules in roots of Solanum lycopersicum L. var. Pisanello at BBCH 22: -M (mock inoculation, control) vs + M (AMF inoculation) (a) (n = 3 and n = 12, respectively); Gigasporaceae (Giga) vs Glomeraceae (Glome) (b) (n = 6). AMF root colonization: -M vs + M (c) (n = 3 and n = 16, respectively). Presence of arbuscules (d) and AMF root colonization (e) at BBCH 62: -M vs + M. At BBCH 89, presence of vesicles: -M vs + M (f); Giga vs Glome (g) (n = 6); Funnelliformis mosseae (F.mos) vs Sclerocystis sinuosa (S.sin) (h) (n = 3). AMF root colonization: F.mos vs S.sin (i) (n = 3). Figure reports only the significant results among all the tested linear orthogonal contrasts (Table S3)
Effect of AM fungal inoculation on tomato yield and fruit nutrient concentrations
In 2019, AM fungal inoculation significantly increased total fruit yield of tomato var. Pisanello accumulated during the productive phase by 32% (Fig. 3a; Table S2). Moreover, there was a difference between the members of Gigasporaceae: tomato plants inoculated with S. pellucida showed higher total yield (+ 27%) than plants inoculated with G. gigantea. Details about yield at each harvest are given in Fig. S4. In 2020, at BBCH 22, AM fungal inoculation increased shoot dry weight (SDW) of var. Rio Grande by 115% (Fig. 3c; Table S3). In addition, significant differences were observed between the SDW of plants inoculated with S. sinuosa (+ 75%) compared with those inoculated with F. mosseae (Fig. 3d). Moreover, total yield of var. Rio Grande was increased by 38% following AM fungal inoculation (Fig. 3e), whereas no differences were recorded among and within families (Table S3).
Total yield (accumulated fruit fresh weight per plant) of Solanum lycopersicum L. var. Pisanello: -M (mock inoculation, control) vs + M (AMF inoculation) (n = 3 and n = 12, respectively) (a); Gigaspora gigantea (G.giga) vs Scutellospora pellucida (S.pellu) (n = 6) (b). Shoot dry weight (SDW) of S. lycopersicum L. var. Rio Grande: -M vs + M (n = 3 and n = 12, respectively) (c); Funnelliformis mosseae (F.mos) vs Sclerocystis sinuosa (S.sin) (n = 3) (d); total yield of S. lycopersicum L. var. Rio Grande: -M vs + M (n = 3 and n = 12, respectively) (e). Figure reports only the significant results among all the tested linear orthogonal contrasts (Table S2 and S3). Data on SDW of var. Rio Grande refer to BBCH 22
In 2019, at first harvest, AM fungal inoculation significantly increased N concentration and decreased Zn concentration in fruits of var. Pisanello (Fig. 4a, c; Table S4). Fruits of plants inoculated with F. mosseae had higher values of both N and Cu concentration than those inoculated with S. sinuosa (Fig. 4b,e). In addition, inoculation with S. pellucida increased Cu concentration compared to G. gigantea (Fig. 4d). At the second harvest, AM fungal inoculation, although decreasing Ca concentration in fruits (Fig. 4h), increased P, Mg, Cu, and Mn concentration compared with control (Fig. 4f, i, l, m). An inter-family variability was recorded, and Mg and Mn concentration in fruits increased in plants inoculated with members of Gigasporaceae compared with those inoculated with Glomeraceae (Fig. 4j, n). Moreover, within Glomeraceae, the inoculation with F. mosseae, similarly to the first harvest, increased K, Mg and Mn concentration as compared to S. sinuosa (Fig. 4g, k, o). Large intra-family differences were also reported in var. Pisanello at the first harvest in the fruit mineral content (Table S5 and S6). Moreover, inter-family differences were recorded in fruit K and Ca content. Similarly, at the second harvest, some inter-family differences were observed in nutrient content of fruits (i.e., K, Mg, Zn and Mn: Gigasporaceae > Glomeraceae).
Concentration of nutrients in fruits of Solanum lycopersicum L. var. Pisanello at 1st harvest (a-e) and 2nd harvest (f-o) and in fruits of var. Rio Grande at 2nd harvest (p-ac). Orthogonal contrasts: -M (mock inoculation, control) vs + M (AMF inoculation) (n = 3 and n = 12, respectively); Gigasporaceae (Giga) vs Glomereaceae (Glome) (n = 6); Gigaspora gigantea (G.giga) vs Scutellospora pellucida (S.pellu) (n = 3); Funnelliformis mosseae (F.mos) vs Sclerocystis sinuosa (S.sin) (n = 3). First and second harvest for var. Pisanello: 22nd July 2019 and 1st August 2019, respectively; second harvest for var. Rio Grande: 10.th August 2020. Figure reports only the significant results among all the tested linear orthogonal contrasts (Table S4)
In 2020, at the second harvest, AM fungal inoculation increased P, K, Zn, Fe, Cu and Mn concentration in fruits of var. Rio Grande compared with control (Fig. 4p, r, v, y, z, ab; Table S4). In addition, the concentration of N, K, Zn, Cu and Mn in fruits of plants inoculated with members of Gigasporaceae was higher than in those inoculated with Glomeraceae (Fig. 4q, s, w, aa, ac). Conversely, Ca concentration was lower in Gigasporaceae than Glomeraceae (Fig. 4u). Finally, significant differences were recorded within Glomeraceae: F. mosseae increased Zn concentration in fruits (Fig. 4x), whereas S. sinuosa increased K concentration (Fig. 4t). By contrast, nutrient content in fruits was not modified by inoculation treatments (Table S7 and S8), with the exception of Ca that was higher in plants inoculated with Glomeraceae.
To summarize the effect of AM fungal inoculation on plant agronomic traits and AM fungal root colonization patterns, PERMANOVAs showed a significant response in total yield and fruit nutrient concentration, as well as in AM fungal traits, in both Pisanello and Rio Grande tomato varieties (Fig. 5; Table S9). In the PCO biplots, the first two principal coordinates explained 30.2 and 39.0% of the total variance in the varieties Pisanello and Rio Grande, respectively (Fig. 5). Pairwise tests highlighted significant differences between F. mosseae and mock-inoculated control (-M) and between G. gigaspora and -M in the var. Pisanello (Table S9). Moreover, significant differences were detected between F. mosseae and -M, G. gigaspora and -M, S.pellucida and -M, as well as between F. mosseae and G. gigaspora, and G.gigaspora and S.sinuosa (Table S9). However, since the two tomato varieties were studied in different years, the results can not be directly compared with each other.
