Abstract
Aims
The aim of the study was to investigate N biogeochemistry of four neighboring, high mountain plant communities and to identify main factors which drive variability among them. We hypothesized that the vegetation types differ in terms of N transformations, and that spatial differentiation of the communities and dominant growth form can reflect an existence of several N-environments along an elevational gradient.
Methods
Plant and soil N characteristics were studied in four vegetation types: heathland, scrub, sward and tall forb. Leaf nitrate reductase activity and total N were measured in the dominant species. Soil pH, total C, N, inorganic and dissolved organic N concentrations were measured. The soil net N mineralization rate was examined.
Results
The DistLM and PERMANOVA analyses revealed that variability among the vegetation types was driven primarily by elevation, soil N–NH4+, soil pH and soil total C. We identified three distinct N-environments along an elevational gradient. The “N-poor alpine” located at the highest altitudes, strongly N-limited and dominated by dwarf-shrub. The "N-mixed subalpine" located in the middle of the gradient and covered by scrub and sward. It was characterized by moderate N turnover rate. The "N-rich subalpine" occurred at lowest locations and was covered by subalpine tall forb community. It exhibited the highest dynamics of N transformations and was rich in inorganic N.
Conclusion
Three main N-environments were identified: N-poor alpine, N-mixed subalpine, N-rich subalpine. Variability among the vegetation types was driven primarily by elevation, soil N–NH4+, soil pH and soil total C.
Similar content being viewed by others
Introduction
Alpine and subalpine ecosystems are particularly vulnerable to climate change, which intensity is predicted to increase in the coming decades (IPCC 2007, 2021; Mietkiewicz et al. 2017). One of the observed effects are changes in nitrogen (N) cycle, which is a consequence of both direct and indirect processes as fossil fuels combustion, N deposition from agricultural systems and systematic annual temperatures increase (Galloway et al. 2008; IPCC 2021). Therefore there is a growing need for data about the N biogeochemistry in high mountain nutrient-poor ecosystems. In this context elevational gradients are considered as a suitable study-systems which allow to investigate potential response of plant communities or ecosystems to changing climatic conditions (Körner 2007). In general the shifts in ecosystem processes along an elevational gradients are driven by changes in temperature mainly, but also numerous community- and ecosystem-level variables (Mayor et al. 2017). Even slight rise in temperature can significantly increase rate of organic matter mineralization and therefore also inorganic N availability (Nadelhoffer et al. 1997; Knorr et al. 2005; Davidson and Janssens 2006). In mountainous areas, concentration of soil inorganic N (IN) (as ammonium or nitrate) decrease with elevation increase (Tanner et al. 1998; Zhang et al. 2012).
Particularly interesting is how N turnover characteristics are spatially differentiated when gradients are covered by neighboring plant communities which altitudinal ranges overlap. On a smaller scale, the specific topography of high-mountain areas affects many environmental characteristics as hydrology, elements redistribution or insolation which in turn leads to ecosystems and communities differentiation. Many of these factors control also quantity and quality of soil N available for plants (Bowman et al. 2003; Fisk et al. 1998; Zhong et al. 2019). On the one hand the differentiation of adjacent vegetation types can be an outcome of spatial heterogeneity of macronutrients biogeochemistry in given habitat, as N availability is a one of the strong environmental filters (Miller and Bowman 2003; Vitousek and Howarth 1991). But on the other hand, the community shapes N biogeochemistry in its environment by the combined effect of species coexisting in that community (Miller et al. 2007). When the area’s topography covered by adjacent vegetation types is similar, the impact of plant community on N turnover can be a remaining missing link of N biogeochemistry. Therefore the spatial mosaic-like pattern of adjacent vegetation types can likely reflect the heterogeneity of soil N transformations, but also drives these transformations by itself. Cruz et al. (2008) showed for Mediterranean ecosystems, that plant cover shapes soil conditions significantly, indicating specific pattern of soil N heterogeneity. The authors demonstrated that the dominant species and its plant functional group (PFG) reflect the spatial heterogeneity of soil characteristics (e.g. pH, nitrification potential). On the species focal-scale, plants differ in their abilities and preferences for absorbing and metabolizing various N chemical forms, (Harrison et al. 2007; Miller and Bowman 2003; Reynolds et al. 2003). The differences can be a result of both species-specific metabolic apparatus as well as an anatomical or phenological traits (Ashton et al. 2008). Furthermore a functional group which plant belongs can reflect its preferences and ability to uptake and metabolize specific N form (e.g. nitrate) as an important N source (Dias et al. 2011). One of the hypotheses that explain species-specific preferences for different soil N forms is that partitioning of the N pool occurs to avoid competition for resources (Harrison et al. 2007; McKane et al. 2002; Reynolds et al. 2003). By this way, coexisting plant species can exploit qualitatively different N pools, which reflect the niche differentiation, due to intraspecific competition is generally stronger than interspecific one (Adler et al. 2018). On the ecosystem focal-scale, also the whole vegetation types can play an important role in macronutrient fluxes, even when they cooccur in the same microclimatic area but are differentiated by species composition or dominant growth form. Acidic soils and low average annual temperatures are conditions typical for Central European mountains such as the Sudetes. Such conditions usually implicate that soil mineralization processes and therefore mineral nitrogen availability are naturally limited (Björk et al. 2007). Thus dissolved organic nitrogen (DON) is predicted to be a dominant N form in soil patches located at the upper part of elevational gradients. According to Read and Perez-Moreno (2003), such areas are predicted to be dominated by species associated with ericoid and ectomycorrhiza, for which ammonium ions and DON are likely the main N source (Björk et al. 2007). On the lower end of altitudinal gradient, soil pH and soil N dynamics are presumed to be higher, which imply domination of plants preferring inorganic N forms as a main N source. Due to higher soil pH and higher temperatures, more intensive soil microbial activity is predicted, leading to higher N dynamics and therefore higher nitrate concentration. Plants dominating on the lower end of the gradient could likely be able to effectively metabolize nitrates as an important N source. Such ability can be reflected by nitrate reductase activity (NRA). Due to nitrate reductase (NR) is the crucial enzyme of nitrate metabolism in plants, the high NRA value means that species is able to assimilate large N-NO3− amounts (Campbell 1988). It was shown previously that leaf NR activity of various plant species can be useful tool for assessment of potential species specific nitrate assimilation abilities (e.g. Gebauer et al. 1988; Lee and Stewart 1979; Rajsz et al. 2019). But because NR is an substrate-induced enzyme, its activity in plant can also reflect an actual soil nitrate availability (Gebauer et al. 1988; Rajsz et al. 2019). Furthermore, due to predicted inorganic N domination in this part of the elevational gradient, the non-mycorrhizal or arbuscular mycorrhiza associations are more probable to occur (Read and Perez-Moreno 2003; Björk et al. 2007). Hence the soil N turnover should be different under domination of different growth form.
