Abstract
Aims
The relative benefits that mycorrhizal fungi confer to host plants and reforestation efforts may differ due to the host tree species and soil nutrient status. Our research objective was to examine the effects of mycorrhizal fungi, host tree species, and soil nutrient availability on nutrient acquisition in tropical reforestation.
Methods
Four tree species (Pinus caribaea, Quercus insignis, Swietenia macrophylla, and Terminalia amazonia) were planted across eight sites and subjected to five nutrient treatments in southern Costa Rica.
Results
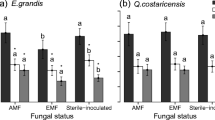
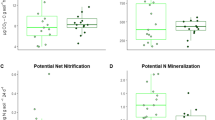
After two years, there were strong growth differences based upon tree species but not mycorrhizal fungi. Site variation, specifically base cation availability, and nutrient treatments also influenced tree growth and tissue nutrient concentrations. In a complementary greenhouse experiment isolating fungal from tree species effects, the ratio of nutrients in treatments was more important to tree growth responses to fungal symbionts than individual nutrients.
Conclusions
These results show that soil nutrient availability may be more important to tree species’ nutrient acquisition than mycorrhizal fungi. This study highlights the importance of tree species selection and replication across sites with different soil nutrient ratios in order to make management recommendations for reforestation success.





Similar content being viewed by others
References
Aldrich-Wolfe L (2007) Distinct mycorrhizal communities on new and established hosts in a transitional tropical plant community. Ecology 88:559–566
Alexander IJ, Hogberg P (1986) Ectomycorrhizas of tropical angiospermous trees. New Phytol 102:541–549
Alexander I, Selosse MA (2009) Mycorrhizas in tropical forests: a neglected research imperative. New Phytol 182:14–16
Allen EB, Allen MF, Egerton-Warburton L, Corkidi L, Gomez-Pompa A (2003) Impacts of Early- and Late-Seral Mycorrhizae during Restoration in Seasonal Tropical Forest, Mexico. Ecol Appl 13:1701–1717
Allsop N, Stock D (1995) Relationship between seed reserves, seedling growth and mycorrhizal responses in 14 related shrubs (Rosidae) from a low nutrient environment. Funct Ecol 9:248–254
Ashton PMS, Gamage S, Gunatilleke IAUN, Gunatilleke CVS (1997) Restoration of a Sri Lankan rainforest: using Caribbean pine Pinus caribaea as a nurse for establishing late-successional tree species. J Appl Ecol 34:915–925
Austin PC, Hux JE (2002) A brief note on overlapping confidence intervals. J Vasc Surg 36:194–195
Baligar VC, Bennett O (1986) NPK-fertilizer efficiency – a situation analysis for the tropics. Fert Res 10:147–164
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Baxter JW, Dighton J (2005) Phosphorus source alters host plant response to ectomycorrhizal diversity. Mycorrhiza 15:513–523
Besford RT (1979) Quantitative aspects of leaf acid phosphatase activity and the phosphorus status of tomato plants. Ann Bot 44:153–161
Bever JD (2002) Negative feedback within a mutualism: host-specific growth of mycorrhizal fungi reduces plant benefit. P Roy Soc Lond B 269:2595–2601
Bloomfield HE, Handley JF, Bradshaw AD (1982) Nutrient deficiencies and the aftercare of reclaimed derelict land. J Appl Ecol 19(15):1–8
Blum JD, Klaue A, Nezat CA, Driscoll CT, Johnson CE, Siccama TG, Eagar C, Fahey TJ, Likens GE (2002) Mycorrhizal weathering of apatite as an important calcium source in base-poor forest ecosystems. Nature 417:729–731
Bradshaw A (2004) The role of nutrients and the importance of function in the assembly of ecosystems. In: Templeton VM, Hobbs R, Nuttle TH, Halle S (eds) Assembly rules and restoration ecology: bridging the gap between theory and practice. Island Press, Washington, D.C., pp. 325–340
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1996) Working with Mycorrhizas in Forestry and Agriculture, vol 32. ACIAR, Pirie, Canberra, Monograph
Burgess TI, Malajczuk N, Groves TS (1993) The ability of 16 ectomycorrhizal fungi to increase growth and phosphorus uptake of Eucalyptus globulus Labill. and E. diversicolor. Plant Soil 153:155–164