Principal Coordinates Analysis (PCO) biplots on the effect of AM fungal inoculation on total yield, fruit nutrient concentration and AMF root traits (arbuscules, vesicles, AMF root colonization) of Solanum lycopersicum L. var. Pisanello (a) and var. Rio Grande (b). Treatments were: -M (mock inoculation, control); Gigaspora gigantea (G.giga); Scutellospora pellucida (S.pellu); Funneliformis mosseae (F.mos); Sclerocystis sinuosa (S.sin). G.giga and S.pellu belong to Gigasporaceae and F.mos and S.sin to Glomeraceae. Response data were fourth-root transformed and normalized, and the resemblance matrix was calculated using the Euclidean distance between samples (n = 3; Table S9)
Effect of AM fungal inoculation on residue decomposition and bacterial and fungal abundance in soil
A significant interaction between AMF inoculation (AMF inoc) and time of field exposure (Time) was observed for litter bags weight (% of remaining mass) and decomposition rate (expressed as a first-order decay constant, k) (P < 0.001; Fig. 6a, b). As expected, for all treatments, the percentage of remaining mass decreased with time, showing the highest values at 40 days and the lowest at 79 days in G. gigantea and in S. sinuosa, respectively (Fig. 6a). At 40 days from litter bag deposition, lower percentages of remaining mass were recorded in all treatments as compared with G. gigantea, whereas at 52 days S. sinuosa showed a significantly lower remaining mass than all the treatments including the not inoculated control (-M). In the S. pellucida treatment, litter bags weights significantly decreased at each sampling time, while in the other treatments a more variable trend was recorded. At the latest sampling time, 86 days from litter bag deposition, -M and F. mosseae showed similar percentage of remaining mass (mean: 8.5%) in comparison with the other AM fungal inoculated treatments (mean: 1.3%).
Interaction between AM fungal inoculation and time on the percentage of remaining mass (a) and on the decomposition coefficient (k) of the litter bags (b) applied in the experiment with Solanum lycopersicum L. var. Rio Grande. Different letters represent significant differences among treatments (Tukey-B test, P ≤ 0.05; n = 3). Treatments were: -M (mock inoculation, control); Gigaspora gigantea (G.giga); Scutellospora pellucida (S.pellu); F. mosseae (F.mos); S. sinuosa (S.sin). G.giga and S.pellu belong to Gigasporaceae and F.mos and S.sin to Glomeraceae. Sampling times were: 40, 52, 67 and 86 days from litter bag deposition (LD). LD corresponds to plant transplanting (23.th May 2020). Fungal:bacterial ratio (F:B) in soil of S. lycopersicum L. var. Pisanello: -M vs + M (AMF inoculation) (c) (n = 3 and n = 12, respectively); Gigasporaceae (Giga) vs Glomeraceae (Glome) (d) (n = 6). F:B in soil of S. lycopersicum L. var. Rio Grande: -M vs + M (e) (n = 3 and n = 12, respectively); Giga vs Glome (f) (n = 6); G.giga vs S.pellu (g) (n = 3). Sampling was performed at BBCH 89. Three biological replicates per treatment were analyzed. Each one was obtained from a triplicate extraction of DNA from the rhizospheric soil collected from a pool of two plants per replicate plot. Figure reports only the significant results among all the tested linear orthogonal contrasts (Table S10 and S11)
The decomposition rate per day, during the starting period of litter bag deposition (1–40 days), was similar among all AM fungal inoculated treatments and -M, whereas during the following 12 days (between 40 and 52 days from litter bag deposition) S. sinuosa strongly promoted the decomposition rate compared to the other treatments (Fig. 6b). In the third period (the following 15 days), S. pellucida determined a faster decomposition as compared to the other treatments, whereas during the fourth period (the latest 15 days), G. gigantea, S. pellucida and S. sinuosa showed higher values of decomposition rate per day compared to -M and F. mosseae.
Total bacterial and fungal abundance as well as fungal:bacterial ratio in the rhizospheric soil of both tomato varieties significantly increased under AM fungal inoculation (Fig. 6c, e; Table S10 and S11). Moreover, fungal abundance and fungal:bacteria ratio increased in the rhizospheric soil of var. Pisanello under Gigasporaceae compared with Glomeraceae (Fig. 6d; Table S10 and S11). Similarly, in the rhizospheric soil of var. Rio Grande, bacterial and fungal abundance and fungal:bacteria ratio increased under Gigasporaceae compared with Glomeraceae (Fig. 6f; Table S10 and S11). In var. Rio Grande, fungal:bacterial ratio was higher under G. gigaspora compared with S. pellucida (Fig. 6g). Finally, we found a significant relationship between the matrix of percentages of remaining mass of litter bags at the four sampling times and the matrix of soil fungal and bacterial abundances (RELATE analysis, ρ = 0.241) (Fig. S5).
Discussion
The present study showed that: (i) AM fungal inoculation increased AM fungal colonization traits at flowering as well as total yield and fruit nutrient concentration (e.g., P, Cu, and Mn) in both tomato varieties; (ii) in the var. Rio Grande AM fungal inocula belonging to Gigasporaceae positively affected the occurrence of arbuscules and vesicles at transplanting and fruit maturity, respectively; (iii) Gigasporaceae promoted the concentration of nutrients in fruits in comparison with Glomeraceae; (iv) a variability of AM fungal colonization and fruit nutrient concentration occurred within members of Glomeraceae in both varieties; (iv) within Gigasporaceae, S. pellucida increased the yield of var. Rio Grande compared to G. gigantea; (v) in var. Rio Grande the inoculation with F. mosseae determined a residue decomposition similar to controls and lower than S. sinuosa and the two species belonging to Gigasporaceae; (vi) in both tomato varieties AM fungal inoculation promoted total bacterial and fungal abundance as well as fungal:bacterial ratio as compared to control; (vii) Gigasporaceae increased fungal:bacterial ratio as compared to Glomeraceae, irrespective of tomato variety.
Mycorrhizal infection potential of the AM fungal inocula and of the experimental soil
The mycorrhizal infection potential (MIP) of the field experimental soil sampled in 2019 and of 2020 were low. The values are consistent with the low MIP recorded in agricultural soils located in the same area (Bedini et al. 2013; Di Bene et al. 2013; Pellegrino et al. 2011). This supports AM fungal inoculation and confirms how agricultural practices (such as type and dose of organic fertilizer; Aguilar et al. 2017) can damage soil AM fungal inoculum potential and impair native AM fungal community, with putatively adverse effects on growth and yield of crops (Gosling et al. 2006; Johnson 1993; Plenchette et al. 2005). On the other side, the AM fungal species utilised as inocula in our study showed values of MIP similar among each other and higher than the field experimental soil. This suggested potential to compete successfully with indigenous AMF and a positive effect on plant growth and nutrient uptake (Smith et al. 1992), in accordance to the site-specificity of AM fungal inoculant establishment and persistence (Kokkoris et al. 2019).
Effect of AM fungal inoculation on tomato root colonization
At flowering, irrespective of AM fungal species, AM fungal inoculation increased percentages of AM fungal root colonization and of root length containing arbuscules of both tomato varieties. These results are in accordance with the positive effect recorded on mycorrhizal colonization parameters assessed at different sampling times in some tomato varieties (TC 2000, PKM-1, Rio Grande, Roma and Perfect Peel) inoculated at nursery by an inoculum composed by five AM fungal species and by single inocula (Bona et al. 2017; Njeru et al. 2017; Subramanian et al. 2006). However, at fully ripening, the lack of difference in mycorrhizal colonization observed in treated and control plants of var. Rio Grande is in contrast with the results of Bona et al. (2017) and Njeru et al. (2017). This inconsistency might be due to differences in soil AM fungal native inoculum potential linked to soil texture and in the responsiveness of the host genotype, or to differences in the adopted agricultural systems (conventional vs organic).
