Abstract
Background and aims
In this study, the potential of non-intrusive root electrical capacitance (CR) measurements for monitoring the seasonal changes and drought response of root activity was tested on two grassland species in a climate change experiment.
Methods
CR was detected between a ground electrode inserted into the soil and a plant electrode attached to the stem of the perennial grass Stipa borysthenica and the biennial herb Crepis rhoeadifolia in control and drought plots throughout two growing seasons. A pilot study revealed that CR was strongly correlated with root biomass for a given time and soil water content. The effect of changing soil water content on the measured CR value was accounted for by means of species-specific experimental calibrations.
Results
Root activity (CR) was found to peak at the flowering stage in late spring (S. borysthenica) or early summer (C. rhoeadifolia). Both the natural shortage of rainfall and the experimental summer drought reduced root activity in both species. Stipa borysthenica displayed great plasticity in root activity, including quick post-treatment recovery during the rainy autumn. The changes observed in root activity were similar to those previously recorded using conventional root investigation techniques (i.e. destructive, minirhizotron, ingrowth core) in temperate grasslands.
Conclusions
Root capacitance measurements proved to be adequate for monitoring root activity in situ in natural grassland. The method could be particularly useful in studies where plant injury or soil disturbance need to be avoided.
Similar content being viewed by others
Introduction
Apart from their main functions on the individual plant scale (i.e. plant anchorage, water and mineral absorption and translocation, food storage and hormonal regulation), roots play a key role in ecosystem processes associated with material and energy flows (Hooker et al. 2000). Root growth dynamics and belowground interactions strongly affect species distribution and relative abundance, and thus plant community structure (Eissenstat et al. 2013). Very few studies focus on collecting relevant information about root functional traits especially in natural vegetation, despite their obvious ecological significance (de Vries et al. 2016). Many authors monitor aboveground phenology, whereas roots are only sampled destructively at the end of the experimental period. However, as root and shoot phenology are often asynchronous and are greatly influenced by growth conditions even in the same species, the tracking of root phenology could be critical for evaluating whole-plant responses to the environment (Radville et al. 2016). The lack of information on root systems is principally due to methodological difficulties in assessing root functions directly in the soil (Milchunas 2012). The conventional destructive techniques (auger sampling, excavation, ingrowth cores) are inherently unsuitable for such studies. In-situ rhizotron and minirhizotron systems are widely used in plant ecology (e.g. Pilon et al. 2013; Bai et al. 2017; Arndal et al. 2018), but the installation requires soil disturbance, they only represent a small fraction of the root system, and they tend to generate artefacts (Vamerali et al. 2012). Advanced methods (e.g. tracers, X-ray imaging, MRI) are expensive, and have low resolution on the scale of active (absorbing) fine roots (Milchunas 2012).
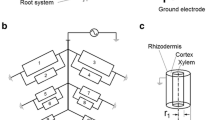
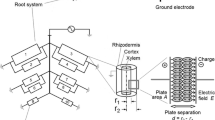
Measuring the electrical capacitance of root–soil systems (CR) is a cheap, simple and rapid non-intrusive method, based on the correlation between CR and the mass, length or surface area of the whole root system (Chloupek 1972). CR is usually detected by driving a low-frequency alternating current (1 kHz AC) between a ground electrode (metal rod) inserted into the soil and a plant electrode (needle or clamp) fixed on the stem base. According to Dalton's (1995) model, root branches act as lossy cylindrical capacitors connected in parallel. Rhizodermal membranes play the role of a dielectric, separating the conductive xylem and phloem sap from the conductive soil solution. Capacitance is formed by the active polarization (charge storage) of the membranes, and its magnitude is proportional to the area of the root–soil interface (Fig. 1). The revised two-dielectric capacitor model considers the capacitive behavior of the surrounding soil as well (Rajkai et al. 2005).
Schematic illustration of root electrical capacitance measurements with the equivalent electrical network of the root system, according to Dalton (1995) and Ellis et al. (2013). Each element (i) consists of a parallel resistor–capacitor (Ri–Ci) circuit. The plant root is represented as an axially symmetric cylindrical capacitor. In the equation for electrical capacitance ε and A are the permittivity and surface area of the root tissue, respectively, r1 is the radius of the xylem and r2 is that of the rhizodermis
The measured CR was found to be linked to root system size, but it could be influenced by variations in root tissue properties (water content, tissue density, suberization, xylem maturation, root decay) and presumably root–soil contact, which could vary with time and environmental conditions (Dalton 1995; Ellis et al. 2013). This shows an advantage of the root capacitance method: CR value seems to comprise root extension and physiological status, could be related to the water (and nutrient) uptake activity of the whole root system (referred to as “root activity” from here) (Cseresnyés et al. 2016). On the other hand, one disadvantage of the technique is the high sensitivity of detected CR values to external factors, including soil composition, soil water content (SWC), and plant electrode position (Ellis et al. 2013), so that data can only be compared when the same species is grown in the same soil type (Chloupek et al. 2010). The capacitance method has mostly been used under controlled pot conditions to estimate root size, and to study the phenology pattern and stress response of root growth in crops (Cseresnyés et al. 2016; Wu et al. 2017), native grasses and herbs (Aulen and Shipley 2012) and tree saplings (Vamerali et al. 2009). Previous studies reported the successful application of CR measurements in field-grown crops (Chloupek et al. 2010; Svačina et al. 2014; Heřmanská et al. 2015), but specific calibrations are a prerequisite for data comparison over time in the case of variable SWC (Cseresnyés et al. 2018).
