Abstract
Purpose
Insect herbivory affects plant growth, nutrient and secondary metabolite concentrations and litter quality. Changes to litter quality due to insect herbivory can alter decomposition, with knock on effects for plant growth mediated through the plant-litter-soil feedback pathway.
Methods
Using a multi-phase glasshouse experiment, we tested how changes in shoot and root litter quality of fast- and slow-growing grass caused by insect herbivores affect the performance of response plants in the soil in which the litter decomposed.
Results
We found that insect herbivory resulted in marginal changes to litter quality and did not affect growth when plants were grown with fast- versus slow-growing litter. Overall, presence of litter resulted in reduced root and shoot growth and this effect was significantly more negative in shoots versus roots. However, this effect was minimal, with a loss of c. 1.4% and 3.1% dry weight biomass in roots versus shoots, respectively. Further, shoot litter exposed to insect herbivory interacted with response plant identity to affect root growth.
Conclusions
Our results suggest that whether litter originates from plant tissues exposed to insect herbivory or not and its interaction with fast- versus slow-growing grasses is of little importance, but species-specific responses to herbivory-conditioned litter can occur. Taken collectively, the overall role of the plant-litter-soil feedback pathway, as well as its interaction with insect herbivory, is unlikely to affect broader ecosystem processes in this system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insect herbivory is omnipresent in terrestrial ecosystems and can create substantial alterations to plant community composition and function (Vidal and Murphy 2018). It is well established that feeding by insects can induce increases in plant defence compounds in both living shoots (Gatehouse 2002; Kaplan et al. 2008; Karban 2011) and roots (Kaplan et al. 2008; van der Putten 2003). Furthermore, insect herbivory can either increase (Ohgushi 2005) or decrease (Johnson et al. 2009; Nykänen and Koricheva 2004) the nutrient content of living plant tissues, depending on plant species, tissue type, metabolites and nutrients considered. Changes to plant tissue chemistry that result in increased concentrations of defensive compounds and lower or higher nutrient content can persist after senescence (Chapman et al. 2003; Lattanzio et al. 2006). Consequently, changes in the quality of the leaf and root litter that reaches the soil could alter the decomposition process. Labile litter is readily decomposable in contrast to poor quality recalcitrant litter. This means recalcitrant litter usually decomposes more slowly because it is less suitable to decomposer organisms (Chomel et al. 2016). For example, Schweitzer et al. (2005) found that litter galled by the leaf galling aphid (Pemphigus betae) contained higher polyphenol and lower N concentrations, which led to decreased leaf litter decomposition rates. Inhibited decomposition can result in fewer nutrients released into the soil, thereby hindering the growth of future plants growing in that soil (Hättenschwiler and Vitousek 2000). Further, herbivore-induced shifts in secondary compounds in plants (Thelen et al. 2005) could result in the build-up of allelopathic compounds in the soil, leading to negative effects in plants that grow in the soil in which the litter decomposes (John and Sarada 2012). Hence, insect-induced changes to the litter decomposition pathway could result in alterations to the plant-litter-soil feedback (PLSF) pathway (Veen et al. 2019b). Despite recent advances in the understanding of the role of litter decomposition in soil nutrient cycling processes (Woodman et al. 2021), and how this may affect plant fitness, few studies have considered how insect herbivory might modify the PLSF pathway (e.g., (Burghardt et al. 2018).
Shoots and roots serve very different functions to the plant, the former providing energy through photosynthesis, and the latter serving to anchor the plant into the soil, gather nutrients and water, and serve as a major storage compartment. After senescence, both shoots and roots become an important source of nutrients in the soil. Shoots and roots – even of the same plant individual—are exposed to a set of very different biotic and abiotic conditions, in terms of herbivore communities and microclimatic conditions. Consequently, chemical composition varies markedly between the above- and belowground plant compartments (Faucon et al. 2017). Therefore, the decomposition of both shoot and root litter tends to be driven by tissue chemistry (Aerts 1997; Silver and Miya 2001), which can be highly plastic, depending on the environment. Shoot litter tends to have higher nitrogen concentrations than root litter, which makes it more labile (i.e., readily decomposable) (Aerts 1997; Cornwell et al. 2008; Reich 2014). Although the shoot and root litter decomposition rates within a species occasionally differ (Hobbie et al. 2010), there is considerable evidence that root and shoot litter decomposition characteristics show similar patterns within most plant species (Freschet et al. 2013).
Substantial research supports the idea of a “plant economic spectrum”, which proposes that plants typically fall on a continuum from “fast” to “slow” growth strategies (Díaz et al. 2016; Reich 2014; Wright et al. 2004). Specifically, fast-growing plants usually have higher productivity, promote faster nutrient cycling, and are less well defended, leading to greater susceptibility to pathogen accumulation (as opposed to mutualists), while slow-growing plants are generally the opposite. Plants at opposite ends of this spectrum tend to produce litter with contrasting decomposition rates; litter of fast growers decomposes quicker, whereas litter of slow growers decomposes slower (Cornwell et al. 2008; Freschet et al. 2012; Santiago 2007). As litter produced by fast-growing plants decomposes, it more easily releases nutrients to the soil and plants growing in the vicinity, leading to more positive plant-litter feedbacks (Freschet et al. 2013). Alterations to the PLSF pathway caused by plant community compositional shifts (i.e., different proportions of fast- versus slow-growing species) have the potential to substantially alter ecosystem function and services through litter input and/or shifts in decomposer communities (Dias et al. 2017; Jongen et al. 2021). Therefore, understanding how litter produced by fast- and slow-growing plant species feeds back to influence the growth of other plants is of critical importance.
We examined how herbivore-induced changes to plant shoot and root litter quality of grasses with contrasting growth strategies (fast versus slow), impact on response plant performance in the soil in which the litter decomposes, via the PLSF pathway. Using a glasshouse experiment with six common, naturally co-occurring grass species with contrasting growth strategies, we tested the following hypotheses: 1) Litter from grasses that were exposed to above- or belowground herbivores will negatively influence response plant biomass, mediated through the PLSF pathway. This is because insect herbivores typically reduce litter nutrient content (Nykänen and Koricheva 2004) and increase secondary metabolites in both shoots and roots (Kaplan et al. 2008), which inhibits decomposition and thereby reduces nutrient access to plants growing in the decomposed litter; 2) Overall, root litter will generate more negative PLSFs than shoot litter because root litter is recalcitrant and shoot litter is labile (Freschet et al. 2013). These effects will interact differently with above- and belowground herbivory, because of the contrasting responses to herbivory in each plant compartment (Kaplan et al. 2008); 3) Litter from fast-growing plant species will generate more positive PLSFs than that of slow-growing plants, because their litter is more labile and less defended (Reich 2014), making it a better source of nutrients for plants growing in the decomposing litter (Cornwell et al. 2008; Freschet et al. 2012; Santiago 2007); and 4) We expect species-specific effects due to the wide interspecific variation in litter nutrient content and secondary metabolites (Faucon et al. 2017).