In this study, we investigated the N biogeochemistry of four neighboring plant communities located in Central European high mountain ecosystems. The communities cover the majority area of the subalpine and alpine belts of the Karkonosze range (Giant Mountains) in the Sudetes. The vegetation types are located at different, but overlapping elevational ranges and strictly differ by growth form of dominant species (in parenthesis): alpine heathlands (dwarf shrub Calluna vulgaris), subalpine swards (grass Nardus stricta), subalpine scrubs (shrub Pinus mugo) and subalpine tall forb (perennial herb Rumex alpinus). We hypothesized that the four plant communities, located in subalpine and alpine belts of the Karkonosze, differ in the soil dissolved N forms, soil total nitrogen and net N mineralization rate as well as leaf nitrate reductase activity and total leaf N content of the dominant species. We also assumed that separate area patches with distinct biotic and abiotic N turnover characteristics, called as N-environments hereafter, can be distinguished along an elevational gradient. Consequently, each N-environment should be covered by different vegetation type, reflecting differences in N biogeochemistry. Thus higher rates of N cycling in the lower elevations will result into higher concentration of soil inorganic N available for plants. By studying spatial variation of plant and soil N characteristics along an elevational gradient in alpine tundra, we aimed to identify the crucial variables driving N biogeochemistry differences of high mountain vegetation.
Materials and methods
Study site and sampling design
Sampling and in situ leaf nitrate reductase activity (NRA) measurements were conducted in the Karkonosze mountains (Karkonosze National Park, SW Poland, 50°46′00″N 15°39′00″E) above the tree line at an elevation of 1150 to 1500 m a.s.l. (subalpine and alpine belts). Mean values of selected climatic variables in the studied area: total annual precipitation 1300 – 1512 mm; temperature in July + 8.5 to + 10.1 °C (Sobik et al. 2013), growing season about 145 days (Pawlak 2009; Gramsz et al. 2010). Granites are the main component of parent rock in all sampling sites (Waroszewski et al. 2013). The area has poor acidic soils of Hyperskeletic Leptosols and Stagnic Albic Podzols (Kabała and Szerszeń 2002). Both measurements and sample collection were conducted in July of 2016 and 2017. Four dominant plant communities, typical for high-mountain ecosystems of Central Europe, were selected for the study: alpine heathlands (referred as “heathland” hereafter) dominated by Calluna vulgaris: Avenello flexuosae-Callunetum vulgaris Zlatnik 1950 (Kočí 2007a), subalpine scrubs (referred as “scrub”) dominated by Pinus mugo: Dryopterido dilatatae-Pinetum mugo Unar in Unar et al. (1985) (Chytrý 2013), acidic swards (referred as “sward”) dominated by Nardus stricta: Carici bigelowii-Nardetum strictae Jeník 1961 (Kočí 2007b) and subalpine tall forb community (referred as “tall forb” hereafter) dominated by Rumex alpinus: Rumicetum alpini Beger 1922 (Kočí 2009). The community coverage on studied area account about 8%, 52%, 15% and 2.5%, for alpine heathlands, subalpine scrubs, acidic swards and the subalpine tall forb respectively. The alpine heathlands occurs at the elevations of 1473 – 1503 m a.s.l, subalpine scrubs and acidic swards form mosaics between 1390 and 1470 m a.s.l, while subalpine tall forbs are found at about 1150 m a.s.l. Nine study plots (30 m x 30 m) for each plant community were selected in the whole mountain range above the tree line. The altitude of each sampling plot was obtained with a GPS device (Garmin GPSMap 62 s) (Fig. 1).
Vegetation description
Vegetation data were collected for each study plot using the relevé method, with plot size ranging from 16 to 100 m2 (Chytrý and Otýpková 2003). Within each plot, homogeneous stands of vegetation were identified based on the dominant species. One relevé was analyzed per stand. The canopy cover for each species was visually estimated as percentage cover. From each plot both plant and soil samples were collected as well as leaf NRA in situ measurement were conducted in the community's dominant species.
Soil and plant analysis
At each plot, the plant material (leaves) was collected randomly from at least nine individuals of the dominant species. Leaf samples were dried at 60 °C and milled with a laboratory mill (Polymix PX-MFC90D, Kinematica AG, Switzerland). Soil samples were collected in three replicates from each site using a stainless steel core (25 cm long and 7 cm in diameter). The samples were collected into a LDPE bags and transported to the laboratory. The plant residue and coarse material were removed and each sample was homogenized. Soil water content was determined as weight loss by drying at 60 °C for 72 h. Soil pH was determined with a pH- meter (Hanna Instruments, Germany) using a 1:5 soil:water ratio (w/v). For mineral N analysis, part of each soil sample was extracted by shaking for 2 h with 1 M KCl (1:5 soil:extractant), centrifuged at 3500 × g (Mistral-1000 MSE, England), and filtered through Whatman42 filter paper (Maynard et al. 2007). To determine N-NO3− and N-NH4+ concentration, extracts were analyzed with a flow injection analyzer (FIA compact—MLE GmbH Germany). Soil dissolved organic nitrogen (DON) concentration was determined with persulfate oxidation method (Cabrera and Beare 1993; Doyle et al. 2004; Hagedorn et al. 1999), by incubating 5 mL aliquots of both the sample and the oxidizing reagent in sealed tubes at 90 °C for 20 h. A set of NH4Cl and glycine standards was also digested to verify oxidation efficiency. The remaining parts of soil samples were dried at 60 °C until constant mass and screened through an automatic sieve shaker (Morek Multiserw LPzE-2e, Poland) with 2 mm mesh size. Total nitrogen concentration was determined with the Kjeldahl method using an automatic steam distillation apparatus (Vapodest 40, Gerhardt GmbH, Germany). Soil total carbon concentration was determined with a carbon and sulfur analyzer (SC 144-DR, Leco Corporation, USA) with a dedicated reference material for C.
Nitrate reductase activity
We used an in vivo assay of nitrate reductase activity (NRA) according to a procedure described by Jaworski (1971), Al Gharbi and Hipkin (1984), Norby et al. (1989), Downs et al. (1993) and Krywult and Bielec (2013). We modified the assay procedure which enabled to conduct measurements in situ with better accuracy (Rajsz et al. 2017). The NR activity was expressed as the amount of nitrite synthesized per gram of plant tissue dry weight per hour (µM NO2− g−1 DW h−1). The measurements were carried out between 10 am and 3 pm GMT only on sunny days, with similar air temperature. Only leaves without damages were chosen for analysis. The incubation buffer pH was set to 7.5. The circles of leaves were cut with a 5-mm diameter hole puncher. Grass leaves and needles of coniferous species were cut into 2–3-mm parts using sterile scissors and immediately placed into incubation buffer. Samples were vacuum infiltrated at 0.33 atm for 5 min. and incubated for 2 h at 25 °C and darkness in a portable water bath (Rajsz et al. 2017). 1 ml of the reaction mixture was mixed with 1 ml of 1% sulphanilamide and 1 ml of 0.02% N-(1-naphthyl)ethylenediamine dihydrochloride. Absorbance was measured at 540 nm using a spectrophotometer (Secomam S-250, Secomam France) (Hageman and Reed 1980). Leaves samples from the assay were dried at 60° to a constant weight. NRA was expressed as the amount of nitrite synthesized per gram of plant tissue dry mass per hour (µM NO2− g−1 DW h−1). A standard curve was prepared before analysis with use of the same buffer solution. The NR activity was expressed as the amount of nitrite synthesized per gram of plant tissue dry weight per hour (µM NO2− g−1 DW h−1).
Net N mineralization rate
Each soil sample was divided into four separate subsamples, packed into LDPE bags and placed in an incubation chamber at 20 °C in darkness. Samples were incubated for 90 days. Moisture content in each sample was monitored to maintain stable conditions. Subsequently, all soil samples were analyzed again for N-NO3−, N-NH4− concentration using the methods described above.