In spite of the promising results in pot experiments, the CR method has not yet been applied in natural plant communities. Since root production accounts for up to 70–90% of total primary production in temperate grasslands, particularly in arid and semiarid areas (Milchunas 2012), root phenology and population dynamics have a great influence on ecosystem functioning and stress (e.g. drought) adaptation, and thus on responses to the changing climate (Carrillo et al. 2014; de Vries et al. 2016). Perennial sand grasslands, which represent valuable patches of remnant vegetation in the Pannonian region in Central Europe, are especially sensitive to climate change (Bartha et al. 2011). Regional climate models predict increasing temperature, severe summer droughts and more frequent rainfall anomalies in the Carpathian Basin for the next few decades (Bartholy et al. 2007).
The objective of this study was to test the potential of the CR method in monitoring the seasonal changes and drought responses of root activity in a native grassland. In particular, the aim was (i) to monitor the seasonal patterns of root activity in a perennial grass and a biennial herb species over two consecutive years, (ii) to evaluate the response of root activity to different annual rainfall patterns, and (iii) to assess root activity responses to an experimental two-month summer drought. Importantly, this study focused on root physiological responses at the species level by sampling individual plants non-destructively. However, the simultaneous application of this in-situ method to several coexisting species could contribute to a better understanding of responses at plant community level.
Materials and methods
Study site and plant material
The study site is located in an inland sand dune area in Central Hungary, near to the village of Fülöpháza (N46°52′17″, E19°25′17″, 107 m asl.). The vegetation is an open perennial sand grassland with 30–40% plant coverage, dominated by Festuca vaginata W. et K. and Stipa borysthenica Klokov. The area is covered by nutrient-poor, coarse-textured calcaric arenosol according to the FAO-WRB (IUSS Working Group 2015) classification (Table 1). The climate is temperate semiarid with a mean (1986–2015) annual temperature of 10.9 °C (−1.2 °C in January and 21.0 °C in July). The mean annual precipitation is 569 mm (with a peak of 78 mm in June), and 404 mm during the vegetation season (March–October). The studied species were the xerophyte, perennial C3 bunchgrass S. borysthenica, and the subordinate, biennial Crepis rhoeadifolia M. B. (Asteraceae).
Pilot experiment: Evaluating the effect of soil water content
This one-day experiment served to parameterize the relationship between CR and SWC for the two studied species in order to make the capacitance data comparable during the course of field monitoring, when SWC varied (Cseresnyés et al. 2018). Six plants of each species with fairly diverse shoot (root) sizes were selected near the experimental area. The sampling was carried out in a dry period (SWC: ~3 v/v%) at the plant flowering stage; in mid-May 2015 for S. borysthenica, and in late June for C. rhoeadifolia (second-year plants).
A PVC cylinder 32 cm in length with a 20 cm inner diameter was gently hammered vertically into the soil around each plant, leaving the upper 1-cm edge above the surface. Volumetric SWC was then measured in the root zone using a Campbell CS620 TDR meter (Campbell Sci. Ltd., Loughborough, UK) calibrated to this soil, equipped with a 20-cm-long probe. Thereafter, CR was detected with an Agilent U1733C handheld digital LCR instrument (Agilent Techn. Co. Ltd., Penang, Malaysia) set to parallel equivalent circuit at 1 kHz and 1 V AC. The ground electrode was a stainless steel rod 20 cm in length and 5 mm in diameter, inserted vertically into the soil to a depth of 18 cm at 5 cm from the plant. The other terminal of the instrument was clamped to the plant through a 4 mm wide aluminum strip, which was smeared with conductivity gel to provide good electrical contact with the plant. The strip was tied around all basal parts of the plant, including stems and leaf bases (S. borysthenica) or basal rosette (C. rhoeadifolia), and positioned as near as possible to the soil surface, but not in contact with it (Svačina et al. 2014). The electrodes were left in place throughout the experiment.
After the capacitance measurement, 250 cm3 of water was poured evenly over the soil inside the PVC cylinder, and further SWC and CR measurements were made about an hour later. The procedure was repeated several times until water leakage prevented a further increase in SWC (field capacity). A big hole was dug right next to the plastic cylinder, and a sharp metal plate (20 × 30 cm) was pushed horizontally immediately below the cylinder. The plate and the cylinder containing the soil and plant were lifted out together and transported to the laboratory. CR was recorded again to check that the excision of deeper roots by the metal plate during plant removal had not strongly influenced root capacitance. The bottom of the cylinder was capped, and the irrigation and measurement steps were continued until the soil became water saturated (the last SWC–CR data pair). Finally, the shoots were cut at the soil surface, and the roots were thoroughly washed free of soil over a 0.2-mm mesh sieve followed by root flotation. After removing debris the roots were oven-dried at 70 °C to determine root dry weight (RDW).
Each SWC value recorded was divided by the predefined saturation water content (0.386 cm3 cm−3) to obtain relative water saturation (θrel). A CR–θrel function was established for each plant of the two species and coefficient of determination (R2) was calculated using ln-transformed CR variables. For a given plant, the CR detected in water-saturated soil (θrel = 1) was considered as the apparent (saturation) root capacitance, CR*. All the CR values were divided by CR* to obtain the relative capacitance, Crel, for each θrel value. Linear regression was performed to evaluate the relationship between ln(Crel) and θrel for each plant. The correlation between CR and RDW was also analyzed for each species using the linear regression method.
The effect of species identity on the Crel–θrel functions was investigated for both species using the linear mixed effect (LME) model, where Crel was a ln-transformed response variable, θrel and species identity were explanatory variables, and individual plants were used as a categorical random effect. As the species effect was significant, separate LME analysis was performed for each species to obtain species-specific Crel–θrel functions that could be used to calculate CR*. In these analyses, θrel and individual plants were considered as explanatory variables. Statistical analyses were performed using the nlme (Pinheiro et al. 2012) and MuMIn packages (Bartoń 2015) in the R ver. 2.15.2 environment (R Core Team 2014).