Materials and methods
Litter conditioning phase
Six grass species were selected to represent fast- (Arrhenatherum elatius L. (P. Beav.), Holcus lanatus L., Lolium perenne L.) and slow- (Agrostis capillaris L., Deschampsia flexuosa L. (Trin.), Festuca ovina L.) growing species. The species were divided into fast- and slow-growing categories based on the literature (Elberse and Berendse 1993; Heinen et al. 2020; Scheurwater et al. 2002) because it is known that species with contrasting growth rates can have different effects on ecosystem processes and functions (Heinen et al. 2020; Steinauer et al. 2020; Vile et al. 2006). In contrast to forbs, grasses have inherently lower concentrations of secondary metabolites (Geisen et al. 2022) and generally lower molecular richness (Defossez et al. 2021). Despite the fact that these compounds are often more abundant in forbs and are usually affected by herbivores (Gatehouse 2002; Kaplan et al. 2008; Karban 2011; van der Putten 2003), we opted to investigate only grass species because grasses are the dominant functional group in grassland systems. Seeds were obtained from Cruydt-Hoeck (Nijeberkoop, The Netherlands) or Pratensis AB (Lönashult, Sweden). The grasses were sown on 15 November 2017 directly into 5-L plastic pots in soil that consisted of 90% gamma irradiated soil (Synergy Health, Ede, The Netherlands) characterised as holtpodzol sandy loam (84% sand, 11% silt, 2% clay, ~ 3% organic matter, pH 5.9, 1150 mg kg−1 N, 61 mg P2O5 100 g−1, 2.4 mmol K kg−1) collected from a grassland near Lange Dreef, Driebergen, The Netherlands (52° 02’ N, 5° 16’ E) and 10% live field soil that was collected from a restored grassland site abandoned from agricultural use in 1996 (“De Mossel”, Ede, The Netherlands, 52° 04´ N, 5° 45´ E) characterised as holtpodzol sandy loam (94% sand, 4% silt, 2% clay, ~ 5% organic matter, 5.2 pH, 1060 mg kg−1 N, 75 mg P2O5 100 g−1 P, 1.9 mmol K kg−1). After the seeds sprouted, each pot of grass seedlings was thinned to obtain a similar visual density. During the entire growing period, the grasses were grown in a glasshouse with climate control (light regime 16:8 h day:night, day temperature 21 °C, night temperature 16 °C) and watered and weeded as needed. D. flexuosa was re-sown due to poor germination rates. Treatments consisted of aboveground herbivory, belowground herbivory, both above- and belowground herbivory and a no herbivory control (n = 5 for each treatment per plant species, yielding a total of 120 pots). All plants were grown in hanging plastic mesh sleeves (BugDorm, Taiwan) for the duration of the herbivory treatment.
Belowground herbivory
The highly polyphagous root-feeding larvae of click beetles (Traugott et al. 2008) (c. 75% Agriotes lineatus and c. 25% A. obscurus), were chosen as the belowground herbivore (hereafter: wireworms). Wireworms are a ubiquitous belowground herbivore that feed upon a variety of plant species, causing significant damage to agricultural crops (e.g., potatoes, maize) (Traugott et al. 2008) and are capable of persisting in the soil for 3–5 years (Toth 1984). The wireworms were collected near Lelystad (52° 54′ 50.35" N, 5° 53′ 68.28" E) in marine sandy loam (c. 7% clay) a few weeks before the start of the experiment and stored at 4 °C until they were used in the experiment. On 20 December 2017, four holes approximately 2–3 cm deep were made in each corner of each pot receiving the belowground herbivory treatment and one wireworm was placed into each hole and covered with soil. Holes were also made in the same manner as described above in the remaining pots (i.e., those that did not receive the belowground herbivory treatment) in order to control for artefact effects. Addition of wireworms to D. flexuosa was delayed by 2 weeks due to re-seeding.
Aboveground herbivory
Caterpillars of the highly polyphagous cabbage moth (Mamestra brassicae), which is ubiquitous within the grasslands from which the plants chosen here originate (Wu et al. 2015), were placed on the plants receiving the aboveground herbivory treatments. The eggs from M. brassicae were obtained from the Department of Entomology at Wageningen University, The Netherlands. The colony has been maintained since at least the early 2000’s on Brassica oleracea var. gemmifera cv. cyrus (Harvey et al. 2008), the original larvae were collected from a cabbage field near Wageningen and fresh caterpillars are added yearly to prevent inbreeding effects. Previous work has shown that M. brassicae performs well and sometimes even prefers grasses over forbs (Heinen et al. 2020). Upon hatching, the larvae were reared in separate groups of 200–300 larvae and provided with artificial diet (140 g agar dissolved in 5 L of boiling water with addition of 800 g maize flour, 250 g beer yeast, 250 g wheat germs, 10 g sorbic acid, 8 g nipagin (methyl-4-hydroxybenzoate), 40 g ascorbic acid and 0.5 g streptomycin), which was regularly refreshed. Caterpillars were placed on the plants in three successive rounds to ensure the plants were sufficiently damaged and thereby the quality of the litter they produced was affected. In the first round of herbivory (20 December 2017), two early L3 larvae were selected and placed on the grass monocultures using a fine-hair brush. In the second round of herbivory (26 December 2017), five late L1 larvae were added to the monocultures, followed by a third round of herbivory (3 January 2018) in which an additional five L2 larvae were added to the monocultures. Larvae at different stages were added to increase the chances of successful establishment. Addition of caterpillars to D. flexuosa was delayed by 2 weeks due to re-seeding.
About one month after the herbivory treatments were initiated (19 January 2018), aboveground damage to the plants was assessed visually as an estimate of total surface area consumed by the caterpillars, and expressed as a percentage of the total surface area. (Please note: Both M. brassicae and Agriotes spp. larvae were still present on the plants and under the ground, respectively.) Assessment of D. flexuosa was delayed by 2 weeks. Regrowth of biomass after herbivory was substantial in A. capillaris and F. ovina, whereas in H. lanatus, A. elatius and L. perenne, regrowth was comparatively less. As a result, in A. capillaris and F. ovina, the estimates of herbivory were comparatively low. Aboveground herbivore visual estimation of damage on the plants exposed to aboveground and above-belowground herbivory ranged between 5–25%. Respectively, aboveground and above-belowground herbivory damage values were as follows: A. capillaris: 5 ± 0% and 5 ± 0%; A. elatius: 16 ± 2% and 15 ± 3%; D. flexuosa: 9 ± 2% and 13 ± 3%; F. ovina: 9 ± 2% and 9% ± 2%; H. lanatus: 21 ± 2.2% and 17 ± 7%; and for L. perenne 8 ± 1% and 10 ± 2%. No aboveground herbivore damage was observed on plants from the control and belowground herbivory treatments. It was not possible to assess belowground herbivore damage, but given that Agriotes spp. are polyphagous and voracious feeders (Hermeziu 2021; Traugott et al. 2008), it is very likely they caused severe damage to the roots of the plants in the belowground and above-belowground herbivory treatments.
After damage was assessed, we stopped watering the plants so that they senesced and their litter could be collected. Again, for D. flexuosa this was delayed by 2 weeks. It is well known that drought causes changes to the chemical composition of plant root and shoot litter (He and Dijkstra 2014; Varela et al. 2016). However, obtaining the litter for this experiment via drought was the only feasible way to ensure the production of enough dead litter in a timely manner. After c. 3 weeks (5 February 2018) the plants were fully senesced and root and shoot litter were harvested. A subsample of litter from each plant was taken, freeze dried and set aside for elemental analyses (see below). Shoots were carefully detached from roots and placed in a paper bag. Roots were then washed clean and left to air-dry overnight, then placed in a paper bag. Both roots and shoots were oven-dried at 40 °C for a minimum of 72 h, and weighed to determine total biomass. Six randomly selected subsamples of 0.5 g of both shoot and root litter were collected from each of the 120 pots. These samples served as litter sources in the decomposition phase of the experiment.