Statistical analysis
To test normality of data distribution the Shapiro–Wilk test was used. The data without normal distribution were log transformed (log(x + 1)). Homoscedasticity was tested with Levene’s and Brown-Forsythe tests (Sokal and Rohlf (2003). The significance of differences in the measured environmental variables among the different plant communities was tested using one-way ANOVA. Nonparametric Kruskal–Wallis statistics was used when the variables did not meet the assumptions required for parametric ANOVA. Basic statistical analysis (means, medians, SD, data normality, significant differences) was conducted with Statistica 12 software (StatSoft, Inc. 2014). All analyses were conducted at a 0.05 significance level.
Principal component analysis (PCA) was used to study the relation between variables and examine if these variables as a whole properly described differences between plant communities. For PCA analysis, an environmental data matrix was used first, and relevés were plotted on the diagram a posteriori.
For multivariate analyses vegetation data (community composition on cover-percentage scale) were square-root transformed and the Bray–Curtis dissimilarity matrix was computed. Environmental data, except for pH and altitude, were log transformed, normalized and resemblance matrices were calculated using Euclidean distance. For results visualization, the data for plant communities where plotted using non-metric multidimensional scaling (NMDS) analysis (Clarke & Gorley 2015). To examine overall relationships between plant communities composition and environmental variables, Mantel-like RELATE tests were performed based on correlations between community composition and all measured environmental variables. To determine which environmental variables components were most influential in the predictor matrices, the relationships between the environmental data and between community composition was tested using a Distance-based Linear Model (DistLM). For visual illustration of the relationships between plant communities assemblage and environmental variables we used distance-based redundancy analysis (dbRDA), a non-parametric ordination method in which axes are constrained as functions of the explanatory variables. Non-parametric, permutational ANOVA (PERMANOVA) was used to test differences in community composition between each vegetation type. Data were analyzed using Primer-E v7 statistical package with PERMANOVA + addon (Clarke & Gorley 2015; Anderson et al. 2008).
Results
Leaf NRA and total leaf N concentration
Considerable differences in leaf NRA values between the dominant species were found (Table 1). NR activity was significantly (more than 30 times) higher in R. alpinus than in the other studied species (K-W, H(3,N = 36) = 26.68; P < 0.0001). The lowest NRA close to detection limit was observed in C. vulgaris. NRA in N. stricta was nearly ten times higher than in C. vulgaris and about four times than in P. mugo. Similarly the highest total leaf nitrogen concentration was observed in R. alpinus and it differed significantly from C. vulgaris and P. mugo (K-W, H(3,N = 36) = 32.84; P < 0.0001) which contained from two to four times less total nitrogen per 100 mg of leaves.
Soil pH, dissolved N forms and total C concentration
Among all the studied communities soil pH was significantly higher in the subalpine tall forb community (F3;16.98 = 17.02; P < 0.0001), but there were no substantial differences in soil reaction between heathland, scrub and sward (Table 1). Soil total N concentration in the tall forb was about three times than in other communities (F3;32 = 4.08 P < 0.05) with significantly lower values in sward. DON concentrations were 5 to 10 times greater than the dissolved inorganic nitrogen (DIN) fraction (N-NO3− + N-NH4+) in all the studied plant communities (Table 1). The largest significant differences in soil DON content were observed between heathland and scrub communities with the highest values in the latter (F3;32 = 4.28 P < 0.05). Plant communities differed markedly in the concentrations of soil exchangeable N forms. Two homogenous groups were observed for soil N-NO3−: group one, with the lowest nitrate concentrations (heathland and scrub), and group two with significantly higher amounts: sward and tall forb (K-W H(3,N = 36) = 26.63; P < 0.001). Overall the highest N-NO3− amounts were observed in tall forb soil and these values were from four (scrub, sward) to eighteen times (heathland) higher than in other communities. A different situation was observed for N-NH4+. The highest ammonium concentrations were measured in scrub soils, and they did not differ significantly from the sward community (F3;16.70 = 15.79 P < 0.0001). In turn the lowest ammonium concentrations were observed in heathland soils, and they were on average from 2.5 to 5 times lower than in the other studied communities (Table 1).
Net soil N mineralization
The overall net N mineralization rate is shown in Fig. 2. A significantly higher total N mineralization rate was observed for soil from the tall forb and scrub communities (K-W: H(3,N = 36) = 22.47 P < 0.001) while the lowest one for heathland. However scrub and tall forb differed in terms of the direction of N transformations (Fig. 2). In tall forb nitrification dominated over ammonification, and it was the community with the highest observed rate of N-NO3− concentration increase during incubation (K-W: H(3,N = 36) = 26.42 P < 0.001). In turn the N-NH4+ increase rate was significantly highest in scrub and sward communities (K-W: H(3,N = 36) = 18.56 P < 0.001) while ammonium concentration decreased during incubation in the tall forb community (a negative increment value in the chart; Fig. 2).
Multivariate analysis
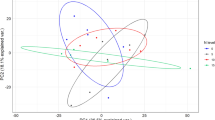
Principal component analysis (PCA) revealed two main ecological gradients (Fig. 3). The first one (PCA1) was mostly related to N-NO3−, H (altitude) and NTot, and to a lesser extent to pH and NO3−/NH4+. The second gradient was linked solely with DON. Eigenvalues for the two first axes (PCA1 and PCA2) were 0.504 and 0.231, respectively and accounted for 73.5% of total variation. Eigenvalues for the two next axes were below 0.1, which indicated their low contribution to total variation. The first gradient clearly distinguished the tall forb community, while the second one distinguished heathland, sward and scrub from each other.
PCA ordination of individual relevés based on environmental variables in relation to the first two axes. Symbols: circle alpine heathlands, triangle acidic swards, white square subalpine scrubs, square subalpine tall forb community. Abbreviations: N-NO3− – soil nitrate nitrogen content, N-NH4+ – soil ammonium nitrogen concentration, DON – soil dissolved organic nitrogen, NO3−/NH4+ – quantitative ratio of soil nitrate nitrogen to ammonium nitrogen content after 90 days of laboratory incubation, C:N – soil total carbon to nitrogen ratio, CTot – soil total carbon, NTot – soil total nitrogen, N-MIN – soil total inorganic nitrogen after 4 months of incubation, pH – soil reaction, H – altitude a.s.l
Mantel-like RELATE test showed significant correlations between community structure and studied environmental variables (RELATE test, Rho = 0.704, P = 0.001). The step-wise DistLM procedure revealed four main, statistically significant environmental variables explaining the biggest part of the variation: altitude, soil N-NH4+, soil pH and soil total C. (Table 2). variability among the vegetation types was driven primarily by elevation, soil N-NH4+, soil pH and soil total C, with significant differences in community structure among the different vegetation types (step-wise DistLM as well as dbRDA; Fig. 4, Table 2). The first coordinate axis revealed 37.4% while the second axis 21.4% of total variation. The NMDS analysis showed clear grouping among four predetermined vegetation types (Electronic supplementary material—ESM1). The PERMANOVA analysis, performed with 999 permutations revealed a significant differences between studied vegetation types: MS = 66.251; Pseudo-F = 18.238; P = 0.001.