Field experiment design
The field trial was conducted during the growing seasons of 2016 and 2017. Twelve plots of 2 × 2 m surrounded by a 0.5 m buffer strip were randomly established within an area of ~0.3 ha. There were two treatments in six replicate plots: an untreated control (CON) and a two-month summer drought treatment (DRO), in which the total rainfall was excluded from 23 June to 25 August 2016 and from 22 June to 23 August 2017 (a similar drought treatment was applied in the summer of 2015). These plots were covered with transparent plastic rainout shelters (with side sheets), placed at 0.8–1.0 m above the soil surface depending on the relief. Intercepted water was channeled well away from the plots. Automatic data loggers were installed 20 and 30 cm above the soil surface to record air temperature (Sensirion SHT75 sensor; Sensirion Co. Ltd., Chicago, IL, USA) and precipitation (Davis DS7852 rain collector; Davis Co. Ltd., Hayward, CA, USA), respectively, and the volumetric SWC in the 0–30 cm layer (Campbell CS616 TDR) at 10-min intervals in each plot.
Both years had close to average temperatures (0.4 °C warmer). In 2016, the annual rainfall (742 mm) exceeded the long-term mean by 30%, but intense drought prevailed from late March to late April (Fig. 2a). The 187 mm summer rain was excluded from the DRO plots. The annual mean SWC was 5.1% in the CON plots, while mean SWC values of 4.9% and 2.8% (with a minimum of 2.2%) were recorded in the CON and DRO plots, respectively, during the drought treatment. The rainout shelters increased the air temperature by 1.4 °C (CON: 22.3 °C; DRO: 23.7 °C). In 2017, the area received 635 mm (12% above the average) total precipitation (Fig. 2b). A one-month “natural” drought occurred between late May and late June (right before the simulated drought), whereas September was extremely wet (106 mm; 260% of the mean). The DRO plots were deprived of 147 mm summer rain. The mean annual SWC was 5.1% in the CON plots, while values of 4.7% and 2.6% (minimum: 2.1%) were recorded in the CON and DRO plots, respectively, during drought exposure. The air temperature increased by 1.7 °C on average as a result of the treatment (CON: 23.6 °C; DRO: 25.3 °C).
Daily precipitation (mm; columns) and volumetric soil water content (SWC %; lines) at the 0–30 cm soil depth in the control (CON) and two-month summer drought (DRO) treatments (indicated by the shaded area) during the growing season in (a) 2016 and (b) 2017. Upper and lower horizontal dashed lines correspond to SWC measured at field capacity and at wilting point, respectively
Monitoring root activity
Two plants of S. borysthenica and two of C. rhoeadifolia were selected and identified with numbered wooden sticks in each CON and DRO plot (n = 12 plants per species) to monitor root activity. The S. borysthenica plants were chosen in early April 2016 and studied over the two vegetation seasons. Second-year C. rhoeadifolia plants (with emerged stem) were chosen in late May 2016, and new second-year specimens had to be selected for the next study year. The field trials were performed on seven occasions from early April to late September for S. borysthenica, and on five occasions from late May to late September for C. rhoeadifolia in both years, including three measurements timed at the beginning, middle and end of the two-month drought period. The SWC of the root zone and the CR value were recorded for each plant with the same instruments and in the same manner as described in the pilot experiment. The electrodes were removed after the CR reading. The main plant phenology stage was determined on each occasion. No destructive investigations were performed to avoid the disturbance of soil or vegetation.
All the measured CR were converted into CR* using the θrel values associated with the relevant CR, based on empirical functions established in the pilot experiment. As the sampled plants were very variable in size (in RDW), the CR* values calculated for the first measurement were highly different. In order to eliminate the plant size effect, each initial CR* calculated for a plant individual in 2016 (and in 2017 for the new second-year plants selected for C. rhoeadifolia) was regarded as 100%. For each individual, the subsequent CR* values were divided by the corresponding initial value, and were expressed as percentage CR* ratios (%).
Data analysis
LME models were applied to investigate the effect of drought treatment on S. borysthenica and C. rhoeadifolia in two growing seasons, using the nlme package (Pinheiro et al. 2012) in the R ver. 2.15.2 environment (R Core Team 2014). The data for the two species were analysed in two separate models. The percentage CR* ratio was taken as the response variable, and the treatment, year and date of measurement within years as categorical fixed factors, while the individual plants were treated as a random effect. The model residuals were visually checked for normality and homoscedasticity. For S. borysthenica, log transformation was applied to the response variable to conform to the normality assumption. The model residuals for C. rhoeadifolia showed heteroscedastic residuals, so the varIdent variance structure was used, which allowed different residual spreads for each date within a year. Contrasts were used to make pairwise comparisons for the effect of the treatment within each date of measurement in the two years, with the help of the contrast package (Kuhn et al. 2016).
Results
Pilot experiment
Plant harvest showed RDW values ranging from 0.68 to 5.88 g and from 0.06 to 0.61 g for S. borysthenica and C. rhoeadifolia, respectively. In water-saturated soil, CR values between 0.42 and 0.92 nanofarads (nF) were detected for S. borysthenica, and between 0.46 and 2.02 nF for C. rhoeadifolia. Strong positive linear correlations (F: 17.9–57.5; R2: 0.825–0.935; p < 0.01) were found between the RDW and CR values recorded in both dry and water-saturated soil for both species (Fig. 3a,b), verifying the reliability of the CR method, irrespective of the soil moisture level.