Litter chemistry analyses
In order to make mechanistic links between litter properties and changes to plant growth during the litter feedback phase (see below), analyses on litter chemistry were performed. After harvest, a subsample of the shoot and root tissue from each pot was also analysed for total carbon (C) and nitrogen (N) content. Each subsample was ground with a ball mill (Schwingmühle Qiagen Tissue Lyser II, Hilden, Germany), placed into a tin capsule and then analysed using a Flash EA1112 elemental analyser (Thermo Fisher Scientific, Inc., Waltham, MA, USA). An additional subsample of ground shoot and root tissue was analysed for total polyphenolic concentrations using the Folin-Denis method (Folin and Denis 1915; Hagerman and Butler 1989). Briefly, 25 mg of freeze-dried root and shoot litter was extracted in 5 ml of a 50:50 2.4 M HCl:MeOH solution heated to 90 °C for 2 h. Extracts were then centrifuged for 10 m at 5000 rpm and the top 2 ml pipetted into an Eppendorf tube and stored at 20 °C until analysis. Upon analysis, extracts were warmed to room temperature. Then, 200 µL was placed into a 2 mL Eppendorf tube along with 200 µL of Folin-Denis reagent and 1 mL of 1.6 M sodium carbonate. Tubes were vortexed for 10 s and then allowed to incubate on the lab bench for 30 m before being centrifuged for 5 m at 14,000 rpm. The top layer of liquid was pipetted into a 96-well plate and absorption was read at 750 nm using on plate reader with Gen5 software (version 1.11.5, BioTek Instruments, Inc., Winooski, Vermont, USA).
Further, a subset of root litter samples (four herbivory treatments × six grass species × three replicates = 72) were analysed for (micro)nutrient concentrations. Root material was oven dried at 70 °C for at least 48 h. Next, 20 mg of dried root material was transferred to glass digestion vials (MG5, Anton Paar GmbH). A mixture of 250 μL 69% HNO3 and 125 μL of 30% H2O2 was added to each vial. The vials were closed with special PEEK screw caps (MG5, Anton Paar GmbH) and disposable PFTE lip-type seals (Anton Paar GmbH) capable of tolerating high temperatures and pressures. Sample digestion was carried out in a microwave oven (Multiwave ECO, Anton Paar GmbH) mounted with a 64-position rotor (64MG5, Anton Paar GmbH). A 10 m ramping period was used to a maximum temperature of 140 °C. The samples were kept at this temperature for 80 m after which the digested samples were left to cool for 10 m. The samples were then transferred to a − 20 °C freezer for 30 m followed by the quick release of the pressure of all samples. This cooling step prevents the loss of volatile elements such as S. Finally, samples were diluted with Milli-Q water to a final concentration of 3.33% HNO3 and filtered using a Whatman Puradisc Aqua 30 filter with CA membrane. Samples were then analysed for Aluminium, (Al), Copper (Cu), Iron (Fe), Potassium (K), Manganese (Mn), Sodium (Na), Nickel (Ni), Phosphorus (P), Sulphur (S) and Zinc (Zn) by an inductively coupled plasma-optical emission spectrometer (ICP-OES, Thermo Scientific iCAP 6500 Duo Instrument with axial and radial view and CID detector microwave digestion system).
Litter feedback phase
On 5–8 March 2018, 1 L pots were filled with 1 kg of soil (a mixture of 90% gamma-irradiated soil and 10% live soil; live soil was collected from the field on 5 March 2018; see above). Using a randomized block design, pots were placed in the glasshouse under the same conditions as mentioned above and watered freely to allow the soil to settle and microbial activity to re-establish. On 12 March 2018, the collected six 0.5 g litter subsamples from all 120 pots from the herbivory treatment phase were placed into individual pots and allowed to decompose in preparation for the litter feedback phase. This resulted in a design as follows: four insect litter legacy treatments (aboveground herbivores, belowground herbivores, both above- and belowground herbivores, or no herbivores) × six ‘litter’ grass species (A. capillaris, A. elatius, D. flexuosa, F. ovina, H. lanatus, L. perenne) × two litter types (shoot, root) × six ‘response’ grass species (A. capillaris, A. elatius, D. flexuosa, F. ovina, H. lanatus, L. perenne) × five replicate blocks = 1,440 pots. In addition, each block included two control pots containing no litter × six response grass species (A. capillaris, A. elatius, D. flexuosa, F. ovina, H. lanatus, L. perenne) × five replicate blocks = 60 no-litter control pots for a total of 1,500 pots (Fig. 1). Due to limited litter production of some species (i.e., D. flexuosa and F. ovina), some replicates were lost, leaving a total of 1,404 pots. Litter was placed onto the surface of each pot and gently pressed into the surface of the soil and then approximately 2 cm of fine quartz sand was placed on top of the litter in order to ensure that the litter was full covered and in contact with the soil substrate below. This helped to retain moisture to ensure decomposition took place and prohibited oviposition into the pots by fungus gnats (superfamily Sciaroidea). Pots were watered as needed over the next three weeks to ensure adequate moisture for decomposition.
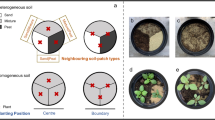
Conceptual diagram of the design of the experiment, which involved exposing fast- (Ae = Arrhenatherum elatius, Hl = Holcus lanatus, Lp = Lolium perenne) and slow- (Ac = Agrostis capillaris, Df = Deschampsia flexuosa, Fo = Festuca ovina) growing grass species to different herbivory treatments (control = no herbivore, aboveground = Mamestra brassicae, belowground = Agriotes spp., above- and belowground Mamestra brassicae + Agriotes spp.), collecting their litter and then growing the same fast- and slow-growing species with the collected litter in a full-factorial experiment. No-litter controls were included to calculate the relativized response of the species to the litter treatments (panel A). Experimental pots before planting, after the litter treatments had been administered. The block design can be clearly seen (panel B). The response plant species several weeks after the feedback phase of the experiment began. Sand was added to the upper layer of the pots to prevent fungus gnat colonisation (panel C)
On 15 March 2018, seeds from the six test species were sown onto sterilised glass beads, watered thoroughly, and placed into the glasshouse (same conditions as mentioned above) to allow for germination. Once species grew large enough for transplantation (approximately 2–3 cm in height), they were moved to the cool room and kept at 4 °C to arrest further growth. On 5 April 2018, a single seedling of the six grass species mentioned above was planted into each pot. All pots were checked daily and watered as necessary and dead seedlings were replaced up until ten days after the initial planting. A total of 57 seedlings were replaced (A. capillaris = 28, A. elatius = 1, D. flexuosa = 8, F. ovina = 3, H. lanatus = 17, L. perenne = zero), which constitutes 4.1% of the total experimental units. Of these 57 seedlings, 33 (c. 58%) were replaced within three days after the initial planting. After the last replacement of dead seedlings, a total of 1.1% of the plants died before the end of the experiment. Beginning on 16 April 2018, each pot was watered every other day with 50 mL of tap water until the harvest of the experiment.
Between 22 and 28 May 2018, the experiment was destructively harvested. Shoots were clipped at the meristem and placed into a paper bag on the first day of the harvest. Pots were subsequently stored in the dark at room temperature until the roots could be washed. Roots were carefully washed clean of soil over a 4 mm sieve and placed on a paper towel overnight to air dry before they were placed into a paper bag. Remaining root litter from the litter treatments was separated from live roots (i.e., the colour and texture were different), while virtually all the shoot litter had decomposed during the course of the experiment and therefore posed no issue. All roots and shoots were placed into an oven and dried for a minimum of 72 h at 40 °C before dry weights were measured. (Please note: for the species D. flexuosa when it was grown with root litter, there were numerous instances where the plants and their root systems were so small that it was not possible to disentangle the roots from the litter. In this case, root measurements could not be taken, but shoot measurements were still recorded. Further, due to contamination in the D. flexuosa seed batch, 12 individuals were actually a Poa spp. instead. These plants were dropped from subsequent analyses.)