Cairney JWG (1999) Intraspecific physiological variation: implications for understanding functional diversity in ectomycorrhizal fungi. Mycorrhiza 9:125–135
Calvo-Alvarado JC, Arias D, Richter DD (2007) Early growth performance of native and introduced fast growing tree species in wet to sub-humid climates of the Southern region of Costa Rica. Forest Ecol Manag 242:227–235
Celentano D, Zahawi RA, Finegan B, Ostertag R, Cole RJ, Holl KD (2011) Litterfall Dynamics under Different Tropical Forest Restoration Strategies in Costa Rica. Biotropica 43:279–287
Chalot M, Brun A (1998) Physiology of organic nitrogen acquisition by ectomycorrhizal fungi and ectomycorrhizas. FEMS Microbiol Rev 22:21–44
Chhetri DBK, Fowler GW (1996) Estimating diameter at breast height and basal diameter of trees from stump measurements in Nepal’s lower temperate broad-leaved forests. Forest Ecol Manag 81:75–84
Clarholm M, Rosengren-Brinck U (1995) Phosphorus and nitrogen fertilization of a Norway spruce forest: effects on needle concentrations and acid phosphatase activity in the humus layer. Plant Soil 175:239–249
Conn C, Dighton J (2000) Litter quality influences on decomposition, ectomycorrhizal community structure and mycorrhizal root surface acid phosphatase activity. Soil Biol Biochem 32:489–496
Development Core Team R (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria http://www.R-project.org
Dickie IA, Koele N, Blum JD, Gleason JD, McGlone MS (2014) Mycorrhizas in changing ecosystems. Botany 92:149–160
Egerton-Warburton L, Allen MF (2001) Endo- and ectomycorrhizas in Quercus agrifolia Nee. (Fagaceae): patterns of root colonization and effects on seedling growth. Mycorrhiza 11:283–290
Gehring CA (2004) Seed reserves and light intensity affect the growth and mycorrhizal development of the seedlings of an Australian rain forest tree. J Trop Ecol 20:345–349
Gehring CA, Whitham TG (1991) Herbivore-driven mycorrhizal mutualism in insect-susceptible pinyon pine. Nature 353:556–557
Godbold D, Feig R, Cremer-Herms A, Huttermann A (1993) Determination of stress bioindicators in three Norway spruce stands in northern Germany. Water Air Soil Pollut 66:231–237
Grayston SJ, Vaughan D, Jones D (1996) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol 5:29–56
Grman E, Robinson TMP (2013) Resource availability and imbalance affect plant–mycorrhizal interactions: a field test of three hypotheses. Ecology 94:62–71
Hedin LO, Vitousek PM, Matson PA (2003) Nutrient losses over four million years of tropical forest development. Ecology 84:2231–2255
Hodge A, Alexander IJ, Gooday GW (1995) Chitinoytic activities of Eucalyptus piluaris and Pinus sylvestris root systems challenged with mycorrhizal and pathogenic fungi. New Phytol 131:255–261
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407
Hogan CM (2014) Talamancan montane forests. World Wildlife Fund. Retrieved from http://www.eoearth.org/view/article/156401/. Accessed 20 November 2015
Holdridge LR (1967) Life zone ecology. Tropical Science Center, San Jose, Costa Rica
Holl KD, Zahawi RA, Cole RJ, Ostertag R, Cordell S (2011) Planting Seedlings in Tree Islands versus Plantations as a Large-Scale Tropical Forest Restoration Strategy. Restor Ecol 19:470–479
Holste EK, Kobe RK, Vriesendorp CF (2011) Seedling growth responses to soil resources in the understory of a wet tropical forest. Ecology 92:1828–1838
Ianson DC, Allen MF (1986) The effects of soil texture on extraction of vesicular-arbuscular mycorrhizal fungal spores from arid sites. Mycologia 78:164–168
ITCR (2004) Atlas Digital de Costa Rica. Escuela de Ingeniería Forestal, Instituto Tecnológico de Costa Rica, Cartago, Costa Rica
Janos JP (1980) Vesicular-arbuscular mycorrhizae affect lowland tropical rainforest plant growth. Ecology 61:151–162
Janos DP (2007) Plant responsiveness to mycorrhizas differs from dependence upon mycorrhizas. Mycorrhiza 17:75–91
Johnson NC (2010) Tansley review: resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647
Kardol P, Wardle DA (2010) How understanding aboveground–belowground linkages can assist restoration ecology. Trends Ecol Evol 25:670–679
Karst J, Marczak L, Jones MD, Turkington R (2008) The mutualism-parasitism continuum in ectomycorrhizas: a quantitative assessment using meta-analysis. Ecology 89:1032–1042