Linear regression between root electrical capacitance (CR in nanofarads, nF) and root dry weight (RDW) in Stipa borysthenica (a) and Crepis rhoeadifolia (b) plants. The markers □ and ■ represent CR data recorded in dry (θrel ~ 0.08) and water-saturated (θrel = 1) soil, respectively. Each regression is significant at the p < 0.01 level
Regression analysis yielded exponentially increasing CR–θrel functions for each plant (Fig. 4a,c). Significant linear correlations were found between ln(CR) and θrel with R2 values of 0.911–0.978 (p < 0.01) for S. borysthenica and 0.859–0.963 (p < 0.05) for C. rhoeadifolia. The relationship between Crel and θrel could be described by the linearized equation ln(Crel) = ln(a) + b·θrel with calculated parameters ranged from 0.184 to 0.231 (a) and from 1.46 to 1.70 (b) for S. borysthenica, and from 0.066 to 0.189 (a) and from 1.61 to 2.69 (b) for C. rhoeadifolia plants (Fig. 4b,d).
Exponential relationships between root electrical capacitance (CR in nanofarads, nF) of (a) Stipa borysthenica and (c)Crepis rhoeadifolia plants and relative water saturation of soil (θrel). St-1...St-6 (S. borysthenica) and Cr-1...Cr-6 (C. rhoeadifolia) are plants in order of increasing root dry weight. (b, d) Relationships for the same species between relative root electrical capacitance (Crel) and θrel. Crel = CR/CR*, where CR* is the capacitance detected for the given plant in water-saturated soil (θrel = 1). The equations were obtained from the model fitted to the whole data set (n = 60; thick line)
According to the LME analysis, the slope and y-intercept of the Crel–θrel functions depended significantly on the species (Table 2). Separate LME analysis for S. borysthenica showed that the individual plant had no significant effect on the function, whereas in the case of C. rhoeadifolia, the function for the “Cr-2” specimen differed significantly from those of “Cr-3” and “Cr-4”. Spearman’s rank correlation analysis was conducted for clarification, but no significant monotonic associations were revealed between RDW and the slope (r = 0.257; p = 0.658) or the y-intercept (r = −0.257; p = 0.658). Therefore, the differences between the function parameters were attributed to the thick taproot of the species, specifically to the weak allometry between taproot traits, derived from the relatively high root weight to surface area (Cseresnyés et al. 2017; Wu et al. 2017), and thus were ignored in further analysis.
In general, species-specific Crel–θrel relationships were found when pooling the data over individual plants (Fig. 4b,d). Based on the Crel = CR/CR* relationship, these could be used to calculate apparent capacitance as CR* = CR · 4.888 · e–1.586θrel for S. borysthenica, and CR* = CR · 8.251 · e–2.099θrel for C. rhoeadifolia. These empirical equations allow us to normalize the field-measured CR to a water-saturated CR*, and thus correct for soil water variations (with an associated uncertainty related to the fit of the equation). In this manner, we could monitor the seasonal changes in root activity, irrespective of SWC (Cseresnyés et al. 2018).
Field monitoring
The SWC in the root zone varied temporally over a wide range in the two years depending on the rainfall conditions (Fig. 5a,b). The t test revealed significantly higher SWC in the DRO plots than in the controls at two measurement times prior to treatment (in early spring and early summer). This was presumably due to the summer drought simulation in the preceding year, which resulted in reduced plant biomass and thus less water uptake from the soil. Drought treatment significantly decreased the SWC during the treatment period (in July and August) in both years.
Volumetric soil water content (SWC; mean ± SD) measured in the root zone of Stipa borysthenica and Crepis rhoeadifolia during the growing season in 2016 (a) and 2017 (b) in the control (CON) and drought (DRO) treatments. Data were pooled over the species. Asterisks indicate the statistical differences shown by t test between the treatments (*p < 0.05; ***p < 0.001; NSnon-significant)
In 2016, the root activity (CR* ratio) of S. borysthenica increased significantly until flowering in early May (210 ± 27%; mean ± SD) in the CON plots, and then gradually decreased till the end of the vegetation period (52 ± 9%; Fig. 6a). Drought treatment markedly decreased the root activity, from 77 ± 12% to 32 ± 6% and from 69 ± 13% to 19 ± 3% in the middle and at the end of treatment, respectively (Table 3). The DRO plants showed a significant increase of up to 45 ± 8% in root activity in the post-treatment period, which was only slightly but significantly lower (p = 0.049) than that of the CON plants.
Changes in root activity represented by the CR* ratio (mean ± SD) of Stipa borysthenica (a) and Crepis rhoeadifolia (b) during the growing season in 2016 and 2017. CR* is the apparent root electrical capacitance. The CR* value calculated at the first sampling time in 2016 (and of 2017 for C. rhoeadifolia) for each plant specimen was regarded as 100%, from which the following CR* ratios were derived. CON: control; DRO: two-month summer drought treatment (indicated by the shaded area)
In 2017, the CR* ratio for S. borysthenica again peaked during the flowering stage (CON: 250 ± 38%), but this seasonal peak proved to be significantly greater than in the preceding year (likely due to more rain in spring). Thereafter, the root activity decreased sharply, and became significantly lower by the end of June than that detected in 2016 (presumably in response to drought in June). The CR* ratio was relatively steady during the summer (62–63%), and subsequently peaked again in late September at 110 ± 17% (due to ample rain), when it was significantly higher than in 2016. Simulated drought induced a substantial reduction in root activity from 63 ± 6% to 31 ± 7% and from 62 ± 9% to 25 ± 5% in the middle and at the end of treatment, respectively. The drought effect disappeared by late September due to the substantial post-treatment increase in the CR* ratio of DRO plants (up to 105 ± 19%).