Distance-based redundancy analysis (dbRDA) ordination relating environmental variables to plant community assemblage data, showing biplot projections for environmental variables. Analysis was performed on principal coordinate axes obtained from Bray–Curtis resemblance matrices of square-root transformed data. Projected environmental variables: soil pH; altitude a.s.l; soil total C – C tot; soil N-NH4+
Discussion
The results showed significant quantitative and qualitative differences in N biogeochemistry between studied communities. Principal component analysis (PCA) confirmed the existence of two main ecological gradients (Fig. 3). PERMANOVA confirmed that studied communities reflect area patches distinct in terms of plant-soil N turnover characteristics which we called here as N-environments (Fig. 4). As we predicted, elevation was indicated as a main, primary factor that shapes variability among studied vegetation types. It was expected as altitude is strongly connected with temperature changes which in turn controls most of environmental variables. One of the well-known relationships between elevation and environmental conditions is temperature decrease with growing altitude (Sundqvist et al. 2013). Besides altitude, the most significant factors driving plant communities differentiation were soil N-NH4+, soil pH and soil total C. All the variables are related to temperature, but are shaped by vegetation also. It was shown for a low temperature ecosystems from high altitudes, that even slight rise in temperature can significantly increase soil organic matter mineralization rate and therefore also inorganic N availability (Nadelhoffer et al. 1997; Knorr et al. 2005; Davidson and Janssens 2006).
Facing the predicted future increase of annual temperatures in alpine ecosystems, changes in nitrogen conditions can be expected. With increasing temperatures, the soil nitrification rate and the level of soil available IN will increase, which will also increase the pH of the soil. In this scenario, some species can migrate upwards, and start to occupy habitats previously unavailable to them. At the same time, rising temperature may cause a decrease environmental harshness, which in turn will lead to changes in the plant-plant interactions. According to stress gradient hypothesis the frequency of positive plant–plant interactions, (among other factors) should increase monotonically with increasing environmental harshness (Anthelme et al. 2014; Michalet and Pugnaire 2016; Soliveres et al. 2015). Thus, under growing temperatures and environmental severity decrease, the facilitative interactions could be replaced by competition mostly. However we did not studied plant-plant interactions directly in presented study, therefore further research is needed to assess the importance of described possibilities.
According to obtained results, three main N-environments with different plant-soil N-turnover characteristics can be identified.
N-poor alpine N-environment
The first N-environment we called “N-poor alpine” as it was located at the highest altitudes and dominated by heathland community. In general it is characterized by N-poorest soils with the lowest both inorganic N (NH4+, NO3−) and DON concentrations as well as one of the lowest soil pH among all studied communities. These observations are in agreement with general knowledge that heathlands are found mostly on acidic soils with low nutrient availability (de Graaf et al. 1998). Nevertheless low soil concentration of given nutrient can be potentially a result of both intensive plant uptake or microbial utilization (Xu et al. 2011). The heathland is dominated by evergreen dwarf-shrub C. vulgaris, which covers the majority of community's area. However it is unlikely that low N-NO3− concentration results from intense uptake and assimilation by C. vulgaris taking into account extremely low leaf NRA, reported also by other authors (de Graff et al. 1998). In addition the lowest net-N mineralization rate in this vegetation type implies, that the soil exhibit a very low nitrification potential. These observations support results reported by other authors (Gebauer et al. 1988; Havill et al. 1974; Högberg et al. 1990; Rajsz et al. 2019) and seems to be typical for the whole Ericaceae family (Smirnoff et al. 1984). Due to very low NRA and soil pH, ammonium seems to be a more essential N source in this N-environment. Higher soil NH4+ concentrations are known to have potential negative effects on many plant species, causing the wide range of physiological disturbances. The sensitivity to ammonium toxicity differ largely between species and ranges from slight symptoms as leaves chlorosis to even mortality increase (Britto and Kronzucker 2002). Furthermore NH4+ accumulation can suppress NRA and therefore inhibit nitrate assimilation, but this effect is also species specific (Dias et al. 2015). It was shown that differences in tolerance to increased ammonium concentrations is a significant factor shaping spatial relations of plant functional groups, giving competitive advantage for some species (Dias et al. 2015). Similar pattern is likely observed in heathland community, as some studies confirm that C. vulgaris is well adapted to high ammonium concentrations (Britto and Kronzucker 2002). Therefore higher soil ammonium availability could act as environmental filter, promoting strong domination of ammonium-tolerant dwarf shrub. In this N-environment however, soil is characterized by the lowest both N-NH4+ and N-NO3− concentrations as well as low rate of N mineralization processes. Hence apart from inorganic N, the probable nutritional role of soil soluble organic N in heathland community is very important. Due to relatively low soil DON concentration it is likely that C. vulgaris uptake and assimilate also soluble organic N. The preference for soil organic N can be related to plant growth-form and plant functional group (Kielland 1994, 1995; Björk et al. 2007; Cruz et al. 2008). C. vulgaris is a slow growing species from Ericaceae family and largely depends on ericoid mycorrhiza association which has been proven to participate in organic N uptake (Stribley and Read 1980; Sokolovski et al. 2002). High altitudes ecosystems are often regarded as dominated by species associated with ericoid and ectomycorrhiza, for which ammonium ions and DON are the main N source (Read and Perez-Moreno 2003; Björk et al. 2007). Mitchell and Gibson (2006) showed that apart from organic N, ericoid mycorrhiza can be involved also in ammonium uptake. The second probable factor causing low soil DON concentration in heathland is a fact that litter produced by C. vulgaris releases N at a very low rate (Gimingham 1972). Both the lowest net N mineralization rate and the second highest soil C:N ratio indicate very low soil microbial activity in this N-environment. Some data show that also soil archaea can have an essential contribution in soil nitrification potential, especially when soil conditions are unfavourable to nitrifying microorganisms (Gubry-Rangin et al. 2010), however still the main nitrifiers in alpine ecosystems seems to be nitrifying bacteria (Xiang et al. 2017).
N-rich subalpine N-environment
The second type of N-environment, we called “N-rich subalpine”. It was located on the opposite site of elevational gradient and occupied by the subalpine tall forb community, dominated by forbs. Soil N-NO3− concentration in this community was the highest observed, comparing to sward, scrub and heathland vegetation types. Albeit tall forb soil pH was relatively highest among studied communities, it reached values ≥ 5.0, which still can be classified as extremely acidic (Osman 2013). Rumex alpinus, the community’s dominant species, was characterized by the highest NRA values (over ten times than in other species) and total leaf N concentration (over 4 times). Constantly high NR activity was shown earlier for many species from the Rumex genus and ranged between 6.9 to 16.6 µM NO2− g−1 DW h−1 (Güleryuz et al. 2008; Langelaan and Troelstra 1992; Rajsz et al. 2019). High NRA values are typically attributed to synanthropic plants, which grow on highly disturbed areas (Al Gharbi and Hipkin 1984; Lee and Stewart 1979). It is evident that nitrification was the dominant process here, as N-NO3− concentration increased over 18 times during soil incubation experiment. A strong increase of nitrate concentration in soil from the R. alpinus community was observed also by Rehder (1982). Interestingly his research was conducted in the Northern Calcareous Alps, while our study shows that the same pattern occurs on acidic soils. Soil pH lower than 4.5 usually inhibit nitrification processes (e.g. Chu and Grogan 2010; Haynes and Swift 1986) however some authors reported nitrification processes occurring even at low soil pH from arctic ecosystems (Chapin et al. 1988; Davy and Taylor 1974; Giblin et al. 1991) where probable nitrifiers are some archaea (Gubry-Rangin et al. 2010). The tall forb was the only community where N-NH4+ concentration decreased during the soil incubation. Similar results were obtained by Güleryuz et al. (2008) for soils dominated by R. olympicus, a species closely related with R. alpinus. Due to soil NO3− is mostly produced by microbial oxidation of ammonium ions, the observed decrease can be a result of a high nitrification rate (Schimel and Bennet 2004). In general the lower C:N the higher net N mineralization rate (Björk et al. 2007; Nadelhoffer et al. 1991). This pattern was clearly visible in the tall forb community where the soil C:N ratio was the lowest, while the net N mineralization rate highest among studied vegetation types.