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bucking H (2011) Reciprocal Rewards Stabilize Cooperation in the Mycorrhizal Symbiosis. Science 333:880–882
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Kobe RK, Iyer M, Walters MB (2010) Optimal partitioning theory revisited: Nonstructural carbohydrates dominate root mass responses to nitrogen. Ecology 91:166–179
Kolari KK, Sarjala T (1995) Acid phosphatase activity and phosphorus nutrition in Scots pine needles. Tree Physiol 15:747–752
Lamb D, Erskine PD, Parrotta JD (2005) Restoration of Degraded Tropical Forest Landscapes. Science 310:1628–1632
Leigh J, Hodge A, Fitter AH (2009) Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol 181:199–207
Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748
Malloch DW, Pirozynski KA, Raven PH (1980) Ecological and evolutionary significance of mycorrhizal symbioses in vascular plants (a review). P Natl Acad Sci USA 77:2113–2118
Marschner H, Dell B (1994) Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159:89–102
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A New Method which Gives an Objective Measure of Colonization of Roots by Vesicular-Arbuscular Mycorrhizal Fungi. New Phytol 115:495–501
McGuire KL, Henkel TW, de la Cerda IG, Villa G, Edmund F, Andrew C (2008) Dual mycorrhizal colonization of forest-dominating tropical trees and the mycorrhizal status of non-dominant tree and liana species. Mycorrhiza 18:217–222
Mehlich A (1984) Mehlich-3 soil test extractant – a modification of mehlich-2 extractant. Commun Soil Sci Plan 15:1409–1416
Meinhardt KA, Gehring CA (2012) Disrupting mycorrhizal mutualisms: a potential mechanism by which exotic tamarisk outcompetes native cottonwoods. Ecol Appl 22:532–549
Peay KG, Kennedy PG, Davies SJ, Tan S, Bruns TD (2010) Potential link between plant and fungal distributions in a dipterocarp rainforest: community and phylogenetic structure of tropical ectomycorrhizal fungi across a plant and soil ecotone. New Phytol 185:529–542
Phillips RP, Fahey TJ (2006) Tree species and mycorrhizal associations influence the magnitude of rhizosphere effects. Ecology 87:1302–1313
Phillips RP, Midgley MG, Brozstek E (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in forests. New Phytol 199:41–51
Picone C (2000) Diversity and abundance of arbuscular-mycorrhizal fungus spores in tropical forest and pasture. Biotropica 32:734–750
Piotto D, Montagnini F, Ugalde L, Kanninen M (2003) Performance of forest plantations in small and medium-sized farms in the Atlantic lowlands of Costa Rica. Forest Ecol Manag 175:195–204
Plenchette C, Fortin JA, Furlan V (1983) Growth responses of several plant species to mycorrhizae in a soil of moderate P fertility. I. Mycorrhizal dependency under field conditions. Plant Soil 70:199–209
Porder S, Vitousek P, Chadwick O, Chamberlain C, Hilley G (2007) Uplift, erosion and phosphorus limitation in terrestrial ecosystems. Ecosystems 10:158–170
Read DJ (1991) Mycorrhizas in ecosystems - Nature’s response to the “Law of the minimum.” In Hawksworth DL. (ed) Frontiers in mycology. CAB International, Wallingford, UK. pp. 101–130
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems – a journey towards relevance? New Phytol 57:475–492
Reynolds HL, Hartley AE, Vogelsang KM, Bever JD, Schultz PA (2005) Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol 167: 869-880
Richards AE, Forrester DI, Bauhus J, Scherer-Lorenzen M (2010) The influence of mixed tree plantations on the nutrition of individual species: a review. Tree Physiol 30:1192–1208
Robertson GP, Tiedje JM (1988) Deforestation alters denitrification in a lowland tropical rain forest. Nature 336:756–759
Ross SM (1993) Organic matter in tropical soils: current conditions, concerns and prospects for conservation. Prog Phys Geogr 17:265–305
Sahani U, Behera N (2001) Impact of deforestation on soil physicochemical characteristics, microbial biomass and microbial activity of tropical soil. Land Degrad Dev 12:93–105
Schenck NC, Pérez Y (1990) Manual for the Identification of VA Mycorrhizal Fungi, 3rd edn. Synergistic Publications, Gainesville, FL