The hypothesis that members of Glomeraceae allocate a larger fraction of fungal biomass inside the roots and members of Gigasporaceae exhibit a limited root colonization, supported by the findings of Maherali and Klironomos (2007), was not confirmed by our results in the field. Indeed, a similar AM fungal root colonization was recorded at flowering in the roots of tomato var. Pisanello inoculated by both AM fungal families, as well as at transplanting, flowering and harvest in tomato var. Rio Grande. Thus, the effect of native AMF on this trait is shown to overcome the life-history colonization strategies characteristic of the two AM fungal families. The Glomeraceae intra-family variability in AM fungal root colonization of both tomato varieties supports previous studies that detected significant differences in AM fungal root colonization among closely related AM fungal species belonging to the same family and colonizing different plant genotypes (Maherali and Klironomos 2012). Indeed, host-specificity and functional diversity in AMF was supported by the opposite trend in the AM fungal root colonization of F. mosseae and S. sinuosa detected in the varities Pisanello and Rio Grande. Nevertheless, further studies with a larger number of AM fungal isolates would better elucidate both inter- and intra-family variability in mycorrhizal colonization traits, since in the field the interactions between inoculated AMF and native microbial community can be an additional factor adding complexity in the outcome of the symbiosis.
Effect of AM fungal inoculation on tomato yield and fruit nutrient concentrations
In accordance with the overall increase of AM fungal colonization in tomato roots following nursery inoculation, total yield was improved by 35%, on average over both inoculated varieties. Our results are in agreement with the yield increase (22%) detected on tomato var. TC 2000 inoculated at nursery by a mixture of five AM fungal species (Bona et al. 2017). By contrast, Subramanian et al. (2006) did not find any effect of nursery AM fungal inoculation by R. intraradices on fruit yield of tomato var. PKM-1, under well watered conditions over a two-year experiment. Similarly, Njeru et al. (2017), nursery inoculating three varities of tomato (Roma, Rio Grande and Perfect Peel), did not find fruit yield changes during a three-year experiment. Nevertheless, during the third year of the study following no cover crops, var. Rio Grande inoculated with a mixture of F. mosseae IMA1 and R. intraradices IMA6 showed 54% higher fruit yield compared with control. The incosistencies in field outcome of the AM fungal inoculation highlight the importance of performing multi-year experiments and verifying the effects on all harvests, taking into consideration the scalability of the tomato fruit ripening. Indeed, Conversa et al. (2013) detected relevant yield increases at some harvest times during the two-year experiment on tomato var. Ercole. Other factors that can affect the field outcome of AM fungal inoculation can be the variability in compatibility between plant genotype and AM fungal isolate, soil water/nutrient availability and soil AM fungal inoculum potential (Bitterlich et al. 2019; Hart and Reader 2002; Subramanian et al. 2006).
Very few studies explored in the field the effect of AM fungal inoculation on nutrients in tomato fruits and they focused only on N and P uptake (Bowles et al. 2016; Conversa et al. 2013). In line with the increases detected in the present study on fruit P concentration following AM fungal inoculation, Conversa et al. (2013) reported P increases at 0 and 26.2 kg P ha−1 fertilizer rates. It is worth pointing out that this effect can be of key importance for tomato production, since it is known that high P uptake is needed for maximizing tomato growth and development (Zhu et al. 2018). Similarly to Bowles et al. (2016), reporting AM fungal mediated increases in fruit N content by 22%, we found a promotion of fruit N concentration in var. Pisanello at 1st harvest. This is also supported by the higher N content detected in roots and shoots of tomato under field AM fungal inoculation (Subramanian et al. 2006).
Among the other seven minerals (K, Ca, Mg, Zn, Fe, Cu and Mn) analysed in tomato fruits, positive responses to AM fungal inoculation were detected for Mg, Cu, and Mn in var. Pisanello and for K, Zn, Fe, Cu and Mn in var. Rio Grande at 2nd harvest, whereas negative responses were detected in var. Pisanello for Zn and Ca at 1st and 2nd harvest, respectively. There is well-documented proof in literature of positive changes in fruit Zn, Cu, Fe and Mn concentration (13%, 34%, 8%, and 19%, respectively) following AM fungal inoculation (Lehmann et al. 2014; Lehmann and Rillig 2015). The increase of K in fruits of the inoculated tomato var. Rio Grande is also in line with Liu et al. (2002) who detected higher K concentration in AM fungal inoculated plants of maize. Indeed, higher K concentration can be a consequence of increased P concentration in plant tissues (Cardoso and Kuyper 2006). The detected increases in fruits of minerals, such as P, K, Mn, Cu and Zn are indeed of relevance for plant nutrition, but also for tomato quality, since ascorbic acid and β-complex vitamins increase in fruits with increasing levels of those mineral elements (Dorais et al. 2008). It is also important to underline that higher K concentration in fruits of tomato were linked to higher values of the health-promoting carotenoids and lycopene (Trudel and Ozbun 1971), and that the mycorrhizal-mediated increases of divalent cations, playing a crucial role in pectin metabolism (Mignani et al. 1995), can also influence tomato tissue softening during ripening. Moreover, Ca, P, and Zn can maintain the integrity of membranes (Marschner 2012), while Zn, Cu, Fe and Mn can govern nonenzymatic and enzymatic components of the anti-oxidative system of plant (Marschner 2012) and enhance plant defence (Poschenrieder et al. 2006).
The absence of differences between the two families in AM fungal root colonization was likely determined by the interactions of the inocula with native microorganisms, whose abundance could have been enanched by inoculation, and reflected in no differences in plant growth and fruit yield. The understanding of the plant growth response to inoculation with members of Gigasporaceae and Glomeraceae is certainly made more complex by the occurrence of native AMF in field conditions. Indeed, Maherali and Klironomos (2007), studying in pot experiments the influence of phylogeny on AM fungal colonization strategies, found contrasting results in comparison with us. Higher root colonization, characteristic of Glomeraceae, correlated with reduced plant growth, whereas lower root colonization together with higher level of extraradical hyphal growth, characteristic of Gigasporaceae, correlated with higher plant growth (Maherali and Klironomos 2007). Thus, we can support that the agroecological relevance of the life-strategies of the two AM fungal families in terms of productivity may have been modulated in our study also by site-specific factors, such as soil disturbance, texture, pH, availabilities of mineral nutrients, plant genotype or the active microbiome shaped by the hyphal exudates (Hart and Reader 2004; Klironomos 2003; Lekberg et al. 2007; van der Heijden and Scheublin 2007; Zhou et al. 2020), as well as by intra-family functional variability (Avio et al. 2006; Mnkvold et al. 2004). The intra-family variability was supported by the higher total yield found in the var. Pisanello inoculated with S. pellucida compared with G. gigantea, together with the higher shoot dry weight found in the var. Rio Grande inoculated with S. sinuosa compared with F. mosseae.