Statistical analyses
All plant data from the glasshouse experiment were analysed using mixed effect models. Data collected after the litter conditioning phase on the root and shoot litter characteristics carbon, nutrients and total polyphenols and root litter nutrients (i.e., Al, Cu, Fe, K, Mn, Na, Ni, P, S and Zn) were analysed in two models. To test for effects of herbivory on litter quality and plant growth rate category on litter properties, the first model included herbivory (control (litter exposed to no insect herbivory), aboveground herbivory (Mamestra brassicae), belowground herbivory (Agriotes spp.), above- and belowground (Mamestra brassicae + Agriotes spp.)) and growth rate category of the species from which the litter was obtained (fast versus slow) as fixed factors. The second model tested for the effects of herbivory and litter species identity on litter properties (i.e., the different grass species from which litter was derived), with both considered fixed factors. In both models, block (i.e., the randomized block design into which all the pots were placed in the glasshouse) was included as a random factor and all interactions were specified.
In order to relativize the response species data collected during the litter feedback phase, the root and shoot biomass of each response plant of each species was subtracted from the average respective root and shoot biomass of the no-litter controls of the same species (i.e., two no-litter control per species present in each of the five blocks for a total of ten no-litter control units across all five blocks). For example, the root biomass of a L. perenne replicate grown with litter exposed to belowground herbivory was subtracted from the average root biomass of the ten L. perenne plants grown without added litter designated for comparison to plants grown with root litter. To investigate our four hypotheses, we created three different models. All models allowed us to test our first hypothesis on the effects of above-belowground herbivory on plant growth response. In the first model, herbivory (as described above), litter type (root versus shoot litter) and response compartment (root or shoot of the response plants) were included as fixed factors, with all interactions specified. The response variable was the relativized value (see above) of the roots and shoots of the response plant; both root and shoot responses were included in the same analysis. Therefore, to account for autocorrelation, the sample identity (i.e., individual from which a particular pairing of root and shoot measurements originated) was included as a random factor. Block, litter species identity and response species identity (i.e., the different grass species that were used as response species) were also included as random factors. This model allowed us to investigate our second hypothesis regarding interactive effects of root versus shoot litter exposed to herbivory on the different compartments (i.e., roots versus shoots) of the response plant species. In the second model, herbivory, litter growth rate category and response species growth rate category (fast versus slow) were included as fixed factors, with all interactions specified. Random factors were as specified in the first model. The response variables were the standardized responses of the roots and shoots of the response plants. Plant roots and shoots that were grown with root versus shoot litter were analysed separately, resulting in four analyses: relativized responses of roots and shoots grown with root litter and relativized responses of roots and shoots grown with shoot litter. This model allowed us to interrogate our third hypothesis regarding the effect of plant growth rate category on the PLSF pathway. In the third model, herbivory, litter species identity and response species identity were included as fixed factors and block was included as a random factor. Response variables were the same as in the second model. The third model was also used to analyse the raw root and shoot biomass data in order to give an overall picture of the raw responses to the treatments. This model allowed us to investigate our fourth hypothesis regarding species-specific effects of litter and herbivory on the response plants. In the three models described immediately above, herbivory was originally analysed as a binary response (i.e., aboveground herbivory yes/no, belowground herbivory yes/no), but these models generated results that were not drastically different from the models that considered herbivory as a single variable with four categories. Therefore, for the sake of simplicity and ease of interpretation, the above models were used.
All of the models described above included an a priori selection of random factors based on the experimental design. However, all possible combinations of random factors listed for each model were compared using the AICcmodavg package in R (Mazerolle and Mazerolle 2017) and the best selection of random factors for each response variable was chosen. Please see footnotes in ANOVA Tables. (Please note: no significant effects were altered when random factors were dropped from the models in accordance with the AIC selection criteria.) All data were transformed as necessary to meet the model assumptions; see ANOVA tables for details. Restricted maximum likelihood (REML) estimation was used to produce an unbiased estimate of variation and covariation (Patterson and Thompson 1971) and Kenward-Roger degrees of freedom approximation was used to reduce bias introduced by a relatively small sample size (Kenward and Roger 1997). Analyses were performed using R software (R Core Team 2020) with the packages lme4/lmerTest (Bates et al. 2015; Kuznetsova et al. 2017). Please note: all root and shoot biomass data presented in Figs. 2–4 were relativized (see above), while raw root and shoot biomass data are presented in Fig. S1.
The effect of root and shoot litter on the relativized responses of roots and shoots of grass species (Agrostis capillaris, Arrhenatherum elatius, Deschampsia flexuosa, Festuca ovina, Holcus lanatus, Lolium perenne) grown with litter that had received different herbivory treatments (control = no herbivore, aboveground = Mamestra brassicae, belowground = Agriotes spp., above- and belowground Mamestra brassicae + Agriotes spp.). Across both panels, groups of bars topped with different uppercase letters differ at p < 0.05 (Tukey’s HSD). Panels show 1st quartile above and below the medians (i.e., line inside each bar), the minimum and maximum values, excluding outliers (i.e., whiskers) and the outliers (i.e., black dots). Data presented are relative change in grams dry weight biomass (g dw) between herbivory litter treatments and no-litter control treatment averages. ANOVA results are presented in Table 1
Results
Litter conditioning phase
Exposure to above- and belowground insect herbivory did not result in changes to root or shoot litter carbon, nitrogen or total polyphenol concentrations (Tables S1-S4). However, compared to roots, shoot C, N and total polyphenol concentrations were c. 3.5%, 24% and 3.5% higher, respectively (Table S1). Root C was 7.3% higher in slow- than in fast-growing plants (Table S2). Analyses on root and shoot C, N and total polyphenol concentrations revealed many species-specific differences; detailed descriptions go beyond the scope of the main text, but fully detailed descriptions can be found in Tables S3-S4 and Text S1.
Analyses on root (micro)nutrient concentrations revealed numerous differences generated by herbivory, litter species identity and their interactions, but litter growth rate category never affected any of the nutrient concentrations (Tables S5-S8). According to the models testing for herbivory and litter growth rate category, as well as herbivory and litter species identity effects, root litter Na concentrations were 14% lower in litter explosed to belowground herbivory only versus litter exposed to both above- and belowground herbivory, but neither differed from control litter or litter exposed to aboveground herbivory (Table S5-S6). According to the model testing for herbivory and litter species identity effects, Ni root litter concentrations were affected by herbivory, but post-hocs revealed no true significant differences (Table S6). However, there was a significant interaction between litter species identity and herbivory on root litter Fe and Ni concentrations, which resulted in numerous species-specific effects (Table S6). For the sake of brevity, these effects will not be described in detail, but means and standard errors, along with post-hoc results can be found in Table S8. Numerous additional species-specific differences between root and shoot litter nutrient concentrations were detected, which are described in detail in Text S1.
Herbivory and root versus shoot litter interactive effects on the PLSF pathway
Although there were no significant interactions between herbivory and litter type (root versus shoot litter) on response plant growth, there was a significant difference between root versus shoot growth in the response plants independent of litter type (root versus shoot) (Table 1, Fig. 2). Specifically, response plant shoot growth was overall negatively affected by litter addition, while root growth was less negatively affected by litter (-0.042 ± 0.010 versus -0.014 ± 0.010).
Herbivory and plant economic spectrum interactive effects on the PLSF pathway
The model to assess the effect of herbivory and plant growth rate category (litter growth rate category and response plant growth rate category) on the PLSF pathway revealed a significant three-way interaction between herbivory, litter species growth rate category and response plant species growth rate category on shoot biomass growth when plants were grown with root litter (Table 2, Fig. 3). However, post-hoc tests revealed no true significant differences. No other significant main or interactive effects of herbivory, litter species growth rate category and/or response species growth rate category were detected.