The root activity of C. rhoeadifolia reached a maximum at the flowering stage in late June 2016 (CON: 137 ± 16%), and subsequently decreased markedly as the plants matured (Fig. 6b). All the plants dried up and died by the end of September, when only “stray” capacitances could be detected. The CR* ratio dropped significantly from 84 ± 12% to 54 ± 13% and from 20 ± 6% to 4 ± 1% in the middle and at the end of the drought treatment, respectively. Drought accelerated the reduction in root activity and plant senescence, leading to the death of most plants by late August.
In 2017, the CR* ratio of C. rhoeadifolia peaked already at the vegetative (stem elongation) growth stage in late May, and then significantly decreased at flowering (CON: 74 ± 12%), when it was substantially lower than in 2016. The lack of rainfall in June led to the formation of fewer, smaller inflorescences, and promoted the senescence and shedding of older leaves from the lower nodes. Root activity decreased continuously throughout the whole measurement period. Drought treatment resulted in a further significant reduction in the CR* ratio (due to accelerated plant maturity), from 54 ± 9% to 44 ± 10% and from 18 ± 4% to 11 ± 4% in the middle and at the end of treatment, respectively. The CON plants exhibited significantly lower root activity during summer 2017 than in 2016.
Discussion
Root phenology
The root system activity, expressed indirectly as the apparent root electrical capacitance, showed characteristic seasonality for both species. The standing root mass and functional activity are the result of root production, growth, mortality and decomposition processes (i.e. root turnover), which occur simultaneously and fluctuate widely over the season (Fukuzawa et al. 2012). Although root phenology is genetically programmed, it is strongly influenced by a variety of endogenous (e.g. photosynthate supply) and exogenous factors, including temperature, soil water regime, nutrient availability and belowground competition (Radville et al. 2016). The root growth pattern also depends on the plant life cycle (Schulze et al. 2005). Herbaceous perennials tend to show markedly greater root activity before and during the flowering stage, which can be attributed to the increasing leaf area index and photosynthetic capacity. Under favorable environmental conditions, the leaf area, whole-plant transpiration and thus root water uptake rate may be transiently maintained by the continuous formation of new leaves in parallel with the dying of old leaves. Biennials (e.g. C. rhoeadifolia) develop a flowering shoot in the second year, with large leaves in many species. These ontogenetic changes are accompanied by substantially enhanced water use, followed by a sharp decrease during plant senescence.
Investigations in European humid grasslands, involving soil coring, rhizotron or minirhizotron methods, revealed seasonal patterns of fine root production, root length and root biomass with peaks in late spring and early summer (May–June) (Sindhøj et al. 2000; Arndal et al. 2018). An early summer (June) maximum of root length and water uptake activity was demonstrated in alluvial grassland vegetation in England by combining chemical tracers with soil core sampling (Fitter 1986). Furthermore, sequential soil coring showed a midsummer peak in the standing root biomass followed by a continuous decline in a North American semiarid mixed grass prairie dominated by perennial C4 grasses (Carrillo et al. 2014).
Nevertheless, Radville et al. (2016) concluded that many species found in arid and semiarid grasslands (such as S. borysthenica) adjusted to severe summer drought by reducing their root growth in midsummer after maximum production in the earlier, more favorable spring season. In a Canadian, continental grassland vegetation dominated by Stipa comata, root production exhibited a bimodal temporal pattern characterized by a major peak in early summer and a second one at the beginning of fall (Steinaker and Wilson 2008). A study on the root lifespan of various native Stipa grasslands demonstrated that the spring maximum of root activity was due to the production of ephemeral roots mainly involved in water and nutrient uptake (these were chiefly decomposed during summer), while the autumn peak was related to the growth of long-lived roots primarily used to store nutrients and produce new laterals (Bai et al. 2017).
The aforementioned observations corroborated the present findings on seasonal changes in root activity, tracked in situ with the capacitance method for the sampled species.
Root response to drought
The present approach demonstrated the influence of both annual deviations in rainfall and of artificially induced water deficit on root activity. The 1.4–1.7 °C increase in the mean air temperature during the summer rainfall exclusion obviously intensified the drought stress. Temperate species frequently respond to intense summer drought by reducing the root growth rate (Fukuzawa et al. 2012). The use of ingrowth cores revealed a significant decrease in root production and root dry weight in a Central European dry Festuca grassland subjected to a 50% reduction in annual rainfall (Fiala et al. 2009). The present results are in accordance with those obtained in a North American native tallgrass prairie by Nippert and Knapp (2007), who demonstrated that summer water shortage resulted in a smaller seasonal peak in the water use patterns of the dominant species, and that this drought effect was eliminated by abundant autumn rains.
The root system response to soil drying depends on the species, on the intensity, timing and duration of stress, and on other concurrent environmental factors (de Micco and Aronne 2012). In many cases, moderate drought either has no effect on root growth or may even stimulate it (via signal transduction pathways) to mitigate the stress, as was demonstrated in grasslands exposed to climate manipulation (Pilon et al. 2013; Arndal et al. 2018). Severe drought may restrict fine root growth and reduce root lifespan either directly (osmotic effect) or indirectly i.e. reduced photosynthesis and carbohydrate supply (Eissenstat et al. 2013), leading to the formation of thicker but shorter root branches and altered root system architecture (de Micco and Aronne 2012). Anatomical and histochemical changes in the roots, especially the accelerated maturation of the exo- and endodermis with enhanced lignin and suberin contents, modify the extra- and intracellular current pathways inside the roots (Cseresnyés et al. 2017; Li et al. 2017). Lignin and suberin have smaller relative permittivity (εr = 2–2.4) than the other main constituents of the root (water: εr ~ 80; cellulose: εr ~ 7.6) (Torgovnikov 1993; Hollertz 2014). As the capacitance is directly proportional to the dielectric permittivity, the extensive lignification (decreased ratio of the absorptive root segments) and the reduced water content in root tissues caused by drought could jointly reduce the measured CR. In addition, decay and death of the shallow roots during drought would decrease the root mass and thus the capacitance. In our case, this effect could arise mainly for S. borysthenica, and points the limitation of using the capacitance method in relation with drought effect. Nevertheless, while Urban et al. (2011) stated that electrical response was chiefly determined by the proximal part of the root–soil interface, experiments by Ellis et al. (2013) suggested that CR was strongly influenced by distal fine roots.