The hypothesis that members of Gigasporaceae, due to their colonization strategy, would have had a stronger effect on nutrient uptake of fruits compared with members of Glomeraceae, was instead confirmed by our results on fruit nutrient concentrations. Indeed, the concentration of several nutrients were higher in fruits inoculated with Gigasporaceae (var. Pisanello: Mg and Mn; var. Rio Grande: N, K, Zn, Cu, Mn), with the only exception of Ca in fruits of tomato var. Rio Grande. Thus, this beneficial functional trait of Gigasporaceae can be of great importance in nursery/field inoculation programmes in order to improve tomato quality by enhancing macro- and micro-nutrient concentration in fruits, especially under the scenario of increased atmospheric CO2, which is reported to lower mineral nutrient uptake by almost all crops (Gojon et al. 2022; Pimenta et al. 2022). Previously, Maherali and Klironomos (2007) reported that inoculation with Gigasporaceae correlated with enhanced P concentration in shoot, whereas inoculation with Glomeraceae determined the opposite response. Accordingly, Powell et al. (2009) found that evolution of increased soil colonization is positively correlated with shoot P content. By contrast, recently, Sorghum sudanense grown in pots and inoculated with members of Glomeraceae family outperformed plants inoculated with Gigasporaceae for shoot P concentrations, whereas other mineral elements (i.s., Zn, K, Mn and Na) were similar between the two families (Horsch et al 2022). Moreover, as regard plant P concentration, the mix of the two families outperformed single-family inocula. Thus, the correlation between fungal colonization strategies and functional host benefits is still to be fully clarified. To generalize host nutrient response to AM fungal functional traits, all factors potentially affecting the relationship should be taken into consideration.
Among the factors potentially affecting the relationship between AM fungal life-history strategies and plant nutrient uptake, intra-family variability was found to play a major role. Indeed, consistent increases of several nutrient concentrations were found in fruits of tomatoes inoculated with F. mosseae compared to S. sinuosa. The behaviour of F. mosseae might be related to peculiar charateristics of this AM fungal species. Funneliformis mosseae is known to be ubiquitous in agricultural soils (Helgason et al. 1998; Pellegrino et al. 2020), quickly colonize plant roots (Chagnon et al. 2013) and facilitate shoot biomass and root length, as well as shoot and fungal P uptake respect to other species belonging to the same family (Avio et al. 2006; Munkvold et al. 2004).
Effect of AM fungal inoculation on residue decomposition and bacterial and fungal abundance in soil
Inoculation by AMF, as expected, increased soil fungal abundance in comparison with controls in both tomato varieties. By contrast, soil bacterial abundance promoted by AM fungal inoculation increased proportionally less than soil fungal abundance, thereby determining a strong increase of the fungal:bacterial (F:B) ratio in inoculated plots. Moreover, based on the higher F:B ratio we expected higher litter decomposition rates and lower percentages of remaining mass in inoculated treatments (García-Palacios et al. 2013; Delgado et al. 2015). In our field study with var. Rio Grande, fungal-dominated communities detected in inoculated treatments were associated with lower percentage of litter remaining mass and higher decomposition rate (i.e., k) at the latest litter bag sampling, with the exception of F. mosseae. This general behaviour is in line with the results of a recent meta-analysis on AMF and soil enzyme activities (Qin et al. 2020). In this work, AMF were reported to increase soil enzyme activities (e.g., N-, P-, C-releasing enzymes), expecially under conditions of neutral pH and small availabilty of P (Qin et al. 2020) that are the conditions found in our study. In support of these findings, AMF were also found to promote litter decomposition (Herman et al. 2012; Hodge et al. 2001; Mei et al. 2022), although they are assumed to facilitate soil C sequestration by increasing C input to soil and by protecting SOC from decomposition via soil aggregation (Pellegrino et al. 2022; Rillig and Mummey 2006).
The fact that Gigasporaceae family allocates a larger fungal biomass in soil compared with Glomeraceae was confirmed by our results on the higher F:B ratios in inoculated soil in both tomato varieties. In addition within Gigasporaceae, the higher F:B ratios found in soil of var. Rio Grande inoculated with G. gigantea compared with S. pellucida can be due to a larger dimension of the spores and a greater mycelium development (Dalpè et al. 2005; Dood et al. 2000). Moreover, our hypothesis that inoculation with Gigasporaceae, associated with a higher soil fungal:bacterial ratio, would have determined a higher residue decomposition, and that Glomeraceae, associated with a lower soil fungal:bacterial ratio, would have determined a lower litter decomposition, was not fully confirmed. Indeed, both members of Gigasporaceae and S. sinuosa showed high decomposition rates per day at the latest sampling. However, the positive relationship found between fungal and bacterial abundance and percentage of remaining mass suggests a good predictivity of the microbial traits for residue decomposition dynamics. Moreover, since AMF improve C input by increasing plant productivity and by producing extraradical mycelium, and reduce C stock by promoting residue decomposition, the assessment of SOC should be integrated in the study of residue decomposition to improve the prediction of the outcome of inoculation in terms of SOM stabilization. Finally, we should take into consideration that the fungal:bacterial ratio obtained in this study using the qPCR DNA assay (Fierer et al. 2015) reflects microbial abundance, but not necessary activity. Thus, the assessment of enzyme activities in further studies would improve our understanding of the mechanisms involved in residue decomposition dynamics.
Conclusions
Our work highlights the importance of applying AM fungal inocula to tomato at nursery for improving yield and fruit nutrient concentrations. Nursery inoculation of vegetables is easy to perform and may generate time and cost benefits compared to soil inoculation. The understanding of the plant responses to AM fungal functional traits turned out to be crucial for more efficient exploitation of AMF in sustainable agricultural biofertilizer programs targeted to the production of high-quality food. Overall Gigasporaceae, which accelerates litter decomposition, might be of great relevance for the uptake and translocation of several macro- and micro-nutrients to tomato fruits and should be included in mixed microbial inoculants for the enhancement of the nutritional value of tomato under organic farming systems. Moreover, due to the variability of environmental conditions in time and space, host benefit could be greater with the application of communities having AMF with diverse life-history stategies. Therefore, we suggest that field inoculation with mixtures of members of Gigasporaceae and Glomeraceae families should should be further studied to improve our knowledge on the significance of AM fungal functional groups for host nutrient response.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon request.
References
Agarwal S, Rao AV (2000) Tomato lycopene and its role in human health and chronic diseases. CMAJ 163:739–744
Aguilar R, Carreón-Abud Y, López-Carmona D, Larsen J (2017) Organic fertilizers alter the composition of pathogens and arbuscular mycorrhizal fungi in maize roots. J Phytopathol 165:448–454. https://doi.org/10.1111/jph.12579
Al-Karaki GN (2000) Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza 10:51–54. https://doi.org/10.1007/s005720000055
Al-Karaki GN, Hammad R, Rusan M (2001) Response of two tomato cultivars differing in salt tolerance to inoculation with mycorrhizal fungi under salt stress. Mycorrhiza 11:43–47. https://doi.org/10.1007/s005720100098
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253. https://doi.org/10.1111/j.1541-0420.2005.00440.x
Anderson MJ, Braak CT (2003) Permutation tests for multi-factorial analysis of variance. J Stat Comput Simul 73:85–113. https://doi.org/10.1080/00949650215733
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693. https://doi.org/10.1111/j.1461-0248.2006.00926.x
Anderson MJ, Gorley RN, Clarke RK (2008) Permanova+ for Primer:Guide to Software and Statistical Methods. Plymouth: Primer-E Limited.
Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM (2004) Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141:221–235. https://doi.org/10.1007/s00442-004-1519-1
Avio L, Pellegrino E, Bonari E, Giovannetti M (2006) Functional diversity of arbuscular mycorrhizal fungal isolates in relation to extraradical mycelial networks. New Phytol 172:347–357
Bago B, Pfeffer PE, Shachar-Hill Y (2000) Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol 124:949–958. https://doi.org/10.1104/pp.124.3.949
Baldi E, Gioacchini P, Montecchio D, Mocali S, Antonielli L, Masoero G, Toselli M (2021) Effect of biofertilizers application on soil biodiversity and litter degradation in a commercial apricot orchard. Agronomy 11:1116. https://doi.org/10.3390/agronomy11061116
Baulcombe D, Crute I, Davies B, Dunwell J, Gale M, Jones J, Pretty J, Sutherland W, Toulmin C (2009) Reaping the benefits: science and the sustainable intensification of global agriculture. Proc R Soc Lond B Biol Sci
Bedini S, Avio L, Sbrana C, Turrini A, Migliorini P, Vazzana C, Giovannetti M (2013) Mycorrhizal activity and diversity in a long-term organic Mediterranean agroecosystem. Biol Fertil Soils 49:781–790. https://doi.org/10.1007/s00374-012-0770-6
Berni R, Romi M, Parrotta L, Cai G, Cantini C (2018) Ancient tomato (Solanum lycopersicum L.) varieties of tuscany have high contents of bioactive compounds. Horticulturae 4:51. https://doi.org/10.3390/horticulturae4040051
Bitterlich M, Franken P, Graefe J (2019) Atmospheric drought and low light impede mycorrhizal effects on leaf photosynthesis - a glasshouse study on tomato under naturally fluctuating environmental conditions. Mycorrhiza 29:13–28. https://doi.org/10.1007/s00572-018-0872-6
Bona E, Cantamessa S, Massa N, Manassero P, Marsano F, Copetta A, Lingua G, D’Agostino G, Gamalero E, Berta G (2017) Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: a field study. Mycorrhiza 27:1–11. https://doi.org/10.1007/s00572-016-0727-y
Bowles TM, Barrios-Masias FH, Carlisle EA, Cavagnaro TR, Jackson LE (2016) Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci Total Environ 566:1223–1234. https://doi.org/10.1016/j.scitotenv.2016.05.178
Bremner JM, Mulvaney CS (1982). Nitrogen - total. In: Page AL, Miller RH, Keeney DR (Eds.), Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties Agronomy Monograph, second ed., vol. 9. American Society of Agronomy, Madison, WI, pp. 595-624
Brundrett MC, Abbott LK, Jasper DA (1999) Glomalean mycorrhizal fungi from tropical Australia. Mycorrhiza 8:305–314. https://doi.org/10.1007/s005720050251
Brundrett MC, Tedersoo L (2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol 220:1108–1115. https://doi.org/10.1111/nph.14976
Bueno CG, Gerz M, Zobel M, Moora M (2019) Conceptual differences lead to divergent trait estimates in empirical and taxonomic approaches to plant mycorrhizal trait assignment. Mycorrhiza 29:1–11. https://doi.org/10.1007/s00572-018-0869-1
Bulgarelli D, Garrido-Oter R, Münch PC, Weiman A, Dröge J, Pan Y, McHardy AC, Schulze-Lefert P (2015) Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17:392–403. https://doi.org/10.1016/j.chom.2015.01.011
Cardini A, Pellegrino E, Declerck S, Calonne-Salmon M, Mazzolai B, Ercoli L (2021) Direct transfer of zinc between plants is channelled by common mycorrhizal network of arbuscular mycorrhizal fungi and evidenced by changes in expression of zinc transporter genes in fungus and plant. Environ Microbiol 23:5883–5900. https://doi.org/10.1111/1462-2920.15542
Cardoso IM, Kuyper TW (2006) Mycorrhizas and tropical soil fertility. Agric Ecosyst Environ 116:72–84. https://doi.org/10.1016/j.agee.2006.03.011
Chaudhary P, Sharma A, Singh B, Nagpal AK (2018) Bioactivities of phytochemicals present in tomato. J Food Sci Technol 55:2833–2849. https://doi.org/10.1007/s13197-018-3221-z
Cheng L, Booker FL, Tu C, Burkey KO, Zhou L, Shew HD, Rufty TW, Hu S (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337:1084–1087. https://doi.org/10.1126/science.1224304
Clarke KR, Gorley RN (2015) Getting started with PRIMER v7. PRIMER-E: Plymouth, Plymouth Marine Laboratory 20(1)
Clarke KR, Warwick R (2001) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth: PRIMER-E
Coccina A, Cavagnaro TR, Pellegrino E, Ercoli L, McLaughlin MJ, Watts-Williams SJ (2019) The mycorrhizal pathway of zinc uptake contributes to zinc accumulation in barley and wheat grain. BMC Plant Biol 19:1–14. https://doi.org/10.1186/s12870-019-1741-y
Conversa G, Lazzizera C, Bonasia A, Elia A (2013) Yield and phosphorus uptake of a processing tomato crop grown at different phosphorus levels in a calcareous soil as affected by mycorrhizal inoculation under field conditions. Biol Fert Soils 49:691–703. https://doi.org/10.1007/s00374-012-0757-3
Corrêa A, Cruz C, Ferrol N (2015) Nitrogen and carbon/nitrogen dynamics in arbuscular mycorrhiza: the great unknown. Mycorrhiza 25:499–515. https://doi.org/10.1007/s00572-015-0627-6
Dalpé Y, Cranenbrouck S, Séguin S, Declerck S (2005) The monoxenic culture of arbuscular mycorrhizal fungi as a tool for systematics and biodiversity. In: In vitro culture of mycorrhizas. Springer, Berlin, Heidelberg, pp. 31-48
Delgado-Baquerizo M, García-Palacios P, Milla R, Gallardo A, Maestre FT (2015) Soil characteristics determine soil carbon and nitrogen availability during leaf litter decomposition regardless of litter quality. Soil Biol Biochem 81:134–142. https://doi.org/10.1016/j.soilbio.2014.11.009
Di Bene C, Pellegrino E, Debolini M, Silvestri N, Bonari E (2013) Short-and long-term effects of olive mill wastewater land spreading on soil chemical and biological properties. Soil Biol Biochem 56:21–30. https://doi.org/10.1016/j.soilbio.2012.02.019
Dorais M, Ehret DL, Papadopoulos AP (2008) Tomato (Solanum lycopersicum) health components: from the seed to the consumer. Phytochem Rev 7:231–250. https://doi.org/10.1007/s11101-007-9085-x
Fierer N, Jackson JA, Vilgalys R, Jackson RB (2005) Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol 71:4117–4120. https://doi.org/10.1128/AEM.71.7.4117-4120.2005
Flores FB, Sanchez-Bel P, Estañ MT, Martinez-Rodriguez MM, Moyano E, Morales B, Campos JF, Garcia-Abellán JO, Egea MI, Fernández-Garcia N, Romojaro F, Bolarín MC (2010) The effectiveness of grafting to improve tomato fruit quality. Sci Hortic 125:211–217. https://doi.org/10.1016/j.scienta.2010.03.026
Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. https://www.fao.org/faostat/en/#data/QCL. Accessed August 2022.