Relativized responses of root and shoot biomass of fast- (Arrhenatherum elatius, Holcus lanatus, Lolium perenne) and slow- (Agrostis capillaris, Deschampsia flexuosa, Festuca ovina) growing grass species that were grown with root (panels A, B) and shoot (panels C, D) litter that had received different herbivory treatments (control = no herbivore, aboveground = Mamestra brassicae, belowground = Agriotes spp., above- and belowground Mamestra brassicae + Agriotes spp.). Panels show 1st quartile above and below the medians (i.e., line inside each bar), the minimum and maximum values, excluding outliers (i.e., whiskers) and the outliers (i.e., black dots). Data presented are relative change in grams dry weight (g dw) biomass between herbivory litter treatments and no-litter control treatment averages. ANOVA results are presented in Table 2
Species-specific effects on the PLSF pathway
The model to assess the species-specific effects of litter and response species identity revealed many response species identity effects (but no litter species identity effects) and an interactive effect between herbivory and response species identity (Table 3, Fig. 4). Shoot biomass response to root litter was positive for L. perenne and A. capillaris (average 0.095 ± 0.040), but negative for all other species. Both L. perenne and A. capillaris differed significantly from H. lanatus and A. elatius (average -0.181 ± 0.033), while no differences were detected between A. capillaris versus F. ovina and F. ovina versus H. lanatus (Fig. 4A). Root biomass response to root litter was positive for A. elatius and H. lanatus (average 0.123 ± 0.033), but negative for all other species. Both A. elatius and H. lanatus differed significantly from A. capillaris and L. perenne (average -0.152 ± 0.029), while no differences were detected between F. ovina versus A. capillaris and A. capillaris versus L. perenne (Fig. 4B). Shoot biomass response to shoot litter was positive for H. lanatus (0.109 ± 0.026) when compared to L. perenne (-0.063 ± 0.028) and F. ovina (-0.325 ± 0.022), but there was no difference in response between H. lanatus versus A. capillaris, A. elatius and D. flexuosa, nor the latter three species and L. perenne (Fig. 4C). Root biomass response to shoot litter was c. 15 times more positive for H. lanatus compared to A. capillaris, A. elatius, D. flexuosa and L. perenne (0.244 ± 0.032 versus 0.016 ± 0.013 averaged across the latter four species). Compared to the other species, F. ovina had a strong negative response (-0.396 ± 0.019). There was a significant interactive effect between herbivory and response species identity on root biomass when plants were grown with shoot litter. For the sake of brevity, these effects will not be described here, but post-hoc letters can be found in Fig. 4D. Raw root and shoot biomass data and accompanying statistical analyses can be found in Fig. S1 and Table S9, respectively. In summary, species-specific effects were the most dominant aspect of the PLSF pathway.
Relativized responses of root and shoot biomass of grass species (Agrostis capillaris, Arrhenatherum elatius, Deschampsia flexuosa, Festuca ovina, Holcus lanatus, Lolium perenne) that were grown with root (panels A, B) and shoot (panels C, D) litter that had received different herbivory treatments (control = no herbivore, aboveground = Mamestra brassicae, belowground = Agriotes spp., above- and belowground Mamestra brassicae + Agriotes spp.). Within each panel, response species followed by different uppercase letters differ at p < 0.05 (Tukey’s HSD). Within panel D, bars topped with different lowercase letters differ at p < 0.05 (Tukey’s HSD). Panels show 1st quartile above and below the medians (i.e., line inside each bar), the minimum and maximum values, excluding outliers (i.e., whiskers) and the outliers (i.e., black dots). Data presented are relative change in grams dry weight (g dw) biomass between herbivory litter treatments and no-litter control treatment averages. ANOVA results are presented in Table 3
Discussion
Here, we examined the effects of above- and belowground insect herbivory on the root and shoot litter quality of three fast- and three slow-growing grass species and the subsequent effects on response plant growth as mitigated via the plant-litter-soil feedback (PLSF) pathway. We found that insect herbivory resulted in few subtle changes to litter quality (i.e., nutrient concentrations), and that insect herbivory did not translate to alterations in the growth of the response plants via the PLSF pathway. Further, in contrast to our hypothesis, there were no significant alterations to response plant growth when plants were grown with fast- versus slow-growing plant litter. However, litter addition (regardless of litter type; i.e., root versus shoot) negatively affected root and shoot growth, but this effect was significantly more negative for the shoots of the response plants versus their roots. We also found an interactive effect on root growth of shoot litter exposed to insect herbivory and response plant identity. Below we discuss possible mechanisms for these effects and relate our findings to potential implications for the PLSF pathway.
Herbivory effects on the PLSF pathway
Our first hypothesis that herbivory would negatively impact on the PLSF pathway was not supported because litter exposed to above- and/or belowground herbivory did not result in changes to plant growth. Despite evidence that insect herbivory can change both leaf nutrient (Nykänen and Koricheva 2004) and polyphenol (Kaplan et al. 2008) concentrations, we found no differences in C, N and polyphenol concentrations across herbivory treatments. This may have been the result of the plants resorbing nutrients before senescence (Vergutz et al. 2012). Further, secondary metabolites such as polyphenols (that are generally present in lower concentrations in grasses versus forbs (Geisen et al. 2022)) that might be responsible for litter allelopathic effects may have broken down rapidly (Bokhari 1978; García Palacios et al. 2016), resultantly erasing chemical differences usually found in live tissue. In a broader sense, polyphenol concentrations could respond differently to herbivory in woody species. For example, Quercus spp. that where defoliated by Lymantria dispar L. (spongy moth) showed an increase in polyphenols, particularly tannins, when the leaves regenerated (Schultz and Baldwin 1982). Tannins and other polyphenols are known to strongly affect nutrient cycling in forests (Kraus et al. 2003). If such herbivore-induced effects persisted after senescence, stronger PLSFs might be realized than were observed here. Finally, although not measured in the current study, the presence of insect frass, which is increasingly being recognized as a driver of soil nutrient dynamics (Poveda 2021), may have had an effect. Although frass pellets generally drop to the soil, a potential microbial (i.e., litter phylloplane) or chemical (i.e., litter quality) contamination of litter cannot be ruled out. Furthermore, frass itself could also be an important direct driver of plant performance (Kagata and Ohgushi 2012). Therefore, the direct and indirect involvement of frass in the interactions between herbivores and PSFs warrants future study.
Herbivory and root versus shoot litter interactive effects on the PLSF pathway
Our second hypothesis that root litter will generate more negative PLSFs than shoot litter, but will vary with herbivory, was not supported because both root and shoot litter resulted in negative effects on response plant growth. Further, there were no interactions between root or shoot litter and herbivory. Herbivory resulted in few changes to litter chemistry, making it unsurprising that interactions with root versus shoot litter did not manifest nor lead to subsequent changes in response plant growth. Yet, independent of herbivory treatment or litter type (root versus shoot), litter had a negative effect on response plant root and shoot growth, but shoots responded more negatively than roots. This (partially) supports other work that has shown inhibitory effects of litter on plant growth (Aldorfová et al. 2022; Veen et al. 2019b; Zhang et al. 2016). It is possible that the litter carried over pathogens that infected the roots of the response plants, leading to the overall negative effects of litter; something that has been suggested in other studies (Aldorfová et al. 2022; van der Putten 2003; Zhang et al. 2016). However, since pathogen carryover was not measured in the current experiment, this cannot be confirmed. Although this finding reinforces the idea that it is important to investigate the different responses of roots and shoots to PLSFs, it must be noted that the reduction of response plant biomass was relatively small. Across all response species, roots were on average 0.998 g and lost 0.014 g (c. 1.402% decrease), while shoots were on average 1.345 g and lost 0.042 g (c. 3.111% decrease) when grown with litter. Therefore, it is likely that this effect is of minimal broad ecological relevance in this system. Finally, the duration of this experiment was likely too short to realise the long-term compensatory effects of this allocation, which is likely relevant for these perennial grass species in the long-term.