In the present experiment, S. borysthenica displayed great plasticity in root activity under variable rainfall conditions. The majority of spring-active grasses are able to develop a particularly dense adventitious root system in the topsoil for more efficient water exploitation; however, these shallow fine roots are also the most sensitive part of the whole root system to soil moisture deficit (Pilon et al. 2013). CR measurements demonstrated that the root activity of S. borysthenica recovered well after soil rewetting. Rapid recovery of this xerophyte species after temporal water stress has been previously described by Kalapos (1994).
The accelerated post-flowering decline in the root activity of C. rhoeadifolia during drought treatment was presumably linked to the stress-induced acceleration of root system mortality in annuals and biennials (Fukuzawa et al. 2012). In the present case, the early-summer drought in 2017 resulted in a continuous decrease in the root activity of this species even during the flowering phase. Drought treatment was observed to shorten the growth cycle of C. rhoeadifolia, probably because the root system could not satisfy the rising water demands of the developing stem and inflorescences (Schulze et al. 2005). Bartha et al. (2011) reported that though sand grassland species were well adapted to stressed environments, serious summer droughts often resulted in their local dieback.
Root capacitance method – Pros and cons
The results demonstrated that the apparent electrical capacitance determined without plant injury could be a good indicator of changes in root system activity. The evaluation of the CR method given above was chiefly based on reviewing previous works that used rhizotron or minirhizotron imaging systems for monitoring root traits in situ.
An evident drawback of the CR method compared with minirhizotrons or ingrowth cores is that it does not make it possible to visualize the root system architecture (e.g. depth distribution, branching order) or to quantify root parameters (e.g. diameter, specific length, growth rate). In contrast, the minirhizotron method is unable to discriminate between the root systems of different plants and species, restricting the results to the plant community as a whole (Sindhøj et al. 2000; Arndal et al. 2018). Plant-level observations require the use of supplementary imaging techniques, such as MRI or positron emission tomography (PET) in these cases (Faget et al. 2013). On the other hand, CR is not confounded by the intermingling of root systems (there is no electrical connection between the neighboring plants), and is thus suitable for detecting the root responses of individual plants (Ellis et al. 2013). The capacitance measured gives a good estimation of the belowground biomass during the physiologically most active plant growth stages (as was shown by destructive sampling in the pilot experiment). However, we are not presently aware of how the morphological and structural components act on the CR signal (Ellis et al. 2013). During periods of fast change in the environment (drying out, rewetting), the change in root capacitance may reflects the change in root activity (root biomass or histological properties). This could be especially important, considering the methodological problems faced when attempting to discern live and dead roots, estimate root longevity or study fine-root functions (Hooker et al. 2000).
The comparability of the capacitance data is restricted to same plant species–soil systems owing to its sensitivity to external factors. Furthermore, CR values cannot be converted directly to root water uptake rate, for instance. The method is less efficient in complex soils than in hydroponics or mineral substrates (Postic and Doussan 2016), such as coarse sand, in which the present measurements were made. This is because chemically and structurally complex soils (e.g. chernozem), and particularly organic substrates (e.g. composts or peat) contain high amount of polarizable charges on colloidal clay minerals and organic compounds (Singh and Uehara 1999). Dielectric character of these growth media could confound root electrical measurements, as indicated by the weaker correlations between CR and root traits (Aulen and Shipley 2012; Cseresnyés et al. 2017). Heterogeneous soil structure often leads to spatially more variable moisture conditions as well (Cseresnyés et al. 2018). Change in SWC is the external factor with the greatest effect on CR. Dalton (1995) suggested that decreasing root capacitance in a drying soil was due to the reduction in the root surface area in contact with soil pore water. On the contrary, Ellis et al. (2013) explained this effect by a reduced electrode–soil contact, considering that root system has substantially higher surface area than has the ground electrode. Nevertheless, as the present study demonstrated, the SWC effect can be eliminated by applying the apparent capacitance (CR*). Although here we obtained species-specific (and likely soil-specific) calibrations for the plants possessing contrasting root traits, further investigations using species with similar root architecture (e.g. different grasses) could be beneficial. Extension of the results to other species could simplify further analyses using the capacitance method. The CR measurements can be quickly performed in the field with a cheap, portable LCR meter. The low voltage (1 V) and current (a few μA) do not injure the plant tissues or disturb the soil. The technique is suitable for monitoring the root dynamics of large plant populations in their natural soil environment. However, more studies will be required to evaluate the potential of the CR method to complement the labor-intensive and destructive routine root observations. The application of the capacitance method concurrently with other conventional or advanced techniques may serve to further validate the measurements.
Conclusions
The capacitance method, taking into account the SWC effect, proved to be an adequate non-invasive tool to follow seasonal patterns in root activity, and to detect the root response to rainfall anomalies, including drought simulation in a natural ecosystem. Plant-level observations provided new insight into population- or species-level root phenology and stress responses. The technique could play a role in diverse fields of ecological research. Monitoring a large number of individual plants may make it possible to detect the influence of environmental conditions (e.g. micrometeorological parameters, enhanced CO2 concentration, slope exposure) and biotic factors (e.g. neighboring species, root–root interactions, shading) on root traits. These results could increase our understanding of natural vegetation processes (e.g. changes in the species abundance or community structure, plant invasiveness, succession) and responses to climate or related environmental changes. Nevertheless, relating the capacitance to root morphology and function is challenging at present, as the electric signal is still poorly understood.