García-Palacios P, Milla R, Álvaro-Sánchez M, Martín-Robles N, Maestro M (2013) Application of a high-throughput laboratory method to assess litter decomposition rates in multiple-species experiments. Soil Biol Biochem 57:929–932. https://doi.org/10.1016/j.soilbio.2012.09.029
Giovannetti M, Avio L, Barale R, Ceccarelli N, Cristofani R, Iezzi A, Scarpato R (2012) Nutraceutical value and safety of tomato fruits produced by mycorrhizal plants. Br J Nutr 107:242–251. https://doi.org/10.1017/S000711451100290X
Gojon A, Cassan O, Bach L, Lejay L, Martin A (2022) The decline of plant mineral nutrition under rising CO2: physiological and molecular aspects of a bad deal. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2022.09.002
González-González MF, Ocampo-Alvarez H, Santacruz-Ruvalcaba F, Sánchez-Hernández CV, Casarrubias-Castillo K, Becerril-Espinosa A, Castañeda-Nava JJ, Hernández-Herrera RM (2020) Physiological, ecological, and biochemical implications in tomato plants of two plant biostimulants: Arbuscular mycorrhizal fungi and seaweed extract. Front Plant Sci 11:999. https://doi.org/10.3389/fpls.2020.00999
Gosling P, Hodge A, Goodlass G, Bending GD (2006) Arbuscular mycorrhizal fungi and organic farming. Agr Ecosyst Environ 113:17–35. https://doi.org/10.1016/j.agee.2005.09.009
Gosling P, Ozaki A, Jones J, Turner Mary Rayns F, Bending GD (2010) Organic management of tilled agricultural soils results in a rapid increase in colonization potential and spore populations of arbuscular mycorrhizal fungi. Agr Ecosyst Environ 139:273–279. https://doi.org/10.1016/j.agee.2010.08.013
Gui H, Hyde K, Xu J, Mortimer P (2017) Arbuscular mycorrhiza enhance the rate of litter decomposition while inhibiting soil microbial community development. Sci Rep 7:1–11. https://doi.org/10.1038/srep42184
Hamel C, Dalpé Y, Furlan V, Parent S (1997) Indigenous populations of arbuscular mycorrhizal fungi and soil aggregate stability are major determinants of leek (Allium porrum L.) response to inoculation with Glomus intraradices Schenck & Smith or Glomus versiforme (Karsten) Berch. Mycorrhiza 7:187–196. https://doi.org/10.1007/s005720050180
Hart M, Ehret DL, Krumbein A, Leung C, Murch S, Turi C, Franken P (2015) Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 25:359–376. https://doi.org/10.1007/s00572-014-0617-0
Hart MM, Reader RJ (2002) Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol 153:335–344. https://doi.org/10.1046/j.0028-646X.2001.00312.x
Hart MM, Reader RJ (2004) Do arbuscular mycorrhizal fungi recover from soil disturbance differently? Trop Ecol 45:97–112
Hart MM, Reader RJ (2005) The role of the external mycelium in early colonization for three arbuscular mycorrhizal fungal species with different colonization strategies. Pedobiologia 49:269–279. https://doi.org/10.1016/j.pedobi.2004.12.001
Heber D (2000) Colorful cancer prevention: α-carotene, lycopene, and lung cancer. Am J Clin Nutr 72:901–902. https://doi.org/10.1093/ajcn/72.4.901
Helgason T, Daniell TJ, Husband R, Fitter AH, Young JPW (1998) Ploughing up the wood-wide web? Nature 394:431–431. https://doi.org/10.1038/28764
Herman DJ, Firestone MK, Nuccio E, Hodge A (2012) Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol Ecol 80:236–247. https://doi.org/10.1111/j.1574-6941.2011.01292.x
Hestrin R, Hammer EC, Mueller CW, Lehmann J (2019) Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun Biol 2:1–9. https://doi.org/10.1038/s42003-019-0481-8
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299. https://doi.org/10.1038/35095041
Hodge A, Storer K (2015) Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386:1–19. https://doi.org/10.1007/s11104-014-2162-1
Horsch CC, Antunes PM, Kallenbach CM (2022) Arbuscular mycorrhizal fungal communities with contrasting life-history traits and trait diversity influence host nutrient acquisition. Preprint from Research Square. https://doi.org/10.21203/rs.3.rs-2052330/v1
Jakobsen I, Abbott LK, Robson A (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol 120:371–380. https://doi.org/10.1111/j.1469-8137.1992.tb01077.x
Javot H, Pumplin N, Harrison MJ (2007) Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant Cell Environ 30:310–322. https://doi.org/10.1111/j.1365-3040.2006.01617.x
Jiang Y, Wang W, Xie Q, Liu N, Liu L, Wang D, Zhang X, Yang C, Chen X, Wang E (2017) Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356:1172–1175. https://doi.org/10.1126/science.aam9970
Jin H, Pfeffer PE, Douds DD, Piotrowski E, Lammers PJ, Shachar-Hill Y (2005) The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol 168:687–696. https://doi.org/10.1111/j.1469-8137.2005.01536.x
Joergensen RG, Wichern F (2008) Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biol Biochem 40:2977–2991. https://doi.org/10.1016/j.soilbio.2008.08.017
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757. https://doi.org/10.2307/1942106
Jones JB Jr, Wolf B, Mills HA (1991) Plant Analysis Handbook II: a practical sampling, preparation, analysis, and interpretation guide. Micro-Macro Publishing Inc., Athens
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301. https://doi.org/10.1890/02-0413
Klironomos JN, Hart MM (2002) Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 12:181–184. https://doi.org/10.1007/s00572-002-0169-6
Kokkoris V, Miles T, Hart MM (2019) The role of in vitro cultivation on asymbiotic trait variation in a single species of arbuscular mycorrhizal fungus. Fungal Biol 123:307–317. https://doi.org/10.1016/j.funbio.2019.01.005
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen-Geiger climate classification updated. Meteorol Z 15:259–263. https://doi.org/10.1127/0941-2948/2006/0130
Lehmann A, Rillig MC (2015) Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops - a meta-analysis. Soil Biol Biochem 81:147–158. https://doi.org/10.1016/j.soilbio.2014.11.013
Lehmann A, Veresoglou SD, Leifheit EF, Rillig MC (2014) Arbuscular mycorrhizal influence on zinc nutrition in crop plants - a meta-analysis. Soil Biol Biochem 69:123–131. https://doi.org/10.1016/j.soilbio.2013.11.001
Leigh J, Fitter AH, Hodge A (2011) Growth and symbiotic effectiveness of an arbuscular mycorrhizal fungus in organic matter in competition with soil bacteria. FEMS Microbiol Ecol 76:428–438. https://doi.org/10.1111/j.1574-6941.2011.01066.x
Lekberg Y, Koide RT, Rohr JR, Aldrich-wolfe L, Morton JB (2007) Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J Ecol 95:95–105. https://doi.org/10.1111/j.1365-2745.2006.01193.x
Liberato CG, Juan AVA, Barros AV, Raquel C, Machado ARA, Nóbrega JA, Schiavo D (2020) Determination of macro and micronutrients in plants using the Agilent 4200 MP AES. Agilent Technologies
Liu A, Hamel C, Elmi A, Costa C, Ma B, Smith DL (2002) Concentrations of K, Ca and Mg in maize colonized by arbuscular mycorrhizal fungi under field conditions. Can J Soil Sci 82:272–278. https://doi.org/10.4141/S01-022
Luginbuehl LH, Menard GN, Kurup S, Van Erp H, Radhakrishnan GV, Breakspear A, Oldroyd GED, Eastmond PJ (2017) Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 356:1175–1178. https://doi.org/10.1126/science.aan0081
Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748. https://doi.org/10.1126/science.1143082
Maherali H, Klironomos JN (2012) Phylogenetic and trait-based assembly of arbuscular mycorrhizal fungal communities. PLoS One 7:e36695. https://doi.org/10.1371/journal.pone.0036695
Maherali H, Oberle B, Stevens PF, Cornwell WK, McGlinn DJ (2016) Mutualism persistence and abandonment during the evolution of the mycorrhizal symbiosis. Am Nat 188:E113–E125
Marschner H (2012) Mineral nutrition of higher plants, vol 3. Academic press, London
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
McLean EO (1982) Soil pH and lime requirement. In: Page AL, Miller RH, Keeney DR (Eds.), Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties Agronomy Monograph, second ed., vol. 9. American Society of Agronomy, Madison, pp. 199-224
Mei L, Zhang P, Cui G, Yang X, Zhang T, Guo J (2022) Arbuscular mycorrhizal fungi promote litter decomposition and alleviate nutrient limitations of soil microbes under warming and nitrogen application. Appl Soil Ecol 171:104318. https://doi.org/10.1016/j.apsoil.2021.104318
Meier U (2001) BBCH-Monograph: growth stages of mono-and dicotyledonous plants. In: Technical Report, 2nd edn. Federal Biological Research Centre for Agriculture and Forestry, pp. 158
Mignani I, Greve LC, Ben-Arie R, Stotz HU, Li C, Shackel K, Labavitch J (1995) The effects of GA3 and divalent cations on aspects of pectin metabolism and tissue softening in ripening tomato pericarp. Physiol Plantarum 93:108–115. https://doi.org/10.1034/j.1399-3054.1995.930116.x
Morton JB, Benny GL (1990) Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): a new order, Glomales, two new suborders, Glomineae and Gigasporineae, and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon 37:471–491
Morton JB, Bentivenga SP, Wheeler WW (1993) Germ plasm in the International Collection of Arbuscular Mycorrhizal Fungi (INVAM) and procedures for culture development, documentation and storage. Mycotaxon 48:491–528
Morton JP (1993) Properties of infective propagules at the suborder level (Glominae versus Gigasporineae). INVAM Newsletter 3: September 1993.