Singmann H, Bolker B, Westfall J, Aust F (2016) Afex: Analysis of Factorial Experiments. R package version 0:16–11 https://CRAN.R-project.org/package=afex
Siqueira JO, Carneiro MAC, Curi N, da Silva Rosado SC, Davide AC (1998) Mycorrhizal colonization and mycotrophic growth of native woody species as related to successional groups in southeastern Brazil. Forest Ecol Manag 107:241–252
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis, 3rd edn. Academic Press, New York
St. Clair SB, Lynch JP (2005) Base cation stimulation of mycorrhization and photosynthesis of sugar maple on acid soils are coupled by foliar nutrient dynamics. New Phytol 165:581–590
Stevens PA, Harrison AF, Jones HE, Williams TG, Hughes S (1993) Nitrate leaching from a Sitka spruce plantation and the effect of fertilization with phosphorus and potassium. Forest Ecol Manag 58:233–247
Tanner EVJ, Kapos V, Freskos S, Healy JR, Theobald AM (1990) Nitrogen and phosphorus fertilization of Jamaican montane forest tress. J Trop Ecol 6:231–238
Ticconi CA, Abel S (2004) Short on phosphate: plant surveillance and countermeasures. Trends Plant Sci 9:548–555
Trappe JM (1962) Fungus associates of ectotrophic mycorrhizae. Bot Rev 28:538–606
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Treseder KK (2013) The extent of mycorrhizal colonization of roots and its influence on plant growth and phosphorus content. Plant Soil 371:1–13
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515
Verbruggen E, Jansa J, Hammer EC, Rillig MC (2016) Do arbuscular mycorrhizal fungi stabilize litter-derived carbon in soil? J Ecol 104:261–269
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar: a simple staining Technique for Arbuscular-Mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Wang B, Qui YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
Wishnie MH, Dent DH, Mariscal E, Deago J, Cedeno N, Ibarra D, Condit R, Ashton PMS (2007) Initial performance and reforestation potential of 24 tropical tree species planted across a precipitation gradient in the Republic of Panama. Forest Ecol Manag 243:39–49
Wright SJ, Yavitt JB, Wurzburger N, Turner BL, Tanner EWJ, Sayer EJ, Santiago LS, Kaspari M, Hedin LO, Harms KE, Garcia MN, Corre MD (2011) Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 92:1616–1625
Yavitt JB, Harms KE, Garcia MN, Mirabello MJ, Wright SJ (2011) Soil fertility and fine root dynamics in response to 4 years of nutrient (N, P, K) fertilization in a lowland tropical moist forest, Panama. Austral Ecol 36:433–445
Yin H, Wheeler E, Phillips RP (2014) Root-induced changes in nutrient cycling in forests depend on exudation rates. Soil Biol Biochem 78:213–221
Zahawi RA, Duran G, Kormann U (2015) Sixty-seven years of land-use change in southern Costa Rica. PLoS One 10:e0143554
Zangaro W, Bononi VLR, Trufen SB (2000) Mycorrhizal dependency, inoculum potential and habitat preference of native woody species in South Brazil. J Trop Ecol 16:603–622
Acknowledgments
We thank S. Chhin, D.E. Rothstein, P. Nzokou, B. Graff and the MSU Agronomy farm for the use of equipment; M. Cook, C.A. Gehring, S.M. Neumann, and P. Nguygen for technical advice; C. Murillo for field help; G. Bayerl, T. Harris, and N. Vannest for laboratory assistance; and OTS and Las Cruces Biological Station staff for support. E.K. Holste was supported by National Science Foundation Graduate Research, Michigan State University Plant Sciences, and the Carol C. Gustafson and Gary S. Murphy Endowed Fellowships while contributing to this research. Funding was provided by the P.E.O. Scholar Award, Rasmussen Fellowship Award, and Organization of Tropical Studies Research Grant. The manuscript benefited from critiques by T.M.P. Robinson, C. Esch, D. Minor, K. Wood, L.O. Brudvig, C.A. Gerhring, G.P. Robertson, M.B. Walters, and two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Peter Christie.
Electronic supplementary material
Table S1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Holste, E.K., Kobe, R.K. Tree species and soil nutrients drive tropical reforestation more than associations with mycorrhizal fungi. Plant Soil 410, 283–297 (2017). https://doi.org/10.1007/s11104-016-3013-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3013-z