Abbreviations
- CR :
-
Root electrical capacitance
- CR*:
-
Apparent root electrical capacitance
- Crel :
-
Relative root electrical capacitance
- LME:
-
Linear mixed effect model
- SWC:
-
Soil water content
- RDW:
-
Root dry weight
- θrel :
-
Relative water saturation
References
Arndal MF, Tolver A, Larsen KS, Beier C, Schmidt IK (2018) Fine root growth and vertical distribution in response to elevated CO2, warming and drought in a mixed heathland–grassland. Ecosystems 21:15–30. https://doi.org/10.1007/s10021-017-0131-2
Aulen M, Shipley B (2012) Non-destructive estimation of root mass using electrical capacitance on ten herbaceous species. Plant Soil 355:41–49. https://doi.org/10.1007/s11104-011-1077-3
Bai WM, Zhou M, Fang Y, Zhang WH (2017) Differences in spatial and temporal root lifespan of three Stipa grasslands in northern China. Biogeochemistry 132:293–306. https://doi.org/10.1107/s10533-017-0302-4
Bartha S, Campetella G, Kertész M, Hahn I, Kröel-Dulay GY, Rédei T, Kun A, Virágh K, Fekete G, Kovács-Láng E (2011) Beta diversity and community differentiation in dry perennial sand grasslands. Ann Bot 1:9–18. https://doi.org/10.4462/annbotrm-9118
Bartholy J, Pongrácz R, Gy G (2007) Regional climate change expected in Hungary for 2071–2100. Appl Ecol Environ Res 5:1–17
Bartoń K (2015) MuMIn: Multi-model inference. R package version 1.10.0 Retrieved 11 March 2017 from http://cran.r-project.org/package=MuMIn
Carrillo Y, Dijkstra FA, LeCain D, Morgan JA, Blumenthal D, Waldron S, Pendall E (2014) Disentangling root responses to climate change in a semiarid grassland. Oecologia 175:699–711. https://doi.org/10.1007/s00442-014-2912-z
Chloupek O (1972) The relationship between electric capacitance and some other parameters of plant roots. Biol Plant 14:227–230. https://doi.org/10.1007/BF02921255
Chloupek O, Dostál V, Středa T, Psota V, Dvořáčková O (2010) Drought tolerance of barley varieties in relation to their root system size. Plant Breed 129:630–636. https://doi.org/10.1111/j.1439-0523.2010.01801.x
Core Team R (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Cseresnyés I, Rajkai K, Takács T (2016) Indirect monitoring of root activity in soybean cultivars under contrasting moisture regimes by measuring electrical capacitance. Acta Physiol Plant 38:121–112. https://doi.org/10.1007/s11738-016-2149-z
Cseresnyés I, Kabos S, Takács T, Végh RK, Vozáry E, Rajkai K (2017) An improved formula for evaluating electrical capacitance using the dissipation factor. Plant Soil 419:237–256. https://doi.org/10.1007/s11104-017-3336-4
Cseresnyés I, Szitár K, Rajkai K, Füzy A, Mikó P, Kovács R, Takács T (2018) Application of electrical capacitance method for prediction of plant root mass and activity in field-grown crops. Front Plant Sci 9:93. https://doi.org/10.3389/fpls.2018.00093
Dalton FN (1995) In-situ root extent measurements by electrical capacitance methods. Plant Soil 173:157–165. https://doi.org/10.1007/BF00155527
de Micco V, Aronne G (2012) Morpho-anatomical traits for plant adaptation to drought. In: Aroca R (ed) Plant responses to drought stress. Springer, Heidelberg, pp 37–62. https://doi.org/10.1007/978-3-642-32653-0
de Vries FT, Brown C, Stevens CJ (2016) Grassland species root response to drought: consequences for soil carbon and nitrogen availability. Plant Soil 409:297–312. https://doi.org/10.1007/11104-016-2964-4
Eissenstat DM, McCormack ML, Du Q (2013) Global change and root lifespan. In: Eshel A, Beeckman T (eds) Plant Roots: The Hidden Half, 4th edn. CRC Press, Boca Raton, pp 27/1–27/2713
Ellis T, Murray W, Paul K, Kavalieris L, Brophy J, Williams C, Maass M (2013) Electrical capacitance as a rapid non-invasive indicator of root length. Tree Physiol 33:3–17. https://doi.org/10.1093/treephys/tps115
Faget M, Nagel KA, Walter A, Herrera JM, Jahnke S, Schurr U, Temperton VM (2013) Root–root interactions: extending our perspective to be more inclusive of the range of theories in ecology and agriculture using in-vivo analyses. Ann Bot 112:253–266. https://doi.org/10.1093/aob/mcs296
Fiala K, Tůma I, Holub P (2009) Effect of manipulated rainfall on root production and plant belowground dry mass of different grassland ecosystems. Ecosystems 12:906–914. https://doi.org/10.1007/s10021-009-9264-2
Fitter AH (1986) Spatial and temporal patterns of root activity in a species-rich alluvial grassland. Oecologia 69:594–599. https://doi.org/10.1007/BF00410368
Fukuzawa K, Dannoura M, Shibata H (2012) Fine root dynamics and root respiration. In: Mancuso S (ed) Measuring roots. Springer, Berlin, pp 291–302. https://doi.org/10.1007/978-3-642-22067-8
Heřmanská A, Středa T, Chloupek O (2015) Improved wheat grain yield by a new method of root selection. Agron Sustain Dev 35:195–202. https://doi.org/10.1007/s13593-014-0227-4
Hollertz R (2014) Dielectric properties of wood fibre components relevant for electrical insulation applications. Dissertation, KTH Royal Institute of Technology, Stockholm
Hooker JE, Hendrick R, Atkinson D (2000) The measurement and analysis of fine root longevity. In: Smit AL, Bengough AG, Engels C, van Noordwijk M, Pellerin S, van de Geijn SC (eds) Root methods: a handbook. Springer, Berlin, pp 273–304. https://doi.org/10.1007/978-3-662-04188-8
IUSS Working GGroup (2015) WWorld Reference Base (WRB) for Soil Resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports 106, FAO, Rome.