Munkvold L, Kjøller R, Vestberg M, Rosendahl S, Jakobsen I (2004) High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol 164:357–364. https://doi.org/10.1111/j.1469-8137.2004.01169.x
Myers SS, Zanobetti A, Kloog I, Huybers P, Leakey ADB, Bloom AJ, Carlisle E, Dietterich LH, Fitzgerald G, Hasegawa T, Holbrook NM, Nelson RL, Ottman MJ, Raboy V, Sakai H, Sartor KA, Schwartz J, Seneweera S, Tausz M, Usui Y (2014) Increasing CO2 threatens human nutrition. Nature 510:139–142. https://doi.org/10.1038/nature13179
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keeney DR (Eds.), Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties Agronomy Monograph, second ed., vol. 9. American Society of Agronomy, Madison, WI, pp. 539-579
Njeru EM, Bocci G, Avio L, Sbrana C, Turrini A, Giovannetti M, Bàrberi P (2017) Functional identity has a stronger effect than diversity on mycorrhizal symbiosis and productivity of field grown organic tomato. Eur J Agron 86:1–11. https://doi.org/10.1016/j.eja.2017.02.007
Nuccio EE, Hodge A, Pett-Ridge J, Herman DJ, Weber PK, Firestone MK (2013) An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ Microbiol 15:1870–1881. https://doi.org/10.1111/1462-2920.12081
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (Eds.), Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties Agronomy Monograph, second ed., vol 9. American Society of Agronomy, Madison, WI, pp 403-430
Parniske M (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol 6:763–775. https://doi.org/10.1038/nrmicro1987
Pellegrino E, Bedini S, Avio L, Bonari E, Giovannetti M (2011) Field inoculation effectiveness of native and exotic arbuscular mycorrhizal fungi in a Mediterranean agricultural soil. Soil Biol Biochem 43:367–376. https://doi.org/10.1016/j.soilbio.2010.11.002
Pellegrino E, Öpik M, Bonari E, Ercoli L (2015) Responses of wheat to arbuscular mycorrhizal fungi: a meta-analysis of field studies from 1975 to 2013. Soil Biol Biochem 84:210–217. https://doi.org/10.1016/j.soilbio.2015.02.020
Pellegrino E, Piazza G, Arduini I, Ercoli L (2020) Field inoculation of bread wheat with Rhizophagus irregularis under organic farming: variability in growth response and nutritional uptake of eleven old genotypes and a modern variety. Agronomy 10:333. https://doi.org/10.3390/agronomy10030333
Pellegrino E, Piazza G, Helgason T, Ercoli L (2021) Eukaryotes in soil aggregates across conservation managements: Major roles of protists, fungi and taxa linkages in soil structuring and C stock. Soil Biol. Biochem 163:108463. https://doi.org/10.1016/j.soilbio.2021.108463
Pellegrino E, Piazza G, Helgason T, Ercoli L (2022) Microbiome structure and interconnection in soil aggregates across conservation and conventional agricultural practices allow to identify main prokaryotic and fungal taxa related to soil functioning. Soil Biol Biochem 175:108833. https://doi.org/10.1016/j.soilbio.2022.108833
Pellegrino E, Turrini A, Gamper HA, Cafà G, Bonari E, Young JPW, Giovannetti M (2012) Establishment, persistence and effectiveness of arbuscular mycorrhizal fungal inoculants in the field revealed using molecular genetic tracing and measurement of yield components. New Phytol 194:810–822. https://doi.org/10.1111/j.1469-8137.2012.04090.x
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. T Brit Mycol Soc 55:158–161. https://doi.org/10.1016/S0007-1536(70)80110-3
Piazza G, Ercoli L, Nuti M, Pellegrino E (2019) Interaction between conservation tillage and nitrogen fertilization shapes prokaryotic and fungal diversity at different soil depths: evidence from a 23-year field experiment in the Mediterranean area. Fron Microbiol 10:2047. https://doi.org/10.3389/fmicb.2019.02047
Pimenta TM, Souza GA, Brito FA, Teixeira LS, Arruda RS, Henschel JM, Zsögön A, Ribeiro DM (2022) The impact of elevated CO2 concentration on fruit size, quality, and mineral nutrient composition in tomato varies with temperature regimen during growing season. Plant Growth Regul 1–12. https://doi.org/10.1007/s10725-022-00889-8
Plenchette C, Clermont-Dauphin C, Meynard JM, Fortin JA (2005) Managing arbuscular mycorrhizal fungi in cropping systems. Can J Plant Sci 85:31–40. https://doi.org/10.4141/P03-159
Poschenrieder C, Tofra R, Barcelo J (2006) Can metals defend plants against biotic stress? Trends Plant Sci 11:288–295. https://doi.org/10.1016/j.tplants.2006.04.007
Powell JR, Parrent JL, Hart MM, Klironomos JN, Rillig MC, Maherali H (2009) Phylogenetic trait conservatism and the evolution of functional trade-offs in arbuscular mycorrhizal fungi. P Roy Soc B-Biol Sci 276:4237–4245. https://doi.org/10.1098/rspb.2009.1015
Qin H, Brookes PC, Xu J, Feng Y (2014) Bacterial degradation of Aroclor 1242 in the mycorrhizosphere soils of zucchini (Cucurbita pepo L.) inoculated with arbuscular mycorrhizal fungi. Environ Sci Pollut Res Int 21:12790–12799. https://doi.org/10.1007/s11356-014-3231-y
Qin M, Zhang Q, Pan J, Jiang S, Liu Y, Bahadur A, Peng Z, Yang Y, Feng H (2020) Effect of arbuscular mycorrhizal fungi on soil enzyme activity is coupled with increased plant biomass. Eur J Soil Sci 71:84–92. https://doi.org/10.1111/ejss.12815
Regione Toscana. Razze e Varietà Locali. http://germoplasma.regione.toscana.it/. Accessed 14th of June 2022
Rillig MC, Mummey DL (2006) Mycorrhizas and soil structure. New Phytol 171:41–53. https://doi.org/10.1111/j.1469-8137.2006.01750.x
Ryan MH, Graham JH (2018) Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol 220:1092–1107. https://doi.org/10.1111/nph.15308
Schalamuk S, Cabello M (2010) Arbuscular mycorrhizal fungal propagules from tillage and no-tillage systems: possible effects on Glomeromycota diversity. Mycologia 102:261–268. https://doi.org/10.3852/08-118