Herbivory and plant economic spectrum interactive effects on the PLSF pathway
Our third hypothesis that litter from fast-growing plant species would generate more positive PLSF effects was not supported because litter from fast- versus slow-growing species did not generate contrasting growth responses. Despite slow-growing root litter having 7% higher total C concentrations, which can be an indicator of more recalcitrant litter and thereby inhibited nutrient release (Cornwell et al. 2008), we found minimal effects of litter growth rate category on the PLSF pathway. There was a three-way interaction between herbivory, litter growth rate category and response plant species rate category on shoot growth when grown with root litter, but post hoc tests detected no true differences, and hence this effect is unlikely to be ecologically relevant. There were no direct or interactive effects of fast- versus slow-growing litter and response species, which may have been because most grasses are positioned closely on the fast-growing side of the plant economic spectrum. In essence, although the grasses used in our study significantly differed from one another in terms of growth rates, grasses in general are relatively fast-growing. As a result, these grasses may have very similar ranges of litter quality that are rather labile and easily decomposable, and lower in secondary metabolites (Defossez et al. 2021), which typically inhibit decomposition and nutrient release (Chomel et al. 2016; Osono and Takeda 2004). Stronger effects of plant growth rate category on the PLSF pathway could be expected if species further apart on the plant economic spectrum were selected, with starker differences in litter nutrient content and secondary metabolites (Díaz et al. 2016).
Species-specific effects on the PLSF pathway
Our fourth hypothesis was partially supported because there were some significant main and interactive effects of herbivory, litter type and response species identity on plant growth. This finding supports other work showing species-specific litter feedback effects (Bueno de Mesquita et al. 2019; Coq et al. 2012). Here, for example, L. perenne showed reduced root growth in response to shoot litter that had been exposed to above-belowground herbivory versus only belowground herbivory. This effect could have been caused by changes to the litter that we did not measure here, such as the litter microbiome (Veen et al. 2019a), that only manifested when plants were exposed to both above- and belowground herbivory. However, no other intraspecific effects of herbivory-exposed litter on response plant growth were detected, suggesting species-specific herbivory effects may be of little ecological significance. On the other hand, species-specific responses to different litter types played a stronger role. One noteworthy example: H. lanatus had an overall positive root growth response to shoot litter, while F. ovina showed a negative response. This contrasts the overall effect of litter on root growth, where litter elicited a negative response. This indicates that species-specific effects can be masked when only composite effects are explored, which is in line with Simpson’s Paradox that suggests the response patterns of individuals may disappear or reverse at the community level (Wagner 1982). Considering the response of individual species to the PLSF pathway is critical, as litter effects could influence dominant and subordinate species responses, with implications for plant community composition (Hassan et al. 2021).
Potential caveats
It is important to highlight a number of potential caveats that may have influenced the results and conclusions presented here. First, the duration (70 days) of the feedback phase of the experiment was rather short because we wanted to avoid the plants becoming root bound in the pots and thereby eclipsing potential differences. Harvesting at this time prevented the plants from (dramatically) exceeding the recommended 1 g plant biomass per liter soil in glasshouse experiments (Poorter et al. 2012). However, plants were likely still building up both above- and belowground biomass, which could have influenced the effects/lack of effects observed. Second, 57 seedlings were replaced during the first 10 days of the litter feedback phase of the experiment began. This constitutes a relatively small percentage of the total experimental plants (c. 4.1%) and such seedling replacement is common within the first two weeks of greenhouse pot experiments that run from 8–12 weeks (Kardol et al. 2007; Kostenko et al. 2012; Spitzer et al. 2021). However, given that the experiment ran for 70 days before harvest, there is a possibility that the full potential feedback effects were not realized on these particular plants. Third, all of the grass species considered here are perennials (Fitter et al. 1992), meaning that the relatively short duration of the litter feedback phase may have detected effects that disappeared later on in the lifespan of the plant or vice versa. Fourth, litter was obtained by killing the plants via drought, which is known to change root and shoot chemistry (Suseela and Tharayil 2018). Fifth, the experiment was conducted under controlled glasshouse conditions with litter buried to a uniform depth, unnatural positioning of litter (i.e., root litter remains in the place where is senesces, while shoot litter is more mobile) and no competition between plants. This could mean that the effects observed here may not hold under field conditions where confounding factors are prevalent. Future studies on the PLSF pathway should utilize experiments with longer feedback phases under more natural conditions.
Conclusions
Although it is well known that insect herbivory induces strong chemical changes in live plant tissues, we show here that effects on litter chemistry are minimal. This may have been due to resorption during senescence or a delay in manifestation of changes that would have occurred later in the life cycle of the plants (e.g., during reproduction). Consequently, effects of insect herbivory on the PLSF pathway were minimal and species-specific. Further, no interactions with fast- versus slow-growing plants (i.e., plant economic spectrum) and insect herbivory on response plant growth were seen, perhaps due to the relatively fast-growing nature of grasses when the entire economic spectrum is considered. An interesting follow up should consider herbivory effects on the litter of plant species that sit further apart on the economic spectrum, with starker differences in initial litter chemistry. Overall, litter amendment demonstrated a small negative impact on the growth of the response plants, with this effect being more negative in shoots versus roots. This finding demonstrates that litter might play a small role in grassland systems, but species-specific effects should be considered. These results pull focus on the need to investigate potential implications of the PLSF pathway on determining plant performance across other species and ecosystems before definitive conclusions can be reached.
Data availability
Data associated with this manuscript is available via Dryad, https://doi.org/10.5061/dryad.fbg79cnxp.
Change history
05 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11104-022-05637-5
Abbreviations
- PLSF:
-
plant-litter-soil feedback
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 79:439–449. https://doi.org/10.2307/3546886
Aldorfová A, Dostálek T, Münzbergová Z (2022) Effects of soil conditioning, root and shoot litter addition interact to determine the intensity of plant–soil feedback. Oikos n/a: e09025. https://doi.org/10.1111/oik.09025.
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. 2015 67: 1–48. https://doi.org/10.18637/jss.v067.i01.
Bokhari U (1978) Allelopathy among prairie grasses and its possible ecological significance. Ann Bot 42:127–136. https://doi.org/10.1093/oxfordjournals.aob.a085432
Bueno de Mesquita CP, Schmidt SK, Suding KN (2019) Litter-driven feedbacks influence plant colonization of a high elevation early successional ecosystem. Plant Soil 444:71–85. https://doi.org/10.1007/s11104-019-04242-3
Burghardt KT, Bradford MA, Schmitz OJ (2018) Acceleration or deceleration of litter decomposition by herbivory depends on nutrient availability through intraspecific differences in induced plant resistance traits. J Ecol 106:2380–2394. https://doi.org/10.1111/1365-2745.13002
Chapman SK, Hart SC, Cobb NS, Whitham TG, Koch GW (2003) Insect herbivory increases litter quality and decomposition: an extension of the acceleration hypothesis. Ecology 84:2867–2876. https://doi.org/10.1890/02-0046
Chomel M, Guittonny-Larcheveque M, Fernandez C, Gallet C, DesRochers A, Pare D, Jackson BG, Baldy V (2016) Plant secondary metabolites: a key driver of litter decomposition and soil nutrient cycling. J Ecol 104:1527–1541. https://doi.org/10.1111/1365-2745.12644
Coq S, Weigel J, Bonal D, Hattenschwiler S (2012) Litter mixture effects on tropical tree seedling growth - a greenhouse experiment. Plant Biol 14:630–640. https://doi.org/10.1111/j.1438-8677.2011.00534.x
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Diaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071. https://doi.org/10.1111/j.1461-0248.2008.01219.x
Defossez E, Pitteloud C, Descombes P, Glauser G, Allard P-M, Walker TW, Fernandez-Conradi P, Wolfender J-L, Pellissier L, Rasmann S (2021) Spatial and evolutionary predictability of phytochemical diversity. Proc Natl Acad Sci 118. https://doi.org/10.1073/pnas.2013344118.