Kalapos T (1994) Leaf water potential – leaf water deficit relationship for ten species of a semiarid grassland community. Plant Soil 160:105–112. https://doi.org/10.1007/BF00150351
Kuhn M, Weston S, Wing J, Forester J (2016) The contrast package. https://cran.r-project.org/web/packages/contrast/vignettes/contrast.pdf
Li MQ, Li JY, Wei XH, Zhu WJ (2017) Early diagnosis and monitoring of nitrogen nutrition stress in tomato leaves using electrical impedance spectroscopy. Int J Agric Biol Eng 10:194–205. https://doi.org/10.3965/j.ijabe.20171003.3188
Milchunas DG (2012) Biases and errors associated with different root production methods and their effects on field estimates of belowground net primary production. In: Mancuso S (ed) Measuring roots. Springer, Berlin, pp 303–339. https://doi.org/10.1007/978-3-642-22067-8
Nippert JB, Knapp AK (2007) Linking water uptake with rooting patterns in grassland species. Oecologia 153:261–272. https://doi.org/10.1007/s00442-007-0745-8
Pilon R, Picon-Cochard C, Bloor JMG, Revaillot S, Kuhn E, Falcimagne R, Balandier P, Soussana J-F (2013) Grassland root demography responses to multiple climate change drivers depend on root morphology. Plant Soil 364:395–408. https://doi.org/10.1007/s11104-012-1371-8
Pinheiro J, Bates D, DebRoy S, Sarkar D and the R Development Core Team (2012) Nlme: linear and nonlinear mixed effects models. R package version 3.1–103. https://cran.r-project.org/web/packages/nlme/nlme.pdf
Postic F, Doussan C (2016) Benchmarking electrical methods for rapid estimation of root biomass. Plant Methods 12:33. https://doi.org/10.1186/s13007-016-0133-7
Radville L, McCormack ML, Post E, Eissenstat DM (2016) Root phenology in a changing climate. J Exp Bot 67:3617–3628. https://doi.org/10.1093/jxb/erw062
Rajkai K, Végh RK, Nacsa T (2005) Electrical capacitance of roots in relation to plant electrodes, measuring frequency and root media. Acta Agron Hung 53:197–210. https://doi.org/10.1556/AAgr.53.2005.2.8
Schulze E-D, Beck E, Müller-Hohenstein K (2005) Plant ecology. Springer, Berlin
Sindhøj E, Hansson A-C, Andrén O, Kätterer T, Marissink M, Pettersson R (2000) Root dynamics in a semi-natural grassland in relation to atmospheric carbon dioxide enrichment, soil water and shoot biomass. Plant Soil 223:253–263. https://doi.org/10.1023/A:1004801718567
Singh U, Uehara G (1999) Electrochemistry of the double layer: principles and applications to soils. In: Sparks DL (ed) Soil physical chemistry, 2nd edn. CRC Press, Boca Raton, pp 1–46
Steinaker DF, Wilson SD (2008) Phenology of fine roots and leaves in forest and grassland. J Ecol 96:1222–1229. https://doi.org/10.1111/j.1365-2745.2008.01439.x
Svačina P, Středa T, Chloupek O (2014) Uncommon selection by root system size increases barley yield. Agron Sustain Dev 34:545–551. https://doi.org/10.1007/s13593-013-0160-y
Torgovnikov GI (1993) Dielectric properties of wood and wood-based materials. Springer, Berlin. https://doi.org/10.1007/978-3-642-77453-9
Urban J, Bequet R, Mainiero R (2011) Assessing the applicability of the earth impedance method for in situ studies of tree root systems. J Exp Bot 62:1857–1869. https://doi.org/10.1093/jxb/erq370
Vamerali T, Bandiera M, Coletto L, Zanetti F, Dickinson NM, Mosca G (2009) Phytoremediation trials on metal- and arsenic-contaminated pyrite wastes (Torvicosa, Italy). Environ Pollut 157:887–894. https://doi.org/10.1016/j.envpol.2008.11.003
Vamerali T, Bandiera M, Mosca G (2012) Minirhizotrons in modern root studies. In: Mancuso S (ed) Measuring Roots. Springer, Berlin, pp 341–361. https://doi.org/10.1007/978-3-642-22067-8
Wu W, Duncan RW, Ma B-L (2017) Quantification of canola root morphological traits under heat and drought stresses with electrical measurements. Plant Soil 415:229–244. https://doi.org/10.1007/s11104-016-3155-z
Acknowledgments
The project was implemented with the support provided from the National Research, Development and Innovation Fund of Hungary (Project No. 112576, 115714 and 129068, financed under the K-16 funding scheme) and a János Bolyai Research Scholarship from the Hungarian Academy of Sciences. The authors thank Prof. Tibor Kalapos for valuable remarks and Barbara Harasztos for language editing.
Funding
Open access funding provided by Centre for Agricultural Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Rafael S. Oliveira .
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cseresnyés, I., Rajkai, K., Szitár, K. et al. Root capacitance measurements allow non-intrusive in-situ monitoring of the seasonal dynamics and drought response of root activity in two grassland species. Plant Soil 449, 423–437 (2020). https://doi.org/10.1007/s11104-020-04505-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04505-4