N-mixed subalpine N-environment
To the third identified N-environment we assigned the working name "N-mixed subalpine". It was located in the middle of the elevational gradient.. In general it was covered by two, most strictly neighboring vegetation types: scrub and sward. The subalpine scrub is strongly dominated by coniferous shrub Pinus mugo. To date little research has been done on P. mugo community and its N biogeochemistry so far; therefore the available literature on this topic is scarce. The leaf NRA observed in the P. mugo was very low and on a similar level as in C. vulgaris. This observation supports the general statement that most angiosperms exhibit constantly low but detectable leaf NR activity (Rajsz et al. 2019; Smirnoff et al. 1984). It clearly corresponds with results by Dias et al. (2011) which presented that plant functional group can reflect the nutritional preferences for specific N form. In addition soil pH in the scrub community was the lowest observed. Soils with such low pH are classified as extremely acidic (Osman 2013). Low soil pH in scrub community (mean pH 4.2) clearly correlate with the highest soil ammonium concentration. It is known that in the communities dominated by coniferous species, ammonium is usually the prevalent soil inorganic N form (Eviner and Chapin 1997). It can be supposed that soil DON can be an important N source for P. mugo. Although many authors reports that plants from nutrient-poor ecosystems are able to utilize DON as a significant N source. further investigations are needed to examine the P. mugo ability to utilize organic N forms (Chapin et al. 1993, Kielland 1994; Miller and Bowman 2003; Raab et al. 1999).
Along with scrub, the sward community covers the “N-mixed subalpine” environment, forming a large mosaic-like area of two distinct communities. The sward's dominant species N. stricta is a grass covering typically more than 90% of the community. The NRA values observed in N. stricta were on a similar level as reported for the species by other authors (Havill et al. 1974; Langelaan and Troelstra 1992). Mean leaf NRA in N. stricta was higher than in P. mugo and C. vulgaris, but still much lower than in R. alpinus. This suggests that N. stricta utilize nitrates more effectively than C. vulgaris and P. mugo, but still far less efficiently than R. alpinus. Although N. stricta typically grows on acidic soils, it shows considerable biomass growth under nitrate feeding (Perkins 1968). Havill et al. (1974) presented N. stricta’s ability to up-regulate leaf NRA to an order of magnitude higher after nitrate feeding, which suggest its wide physiological plasticity for nitrate assimilation. It is visible also by large standard deviation value obtained for NRA in N. stricta. Nitrification was on similar level as ammonification in sward soil N incubation experiment, but also total inorganic N production was comparable between the sward and scrub. Therefore the quantitative and qualitative relations between different inorganic N forms seems to be relatively balanced in sward community. Falkengren-Grerup (1995) showed that many species from the Poaceae family can utilize both NO3− and NH4+ in relatively balanced proportions (40% NO3−; 60% NH4+). The versatile preference for different soil N forms is known for N. stricta for a long time (e.g. Gigon and Rorison 1972). This phenomenon can be regarded as species adaptation to spatial heterogeneity of soil inorganic N. Due to N. stricta rooting system is not as long as in neighbouring P. mugo, the versatile N preferences have developed in N. stricta to avoid potential N deficiency. Hence each clump can exhibit domination of different N assimilation pathway, and therefore particular clumps can differ in terms of N metabolism, more than individuals of other studied species. It is supported also by the fact that leaf N concentration in N. stricta strongly depends on nitrogen availability (Hicks et al. 2000). Moreover the ability to soil organic N utilization can also be important in this species nutrition. Weigelt et al. (2005) presented preference of N. stricta for serine, even over inorganic N. A different strategy is likely observed in P. mugo. Although P. mugo can potentially reproduce by seeds, in Karkonosze mountains it is known to prosper as clonal species mostly. Clonal plants can exploit soil grains rich in nutrients through connecting rhizomes or stolons (Xue et al. 2019). Therefore even distant N-abundant soil patches can be reached by such species. These differences between scrub and sward communities can be a one of the key factors leading to distinct differentiation of these vegetation types, despite the fact that they occur at the same altitude and closely adjoin each other, forming a mosaic-like spatial pattern.
Comparative analysis of the identified N-environments
Although both increase and decrease of N transformations rate along increasing elevation have been reported for different ecosystems (Marrs et al. 1988; Kitayama et al. 1998; Knoepp and Swank 1998; Zhang et al. 2012), the decreasing N turnover rate with increasing altitude is visible in our results. The N-poorest as well as most acidic is the “N-poor alpine” environment located at the upper end of elevational gradient. Dias et al. (2015) showed for species from Mediterranean ecosystems, that ammonium can inhibit NRA even when nitrates are abundant in the soil. However the effect was dependent on plant functional group which given species belonged to. Because in each of studied communities NH4+ was relatively abundant, further research are needed to assess the species reaction to different ammonium concentrations in controlled conditions. Although in tall forb community the soil N-NH4+ concentration was similar to sward, scrub and even heathland, the multivariate analysis indicated soil ammonium as a one of most important factors explaining studied variation. The observed pattern can be a result of different soil nitrification/ammonification potentials in studied N-environments. In the heathland community, very low soil ammonium concentration resulted from low soil N-dynamics and therefore low ammonium production, while in the tall forb community the larger amounts of soil N-NH4+ was likely produced but in the same time it was oxidized by microorganisms, and partially absorbed by plants. However further research are needed, for examination of temporal variation of N turnover in each vegetation type. Total leaf N concentrations were lowest in both P. mugo and C. vulgaris which support the statement that nutrient concentration in mature leaves of evergreen species is usually lower than in fast growing deciduous plants due to a specific nutritional strategy focused on slow accumulation (Aerts 1996; Diehl et al. 2003; Knops and Koenig 1997). Therefore it is likely that observed differences in N-turnover partially resulting from dominance of different functional group. The C. vulgaris – woody dwarf shrub dominating in heathland is focused on slow nutrient accumulation, but also low N deposition. On the other hand, deciduous herb—R. alpinus occupying the tall forb community, accumulate high nitrogen amounts as can be observed in the total leaf N concentration (Table 1) but it also release high N amounts at the end of growth season. Cruz et al. (2008), and Rutigliano et al. (2004) showed that the one of the key factors influencing soil properties is the plant cover type. The shape and structure of the crowns, growth form of the dominant species as well as the leaf morphology may significantly affect the distribution of light and water availability, as well as the soil temperature and in consequence the type of soil N transformations. Water availability and soil/air humidity is regarded as a one of the main factors shaping the relationships between plants in a community, and therefore likely between entire communities (Liancourt et al. 2005; Brooker et al. 2008). Results presented in our previous paper showed that in each of the communities soil moisture was very similar (Rajsz et al. 2017). Nevertheless single measurements give little information on the actual water and humidity conditions, therefore further research are needed to explore both daily and seasonal moisture and temperature changes in studied ecosystems.