Dias ATC, Cornelissen JHC, Berg MP (2017) Litter for life: assessing the multifunctional legacy of plant traits. J Ecol 105:1163–1168. https://doi.org/10.1111/1365-2745.12763
Díaz S, Kattge J, Cornelissen JHC, Wright IJ, Lavorel S, Dray S, Reu B, Kleyer M, Wirth C, Prentice IC, Garnier E, Bönisch G, Westoby M, Poorter H, Reich PB, Moles AT, Dickie J, Gillison AN, Zanne AE, Chave J, Wright SJ, Sheremet’ev SN, Jactel H, Baraloto C, Cerabolini B, Pierce S, Shipley B, Kirkup D, Casanoves F, Joswig JS, Günther A, Falczuk V, Rüger N, Mahecha MD, Gorne LD (2016) The global spectrum of plant form and function. Nature 529:167–173. https://doi.org/10.1038/nature16489
Elberse WT, Berendse F (1993) A comparative study of the growth and morphology of eight grass species from habitats with different nutrient availabilities. Funct Ecol 223–229:2389891
Faucon M-P, Houben D, Lambers H (2017) Plant functional traits: soil and ecosystem services. Trends Plant Sci 22:385–394. https://doi.org/10.1016/j.tplants.2017.01.005
Fitter R, Fitter A, Farrer A (1992) Grasses Sedges, Rushes and Ferns of Britain and Northern Europe, Collins Pocket Guide. Harper Collins Manufacturing, Glasgow.
Folin O, Denis W (1915) A colorimetric method for the determination of phenols (and phenol derivatives) in urine. J Biol Chem 22:305–308. https://doi.org/10.1016/S0021-9258(18)87648-7
Freschet GT, Aerts R, Cornelissen JHC (2012) A plant economics spectrum of litter decomposability. Funct Ecol 26:56–65. https://doi.org/10.1111/j.1365-2435.2011.01913.x
Freschet GT, Cornwell WK, Wardle DA, Elumeeva TG, Liu WD, Jackson BG, Onipchenko VG, Soudzilovskaia NA, Tao JP, Cornelissen JHC (2013) Linking litter decomposition of above- and below-ground organs to plant-soil feedbacks worldwide. J Ecol 101:943–952. https://doi.org/10.1111/1365-2745.12092
García Palacios P, Shaw EA, Wall DH, Hättenschwiler S (2016) Temporal dynamics of biotic and abiotic drivers of litter decomposition. Ecol Lett 19:554–563. https://doi.org/10.1111/ele.12590
Gatehouse JA (2002) Plant resistance towards insect herbivores: a dynamic interaction. New Phytol 156:145–169. https://doi.org/10.1046/j.1469-8137.2002.00519.x
Geisen S, Heinen R, Andreou E, van Lent T, Ten Hooven FC, Thakur MP (2022) Contrasting effects of soil microbial interactions on growth–defence relationships between early-and mid-successional plant communities. New Phytol 233:1345–1357. https://doi.org/10.1111/nph.17609
Hagerman AE, Butler LG (1989) Choosing appropriate methods and standards for assaying tannin. J Chem Ecol 15:1795–1810. https://doi.org/10.1007/bf01012267
Harvey JA, Bezemer TM, Gols R, Nakamatsu Y, Tanaka T (2008) Comparing the physiological effects and function of larval feeding in closely-related endoparasitoids (Braconidae: Microgastrinae). Physiol Entomol 33:217–225. https://doi.org/10.1111/j.1365-3032.2008.00623.x
Hassan N, Sher K, Rab A, Abdullah I, Zeb U, Naeem I, Shuaib M, Khan H, Khan W, Khan A (2021) Effects and mechanism of plant litter on grassland ecosystem: A review. Acta Ecol Sin. https://doi.org/10.1016/j.chnaes.2021.02.006
Hättenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15:238–243. https://doi.org/10.1016/s0169-5347(00)01861-9
He MZ, Dijkstra FA (2014) Drought effect on plant nitrogen and phosphorus: a metaanalysis. New Phytol 204:924–931. https://doi.org/10.1111/nph.12952
Heinen R, Biere A, Bezemer TM (2020) Plant traits shape soil legacy effects on individual plant–insect interactions. Oikos 129:261–273. https://doi.org/10.1111/oik.06812
Hermeziu M (2021) Possibility to limit the wireworms (Agriotes spp.) damages on potato crops. Romanian Agricultural Research 38:381–388
Hobbie SE, Oleksyn J, Eissenstat DM, Reich PB (2010) Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 162:505–513. https://doi.org/10.1007/s00442-009-1479-6
John J, Sarada S (2012) Role of phenolics in allelopathic interactions. Allelopathy Journal 29
Johnson SN, Hawes C, Karley AJ (2009) Reappraising the role of plant nutrients as mediators of interactions between root- and foliar-feeding insects. Funct Ecol 23:699–706. https://doi.org/10.1111/j.1365-2435.2009.01550.x
Jongen R, Hannula SE, De Long JR, Heinen R, Huberty M, Steinauer K, Bezemer TM (2021) Plant community legacy effects on nutrient cycling, fungal decomposer communities and decomposition in a temperate grassland. Soil Biology and Biochemistry: 108450. https://doi.org/10.1016/j.soilbio.2021.108450.
Kagata H, Ohgushi T (2012) Positive and negative impacts of insect frass quality on soil nitrogen availability and plant growth. Popul Ecol 54:75–82. https://doi.org/10.1007/s10144-011-0281-6
Kaplan I, Halitschke R, Kessler A, Sardanelli S, Denno RF (2008) Constitutive and induced defenses to herbivory in above- and belowground plant tissues. Ecology 89:392–406. https://doi.org/10.1890/07-0471.1
Karban R (2011) The ecology and evolution of induced resistance against herbivores. Funct Ecol 25:339–347. https://doi.org/10.1111/j.1365-2435.2010.01789.x
Kardol P, Cornips NJ, van Kempen MML, Bakx-Schotman JMT, van der Putten WH (2007) Microbe-mediated plant-soil feedback causes historical contingency effects in plant community assembly. Ecol Monogr 77:147–162. https://doi.org/10.1890/06-0502
Kenward MG, Roger JH (1997) Small Sample Inference for Fixed Effects from Restricted Maximum Likelihood. Biometrics 53:983–997. https://doi.org/10.2307/2533558
Kostenko O, van de Voorde TFJ, Mulder PPJ, van der Putten WH, Bezemer TM (2012) Legacy effects of aboveground–belowground interactions. Ecol Lett 15:813–821. https://doi.org/10.1111/j.1461-0248.2012.01801.x
Kraus TEC, Dahlgren RA, Zasoski RJ (2003) Tannins in nutrient dynamics of forest ecosystems - a review. Plant Soil 256:41–66. https://doi.org/10.1023/A:1026206511084
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. 2017 82: 26. https://doi.org/10.18637/jss.v082.i13.