Scharff LB, Saltenis VL, Jensen PE, Baekelandt A, Burgess AJ, Burow M, Ceriotti A, Cohan J-P, Geu-Flores F, Halkier BA, Haslam RP, Inzé D, Lankhorst RK, Murchie EH, Napier JA, Nacry P, Parry MAJ, Santino A, Scarano A, Sparvoli F, Wilhelm R, Pribil M (2022) Prospects to improve the nutritional quality of crops. Food Energy Secur 11:e327. https://doi.org/10.1002/fes3.327
Sharma P, Aggarwal P, Kaur A (2017) Biofortification: A new approach to eradicate hidden hunger. Food Rev Int 33:1–21. https://doi.org/10.1080/87559129.2015.1137309
Singh M, Singh P, Singh S, Saini RK, Angadi SV (2021) A global meta-analysis of yield and water productivity responses of vegetables to deficit irrigation. Sci Rep 11:1–13. https://doi.org/10.1038/s41598-021-01433-w
Smith FA, Jakobsen I, Smith SE (2000) Spatial differences in acquisition of soil phosphate between two arbuscular mycorrhizal fungi in symbiosis with Medicago truncatula. New Phytol 147:357–366. https://doi.org/10.1046/j.1469-8137.2000.00695.x
Smith SE, Read D (2008) Mycorrhizal Symbiosis, vol 3. Academic Press, London
Smith SE, Robson AD, Abbott LK (1992) The involvement of mycorrhizas in assessment of genetically dependent efficiency of nutrient uptake and use. Plant Soil 146:169–217. https://doi.org/10.1007/BF00012010
Subramanian KS, Santhanakrishnan P, Balasubramanian P (2006) Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci Hortic 107:245–253. https://doi.org/10.1016/j.scienta.2005.07.006
Tedersoo L, Sánchez-Ramírez S, Kõljalg U, Bahram M, Döring M, Schigel D, May T, Ryberg M, Abarenkov K (2018) High-level classification of the fungi and a tool for evolutionary ecological analyses. Fungal Divers 90:135–159. https://doi.org/10.1007/s13225-018-0401-0
Thomas GW (1982) Exchangeable cations. In: Page AL, Miller RH, Keeney DR (Eds.), Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties Agronomy Monograph, second ed., vol. 9. American Society of Agronomy, Madison, WI, pp. 159-165
Tommerup I, Abbott L (1981) Prolonged survival and viability of VA mycorrhizal hyphae after root death. Soil Biol Biochem 13:431–433. https://doi.org/10.1016/0038-0717(81)90090-0
Toor RK, Savage GP (2005) Antioxidant activity in different fractions of tomatoes. Food Res Int 38:487–494. https://doi.org/10.1016/j.foodres.2004.10.016
Trudel MJ, Ozbun JL (1971) Influence of potassium on carotenoid content of tomato fruit. J Amer Soc Hort Sci 96:763–765. https://doi.org/10.21273/JASHS.96.6.763
USDA (1975) Color classification requirements in tomatoes. Visual Aid TM‐L‐I.
van der Heijden MG, Scheublin TR (2007) Functional traits in mycorrhizal ecology: their use for predicting the impact of arbuscular mycorrhizal fungal communities on plant growth and ecosystem functioning. New Phytol 17:244–250. https://doi.org/10.1111/j.1469-8137.2007.02041.x
Watts-Williams SJ, Cavagnaro TR (2014) Nutrient interactions and arbuscular mycorrhizas: a meta-analysis of a mycorrhiza-defective mutant and wild-type tomato genotype pair. Plant Soil 384:79–92. https://doi.org/10.1007/s11104-014-2140-7
Zhang S, Lehmann A, Zheng W, You Z, Rillig MC (2019) Arbuscular mycorrhizal fungi increase grain yields: A meta-analysis. New Phytol 222:543–555. https://doi.org/10.1111/nph.15570
Zhou J, Chai X, Zhang L, Wang F, Feng G (2020) Different Arbuscular Mycorrhizal Fungi Cocolonizing on a Single Plant Root System Recruit Distinct Microbiomes Msystems 5:e00929-e1020. https://doi.org/10.1128/mSystems.00929-20
Zhu Q, Ozores-Hampton M, Li YC, Morgan KT (2018) Phosphorus application rates affected phosphorus partitioning and use efficiency in tomato production. Agron J 110:2050–2058. https://doi.org/10.2134/agronj2018.03.0152
Zhu HH, Yao Q (2004) Localized and systemic increase of phenols in tomato roots induced by Glomus versiforme inhibits Ralstonia solanacearum. J Phytopathol 152:537–542. https://doi.org/10.1111/j.1439-0434.2004.00892.x
Acknowledgements
The authours would like to thank Alessandro Agostini Venerosi della Seta for allowing to run the field experiments at the “Fattoria Le Prata” farm (Pisa, Italy), Giorgio Masoero of the “Accademia di Agricoltura” of Torino giving us valuable advices for litter bag experiment set-up and interpretation of results and Giusto Giovannetti of the CCS Aosta for providing us some of AM fungal isolates.
Funding
Open access funding provided by Scuola Superiore Sant'Anna within the CRUI-CARE Agreement. This work was funded by the the European Agricultural Fund for Rural Development 2007–2013 for Tuscany (Italy), measure 16.1 (FERTIBIO project; CUP Artea 743548) project leader Dr. Elisa Pellegrino and measure 16.2 (FERTIBIO project; CUP Artea 828090) project leader Prof. Laura Ercoli. Myriam Arcidiacono was supported by a PhD scholarship from Scuola Superiore Sant’Anna (SSSA, Pisa, Italy).
Author information
Authors and Affiliations
Contributions
EP and LE acquired funding, conceived and designed the study. MA carried out the experimental procedures. EP and MA performed the molecular study, collected the data, performed the statistical analyses and wrote the first draft of the manuscript. LE and MN contributed to the discussion of the data. All authors commented on the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. All authours have no conflict of interests to declare.
Additional information
Responsible Editor: Jan Jansa.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arcidiacono, M., Pellegrino, E., Nuti, M. et al. Field inoculation by arbuscular mycorrhizal fungi with contrasting life-history strategies differently affects tomato nutrient uptake and residue decomposition dynamics. Plant Soil (2023). https://doi.org/10.1007/s11104-023-05995-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-023-05995-8