Conclusions
The environmental factors connected with N biogeochemistry explaining the largest part of variation in neighboring, subalpine and alpine vegetation types in Karkonosze mountains are: altitude, soil pH, soil ammonium concentration and soil total C. The three main, distinct N-environments can be identified along small elevational gradient: the N-poor alpine, N-mixed subalpine, and N-rich subalpine. The specific vegetation types are occupying these N-environments, and differ by growth form of dominant species, reflecting different strategies of nitrogen use.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Change history
25 October 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11104-022-05751-4
References
Adler PB, Smull D, Beard KH, Choi RT, Furniss T, Kulmatiski A, Meiners JM, Tredennick AT, Veblen KE (2018) Competition and coexistence in plant communities: intraspecific competition is stronger than interspecific competition. Ecol Lett 21(9):1319–1329
Aerts R (1996) Nutrient Resorption from Senescing Leaves of Perennials: Are there General Patterns? J Ecol 84:597. https://doi.org/10.2307/2261481
Al Gharbi A, Hipkin CR (1984) Studies on nitrate reductase in British angiosperms. New Phytol 97(4):629–639
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. PRIMER-E: Plymouth, UK
Anthelme F, Cavieres LA, Dangles O (2014) Facilitation among plants in alpine environments in the face of climate change. Frontiers in plant science 5:387
Ashton IW, Miller AE, Bowman WD, Suding KN (2008) Nitrogen preferences and plant-soil feedbacks as influenced by neighbors in the alpine tundra. Oecologia 156(3):625–636
Björk RG, Klemedtsson L, Molau U et al (2007) Linkages between N turnover and plant community structure in a tundra landscape. Plant Soil 294:247–261. https://doi.org/10.1007/s11104-007-9250-4
Bowman WD, Bahn L, Damm M (2003) Alpine landscape variation in foliar nitrogen and phosphorus concentrations and the relation to soil nitrogen and phosphorus availability. Arct Antarct Alp Res 35(2):144–149
Britto DT, Kronzucker HJ (2002) NH4+ toxicity in higher plants: A critical review. J Plant Physiol 159:567–584. https://doi.org/10.1078/0176-1617-0774
Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, ... & Michalet R (2008) Facilitation in plant communities: the past, the present, and the future. J Ecol 96(1): 18-34. https://doi.org/10.1111/j.1365-2745.2007.01295.x
Cabrera ML, Beare MH (1993) Alkaline Persulfate Oxidation for Determining Total Nitrogen in Microbial Biomass Extracts. Soil Sci Soc Am J 57:1007–1012. https://doi.org/10.2136/sssaj1993.03615995005700040021x
Campbell GW (1988) Measurements of nitrogen dioxide concentrations at rural sites in the United Kingdom using diffusion tubes. Environmental Pollution 55(4):251–270
Chapin FS III, Jefferies RL, Reynolds JF, Shaver GR, Svoboda J, Chu EW (1993) Arctic ecosystems in a changing climate: an ecophysiological perspective. Academic Press
Chapin FS III, Fetcher N, Kielland K, Everett KR, Linkins AE (1988) Productivity and Nutrient Cycling of Alaskan Tundra: Enhancement by Flowing Soil Water. Ecology 69:693–702. https://doi.org/10.2307/1941017
Chu H, Grogan P (2010) Soil microbial biomass, nutrient availability and nitrogen mineralization potential among vegetation-types in a low arctic tundra landscape. Plant Soil 329:411–420. https://doi.org/10.1007/s11104-009-0167-y
Chytrý M (2013) Dryopteridodilatatae-Pinetum mugo Unar in Unar et al. 1985. – In: Chytrý M. (ed.), Vegetation of the Czech Republic 4. Forest and shrub vegetation, p. 159–163, Academia, Praha
Chytrý M, Otýpková Z (2003) Plot sizes used for phytosociological sampling of European vegetation. J Veg Sci 14:563–570. https://doi.org/10.1111/j.1654-1103.2003.tb02183.x
Clarke KR, Gorley RN (2015) PRIMER v7: User Manual/Tutorial. Auckland, New Zealand
Cruz C, Bio AM, Jullioti A, Tavares A, Dias T, Martins-Loução MA (2008) Heterogeneity of soil surface ammonium concentration and other characteristics, related to plant specific variability in a Mediterranean-type ecosystem. Environ Pollut 154(3):414–423
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440(7081):165–173
Davy AJ, Taylor K (1974) Seasonal Patterns of Nitrogen Availability in Contrasting Soils in the Chiltern Hills. J Ecol 62:793. https://doi.org/10.2307/2258955
de Graaf MCC, Bobbink R, Roelofs JGM, Verbeek PJM (1998) Differential effects of ammonium and nitrate on three heathland species. Plant Ecol 135:185–196. https://doi.org/10.1023/A:1009717613380
Dias T, Martins-Loução MA, Sheppard L, Cruz C (2015) Plant tolerance of ammonium varies between co-existing Mediterranean species. Plant Soil 395(1):243–252
Dias T, Neto D, Martins-Loução MA, Sheppard L, Cruz C (2011) Patterns of nitrate reductase activity vary according to the plant functional group in a Mediterranean maquis. Plant Soil 347(1):363–376
Diehl P, Mazzarino MJ, Funes F et al (2003) Nutrient conservation strategies in native Andean-Patagonian forests. J Veg Sci 14:63–70. https://doi.org/10.1111/j.1654-1103.2003.tb02128.x
Downs MR, Nadelhoffer KJ, Melillo JM, Aber JD (1993) Foliar and fine root nitrate reductase activity in seedlings of four forest tree species in relation to nitrogen availability. Trees 7(4):233–236
Doyle A, Weintraub MN, Schimel JP (2004) Persulfate Digestion and Simultaneous Colorimetric Analysis of Carbon and Nitrogen in Soil Extracts. Soil Sci Soc Am J 68:669–676. https://doi.org/10.2136/sssaj2004.6690
Eviner VT, Chapin FS (1997) Plant–microbial interactions. Nature 385(6611):26–27
Falkengren-Grerup U (1995) Interspecies differences in the preference of ammonium and nitrate in vascular plants. Oecologia 102:305–311. https://doi.org/10.1007/BF00329797
Fisk MC, Schmidt SK, Seastedt TR (1998) Topographic patterns of above-and belowground production and nitrogen cycling in alpine tundra. Ecology 79(7):2253–2266
Galloway JN, Townsend AR, Erisman JW et al (2008) Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 320:889–892
Gebauer G, Rehder H, Wollenweber B (1988) Nitrate, nitrate reduction and organic nitrogen in plants from different ecological and taxonomic groups of Central Europe. Oecologia 75:371–385
Giblin AE, Nadelhoffer KJ, Shaver GR et al (1991) Biogeochemical Diversity Along a Riverside Toposequence in Arctic Alaska. EcolMonogr 61:415–435. https://doi.org/10.2307/2937049
Gigon A, Rorison IH (1972) The Response of Some Ecologically Distinct Plant Species to Nitrate- and to Ammonium-Nitrogen. J Ecol 60:93. https://doi.org/10.2307/2258043
Gimingham CH (1972) Ecology of heathlands. Chapmann Hall, London