Lattanzio V, Lattanzio VMT, Cardinali A (2006) Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects
Mazerolle MJ, Mazerolle MMJ (2017) Package ‘AICcmodavg’. R package 281
Nykänen H, Koricheva J (2004) Damage-induced changes in woody plants and their effects on insect herbivore performance: a meta-analysis. Oikos 104:247–268. https://doi.org/10.1111/j.0030-1299.2004.12768.x
Ohgushi T (2005) Indirect interaction webs: Herbivore-induced effects through trait change in plants. Annu Rev Ecol Evol Syst 36:81–105. https://doi.org/10.1146/annurev.ecolsys.36.091704.175523
Osono T, Takeda H (2004) Accumulation and release of nitrogen and phosphorus in relation to lignin decomposition in leaf litter of 14 tree species. Ecol Res 19:593–602. https://doi.org/10.1111/j.1440-1703.2004.00675.x
Patterson HD, Thompson R (1971) Recovery of inter-block information when block sizes are unequal. Biometrika 58:545–554. https://doi.org/10.1093/biomet/58.3.545
Poorter H, Bühler J, van Dusschoten D, Climent J, Postma JA (2012) Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Funct Plant Biol 39:839–850. https://doi.org/10.1071/FP12049
Poveda J (2021) Insect frass in the development of sustainable agriculture. A Review. Agron Sustain Dev 41:1–10. https://doi.org/10.1007/s13593-020-00656-x
R Core Team (2020) R: A Language and environment for statistical computing
Reich PB (2014) The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J Ecol 102:275–301. https://doi.org/10.1111/1365-2745.12211
Santiago LS (2007) Extending the leaf economics spectrum to decomposition: Evidence from a tropical forest. Ecology 88:1126–1131. https://doi.org/10.1890/06-1841
Scheurwater I, Koren M, Lambers H, Atkin O (2002) The contribution of roots and shoots to whole plant nitrate reduction in fast-and slow-growing grass species. J Exp Bot 53:1635–1642. https://doi.org/10.1093/jxb/erf008
Schultz JC, Baldwin IT (1982) Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217:149–151. https://doi.org/10.1126/science.217.4555.149
Schweitzer JA, Bailey JK, Hart SC, Wimp GM, Chapman SK, Whitham TG (2005) The interaction of plant genotype and herbivory decelerate leaf litter decomposition and alter nutrient dynamics. Oikos 110:133–145. https://doi.org/10.1111/j.0030-1299.2005.13650.x
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419. https://doi.org/10.1007/s004420100740
Spitzer CM, Lindahl B, Wardle DA, Sundqvist MK, Gundale MJ, Fanin N, Kardol P (2021) Root trait–microbial relationships across tundra plant species. New Phytol 229:1508–1520. https://doi.org/10.1111/nph.16982
Steinauer K, Heinen R, Hannula SE, De Long JR, Huberty M, Jongen R, Wang M, Bezemer TM (2020) Above-belowground linkages of functionally dissimilar plant communities and soil properties in a grassland experiment. Ecosphere 11:e03246. https://doi.org/10.1002/ecs2.3246
Suseela V, Tharayil N (2018) Decoupling the direct and indirect effects of climate on plant litter decomposition: Accounting for stress-induced modifications in plant chemistry. Glob Change Biol 24:1428–1451. https://doi.org/10.1111/gcb.13923
Thelen GC, Vivanco JM, Newingham B, Good W, Bais HP, Landres P, Caesar A, Callaway RM (2005) Insect herbivory stimulates allelopathic exudation by an invasive plant and the suppression of natives. Ecol Lett 8:209–217. https://doi.org/10.1111/j.1461-0248.2004.00713.x
Toth Z (1984) Click beetles (Elateridae) in the soils of Central Europe. Their distribution and description. Part I.(Gen.: Agriotes). http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=9635227.
Traugott M, Schallhart N, Kaufmann R, Juen A (2008) The feeding ecology of elaterid larvae in central European arable land: new perspectives based on naturally occurring stable isotopes. Soil Biol Biochem 40:342–349. https://doi.org/10.1016/j.soilbio.2007.08.013
van der Putten WH (2003) Plant defense belowground and spatiotemporal processes in natural vegetation. Ecology 84:2269–2280. https://doi.org/10.1890/02-0284
Varela MC, Arslan I, Reginato MA, Cenzano AM, Luna MV (2016) Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiol Biochem 104:81–91. https://doi.org/10.1016/j.plaphy.2016.03.014
Veen G, Snoek BL, Bakx-Schotman T, Wardle DA, van der Putten WH (2019a) Relationships between fungal community composition in decomposing leaf litter and home-field advantage effects. Funct Ecol 33:1524–1535. https://doi.org/10.1111/1365-2435.13351
Veen GF, Fry EL, ten Hooven FC, Kardol P, Morriën E, De Long JR (2019b) The Role of Plant Litter in Driving Plant-Soil Feedbacks. Frontiers in Environmental Science 7. https://doi.org/10.3389/fenvs.2019.00168.
Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82:205–220. https://doi.org/10.1890/11-0416.1
Vidal MC, Murphy SM (2018) Bottom-up vs. top-down effects on terrestrial insect herbivores: a meta-analysis. Ecol Lett 21:138–150. https://doi.org/10.1111/ele.12874
Vile D, Shipley B, Garnier E (2006) Ecosystem productivity can be predicted from potential relative growth rate and species abundance. Ecol Lett 9:1061–1067. https://doi.org/10.1111/j.1461-0248.2006.00958.x
Wagner CH (1982) Simpson’s Paradox in Real Life. Am Stat 36:46–48. https://doi.org/10.1080/00031305.1982.10482778
Woodman SG, Khoury S, Fournier RE, Emilson EJ, Gunn JM, Rusak JA, Tanentzap AJ (2021) Forest defoliator outbreaks alter nutrient cycling in northern waters. Nat Commun 12:1–8. https://doi.org/10.1038/s41467-021-26666-1
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Wu X, Fu X, Guo J, Zhao X, Wu K (2015) Annual migration of cabbage moth, Mamestra brassicae L.(Lepidoptera: Noctuidae), over the sea in northern China. PLoS One 10:e0132904. https://doi.org/10.1371/journal.pone.0132904
Zhang NL, Van der Putten WH, Veen GF (2016) Effects of root decomposition on plant-soil feedback of early- and mid-successional plant species. New Phytol 212:220–231. https://doi.org/10.1111/nph.14007
Acknowledgements
We would like to thank Ivor Keesmaat and Martine Huberty for help in the glasshouse and lab. Thanks to G. Ciska Veen and Jasper Wubs for discussions about the statistical analyses.
Funding
This work was funded by the Netherlands Organization for Scientific Research (NWO VICI Grant 865.14.006).
Author information
Authors and Affiliations
Contributions
J.R.D.L., R.H. and T.M.B. conceived the experimental design; J.R.D.L., R.H., S.E.H., R.J. and K.S. collected the data; J.R.D.L. and R.H. analysed the data; J.R.D.L. and R.H. led the writing of the manuscript. All authors contributed to revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests of any nature.
Additional information
Responsible Editor: Luca Bragazza.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Long, J.R., Heinen, R., Hannula, S.E. et al. Plant-litter-soil feedbacks in common grass species are slightly negative and only marginally modified by litter exposed to insect herbivory. Plant Soil 485, 227–244 (2023). https://doi.org/10.1007/s11104-022-05590-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05590-3