Gramsz R, Potocka J, Kociánová M (2010) Essential climatic conditions in the Giant Mts compared with Northern Scandinavia along Andøya - Kiruna profile. Opera Corcontica 47:29–54
Gubry-Rangin C, Nicol GW, Prosser JI (2010) Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol Ecol 74(3):566–574
Güleryüz G, Titrek E, Arslan H (2008) Nitrogen mineralization in the ruderal sub-alpine communities in Mount Uludağ. Turkey. European Journal of Soil Biology 44(4):408–418
Hagedorn F, Mohn J, Schleppi P, Flu¨hler H (1999) The Role of Rapid Flow Paths for Nitrogen Transformation in a Forest Soil A Field Study with Micro Suction Cups. Soil Sci Soc Am J 63:1915–1923. https://doi.org/10.2136/sssaj1999.6361915x
Hageman RH, Reed AJ (1980) Nitrate reductase from higher plants. In Methods in enzymology (Vol. 69, pp. 270–280). Academic Press
Harrison KA, Bol R, Bardgett RD (2007) Preferences for different nitrogen forms by coexisting plant species and soil microbes. Ecology 88:989–999. https://doi.org/10.1890/06-1018
Havill DC, Lee JA, Stewart GR (1974) Nitrate utilization by species from acidic and calcareous soils. New Phytol 73:1221–1231. https://doi.org/10.1111/j.1469-8137.1974.tb02151.x
Haynes RJ, Swift RS (1986) Effect of soil amendments and sawdust mulching on growth, yield and leaf nutrient content of highbush blueberry plants. Sci Hortic (Amsterdam) 29:229–238. https://doi.org/10.1016/0304-4238(86)90066-X
Hicks WK, Leith ID, Woodin SJ, Fowler D (2000) Can the foliar nitrogen concentration of upland vegetation be used for predicting atmospheric nitrogen deposition? Evidence from field surveys. Environ Pollut 107:367–376. https://doi.org/10.1016/S0269-7491(99)00166-9
Högberg P, Johannisson C, Nicklasson H, Högbom L (1990) Shoot nitrate reductase activities of field-layer species in different forest types: I. preliminary surveys in Northern Sweden. Scand J for Res 5:449–456. https://doi.org/10.1080/02827589009382627
IPCC (2007) Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Geneva, Switzerland
IPCC (2021) Climate change 2021: the physical science basis. In: Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Zhou B (eds) Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change, 2. Cambridge University Press, Cambridge
Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Biochemical and biophysical research communications 43(6):1274–1279
Kabała C, Szerszeń L (2002) Profile distributions of lead, zinc, and copper in Dystric Cambisols developed from granite and gneiss of the Sudetes Mountains, Poland. Water Air Soil Pollut 138:307–317. https://doi.org/10.1023/A:1015591607154
Kielland K (1994) Amino acid absorption by Arctic plants: Implications for plant nutrition and nitrogen cycling. Ecology 75:2373–2383. https://doi.org/10.2307/1940891
Kielland K (1995) Landscape patterns of free amino acids in arctic tundra soils. Biogeochemistry 31(2):85–98
Kitayama K, Aiba SI, Majalap-Lee N, Ohsawa M (1998) Soil nitrogen mineralization rates of rainforests in a matrix of elevations and geological substrates on Mount Kinabalu, Borneo. Ecological Research 13(3):301–312
Knoepp JD, Swank WT (1998) Rates of nitrogen mineralization across an elevation and vegetation gradient in the southern Appalachians. Plant Soil 204(2):235–241
Knops JMH, Koenig WD (1997) Site fertility and leaf nutrients of sympatric evergreen and deciduous species of Quercus in central coastal California. Plant Ecol 130:121–131. https://doi.org/10.1023/A:1009798327200
Knorr W, Prentice IC, House JI, Holland EA (2005) Long-term sensitivity of soil carbon turnover to warming. Nature 433(7023):298–301
Kočí M (2009) Rumicetum alpini Beger 1922. – In: Chytrý M. (ed.), Vegetation of the Czech Republic 2. Ruderal, weed, rock and scree vegetation, p. 377–378, Academia, Praha
Kočí M (2007a) Avenello flexuosae-Callunetum vulgaris Zlatník 1925. – In: Chytrý M. (ed.), Vegetation of the Czech Republic. 1. Grassland and Heathland Vegetation, p. 67–69, Academia, Praha
Kočí M (2007b) Carici bigelowii-Nardetum strictae (Zlatník 1928) Jeník 1961. – In: Chytrý M. (ed.), Vegetation of the Czech Republic. 1. Grassland and Heathland Vegetation, p. 81–83, Academia, Praha
Körner C (2007) The use of ‘altitude’ in ecological research. Trends Ecol Evol 22:569–574
Krywult M., Bielec D (2013) Method of measurement of nitrate reductase activity in field conditions. J Ecol Eng, 14(1)
Langelaan JG, Troelstra SR (1992) Growth, chemical composition, and nitrate reductase activity of Rumex species in relation to form and level of N supply. Plant Soil 145:215–229. https://doi.org/10.1007/BF00010350
Lee JA, Stewart GR (1979) Ecological Aspects of Nitrogen Assimilation. Adv Bot Res 6:1–43. https://doi.org/10.1016/S0065-2296(08)60328-6
Liancourt P, Corcket E, Michalet R (2005) Stress tolerance abilities and competitive responses in a watering and fertilization field experiment. J Veg Sci 16(6):713–722. https://doi.org/10.1111/j.1654-1103.2005.tb02414.x
Marrs RH, Proctor J, Heaney A, Mountford MD (1988) Changes in soil nitrogen-mineralization and nitrification along an altitudinal transect in tropical rain forest in Costa Rica. The Journal of Ecology 76(2):466–482. https://doi.org/10.2307/2260606
Maynard DG, Kalra YP, Crumbaugh JA (2007) Nitrate and exchangeable ammonium nitrogen. In: M.R. Carter and E.G. Gregorich, editors, Soil sampling and methods of analysis. 2nd ed. CRC Press, Boca Raton, FL
Mayor JR, Sanders NJ, Classen AT, Bardgett RD, Clement JC, Fajardo A, Lavorel S, Sundqvist MA, Bahn M, Chisholm C, Cieraad E, Gedalof Z, Grigulis K, Kudo G, Oberski DL, Wardle DA (2017) Elevation alters ecosystem properties across temperate treelines globally. Nature 542(7639):91–95
McKane RB, Johnson LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, Fry B, Murray G (2002) Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415(6867):68
Michalet R, Pugnaire FI (2016) Facilitation in communities. Funct Ecol 30(1):3–9
Mietkiewicz N, Kulakowski D, Rogan J, Bebi P (2017) Long-term change in subalpine forest cover, tree line and species composition in the Swiss Alps. J Veg Sci 28(5):951–964
Miller AE, Bowman WD (2003) Alpine plants show species-level differences in the uptake of organic and inorganic nitrogen. Plant Soil 250(2):283–292
Miller AE, Bowman WD, Suding KN (2007) Plant uptake of inorganic and organic nitrogen: neighbor identity matters. Ecology 88(7):1832–1840
Mitchell DT, Gibson BR (2006) Ericoid mycorrhizal association: ability to adapt to a broad range of habitats. Mycologist 20(1):2–9
Nadelhoffer KJ, Giblin AE, Shaver GR, Laundre JA (1991) Effects of temperature and substrate quality on element mineralization in six Arctic soils. Ecology 72:242–253. https://doi.org/10.2307/1938918
Nadelhoffer KJ, Shaver GR, Giblin A, Rastetter EB (1997) Potential impacts of climate change on nutrient cycling, decomposition, and productivity in arctic ecosystems. In Global change and arctic terrestrial ecosystems (pp. 349–364). Springer, New York, NY
Norby RJ, Weerasuriya Y, Hanson PJ (1989) Induction of nitrate reductase activity in red spruce needles by NO2 and HNO3 vapor. Canadian Journal of Forest Research 19(7):889–896
Osman KT (2013) Soils. Principles, properties and management. Springer, Dordrecht
Pawlak W (ed.) 2009 The second edition of the Atlas of Lower and Opole Silesia. Polish Cartographical Review, 41 (4), Polskie Towarzystwo Geograficzne, Oddział Kartograficzny, Warsaw, pp330–343
Perkins DF (1968) Ecology of Nardus Stricta L.: I. Annual Growth in Relation to Tiller Phenology. J Ecol 56:633. https://doi.org/10.2307/2258096
Raab TK, Lipson DA, Monson RK (1999) Soil amino acid utilization among species of the Cyperaceae: plant and soil processes. Ecology 80(7):2408–2419
Rajsz A, Wojtuń B, Bytnerowicz A (2017) In situ assay of nitrate reductase activity using portable water bath. Environ Monit Assess 189:1–7. https://doi.org/10.1007/s10661-017-6045-9
Rajsz A, Wojtuń B, Mróz L et al (2019) Nitrate reductase activity in high-mountain plants: A test across species, growth form and habitat type. J Plant Ecol 12:519–530. https://doi.org/10.1093/jpe/rty044
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems–a journey towards relevance? New Phytol 157(3):475–492
Rehder H (1982) Nitrogen relations of ruderal communities (Rumicion alpini) in the Northern Calcareous Alps. Oecologia 55(1):120–129
Reynolds HL, Packer A, Bever JD, Clay K (2003) Grassroots ecology: Plant-microbe-soil interactions as drivers of plant community structure and dynamics. Ecology 84:2281–2291
Rutigliano FA, D’ascoli R, De Santo AV (2004) Soil microbial metabolism and nutrient status in a Mediterranean area as affected by plant cover. Soil Biology and Biochemistry 36(11):1719–1729
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85(3):591–602
Smirnoff N, Todd P, Stewart GR (1984) The Occurrence of Nitrate Reduction in the Leaves of Woody Plants. Ann Bot 54:363–374. https://doi.org/10.1093/oxfordjournals.aob.a086806
Sobik M, Błaś M, Migała K, Godek M, Nasiółkowski T (2013) Klimat. In: Knapik R. & Raj A. (eds.), Przyroda Karkonoskiego Parku Narodowego. Karkonoski Park narodowy, Jelenia Góra, pp 147–186
Sokal RR, Rohlf FJ, (2003) Biometry: The principles and practices of statistics in biological research. W.H. Freeman and Company, New York, New York
Sokolovski SG, Meharg AA, Maathuis FJ (2002) Calluna vulgaris root cells show increased capacity for amino acid uptake when colonized with the mycorrhizal fungus Hymenoscyphus ericae. New Phytol 155(3):525–530
Soliveres S, Smit C, Maestre FT (2015) Moving forward on facilitation research: response to changing environments and effects on the diversity, functioning and evolution of plant communities. Biol Rev 90(1):297–313
Statsoft Inc., Statistica (2014) Data analysis software system. Version 12.0. Available at www.statsoft.com
Stribley DP, Read DJ (1980) The biology of mycorrhiza in the Ericaceae. New Phytol 86(4):365–371
Sundqvist MK, Sanders NJ, Wardle DA (2013) Community and ecosystem responses to elevational gradients: processes, mechanisms, and insights for global change. Annu Rev Ecol Evol Syst 44:261–280
Tanner EVJ, Vitousek PA, Cuevas E (1998) Experimental investigation of nutrient limitation of forest growth on wet tropical mountains. Ecology 79(1):10–22
Unar J, Unarová M, Šmarda J (1985) Vegetační poměry Tomanovy doliny a Žlebu spod Diery v Západních Tatrách: Charakteristika přírodních poměrů a rostlinných společenstev. Č. 2. Univerzita JE Purkyně
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 13:87–115. https://doi.org/10.1007/BF00002772
Waroszewski J, Kalinski K, Malkiewicz M et al (2013) Pleistocene-Holocene cover-beds on granite regolith as parent material for Podzols - An example from the Sudeten Mountains. CATENA 104:161–173. https://doi.org/10.1016/j.catena.2012.11.006
Weigelt A, Bol R, Bardgett RD (2005) Preferential uptake of soil nitrogen forms by grassland plant species. Oecologia 142(4):627–635
Xiang X, He D, He JS, Myrold DD, Chu H (2017) Ammonia-oxidizing bacteria rather than archaea respond to short-term urea amendment in an alpine grassland. Soil Biol Biochem 107:218–225
Xu X, Ouyang H, Richter A, Wanek W, Cao G, Kuzyakov Y (2011) Spatio-temporal variations determine plant–microbe competition for inorganic nitrogen in an alpine meadow. J Ecol 99(2):563–571
Xue W, Bezemer TM, Berendse F (2019) Soil heterogeneity and plant species diversity in experimental grassland communities: contrasting effects of soil nutrients and pH at different spatial scales. Plant Soil 442(1):497–509
Zhang S, Chen D, Sun D, Wang X, Smith JL, Du G (2012) Impacts of altitude and position on the rates of soil nitrogen mineralization and nitrification in alpine meadows on the eastern Qinghai-Tibetan Plateau, China. Biology and Fertility of Soils 48(4):393–400
Zhong Q, Zhang S, Chen H, Li T, Zhang C, Xu X, Mao Z, Gong G, Deng O, Zhang Y, Pu Y, Wang L (2019) The influence of climate, topography, parent material and vegetation on soil nitrogen fractions. CATENA 175:329–338
Acknowledgements
We thank Andrzej Leszek Rudecki and Teresa Łysiak for their assistance with chemical analysis.
Author information
Authors and Affiliations
Contributions
Adam Rajsz: data collection, data analysis and interpretation, drafting the article, methodology, figures, statistical analysis; Bronisław Wojtuń: data collection, data analysis and interpretation, drafting the article, methodology, statistical analysis; Aleksandra Samecka-Cymerman: data analysis and interpretation, consulting statistical analysis, critically revising the article draft for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest/Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Jorge Durán.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
Supplementary file1 (DOC 102 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rajsz, A., Wojtuń, B. & Samecka-Cymerman, A. Community-specific patterns of nitrogen transformations along an elevational gradient in alpine and subalpine ecosystems. Plant Soil 479, 699–713 (2022). https://doi.org/10.1007/s11104-022-05555-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05555-6