Abstract
Aims
In Brazil, cerrado and seasonal forest occur in close proximity but on soils with very different chemistry and texture. We aimed to compare species from these two vegetation types in terms of leaf N and P concentrations (of green and senesced leaves) and proportional nutrient resorption, quantifying the relationships among these traits, with other key leaf traits, and with soil properties.
Methods
We collected topsoil at 100 25 m2 sample plots in south-eastern Brazil and measured leaf traits of 89 woody species occurring therein, expressing them as community-weighted means. Soil nutrient status was indexed using eight standard variables.
Results
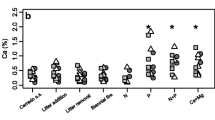
In terms of properties such as pH, clay content, and cation exchange capacity, cerrado soils were deemed as being less “fertile” than forest soils, yet cerrado and forest sites did not differ in soil total N or available P (which themselves were negatively correlated). On average, forest species showed higher proportional P resorption but lower N resorption. Leaves with higher nutrient concentrations were less scleromorphic.
Conclusion
In Brazilian cerrado and forests, variation in green- and senesced-leaf nutrients was better aligned with generalised measures of soil fertility than with total N or available P and showed far more clear patterns than nutrient resorption efficiencies.




Similar content being viewed by others
References
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 84:597–608
Aerts R, Chapin FS (1999) The mineral nutrition of wild plants revisited. Adv Ecol Res 30:1–67
Batalha MA, Mantovani W (1999) Chaves de identificação das espécies vegetais vasculares baseada em caracteres vegetativos para a ARIE Cerrado Pé-de-Gigante (Santa Rita do Passa Quatro, SP). Revista do Instituto Florestal 11:137–158
Batalha MA, Mantovani W (2005) Alguns aspectos das comunidades vegetais. In: Pivello VR, Varanda EM (eds) O cerrado Pé-de-Gigante: ecologia e conservação - Parque Estadual de Vassununga. Secretaria do Meio Ambiente, São Paulo
Batalha MA, Aragaki S, Mantovani W (1998) Chave de identificação das espécies vasculares do cerrado em Emas (Pirassununga, SP) baseada em caracteres vegetativos. Boletim de Botânica da Universidade de São Paulo 17:85–108
Bradstreet RB (1965) The Kjeldahl method for organic nitrogen. Academic, New York
Chapin FS III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Cole MM (1986) The savannas biogeography and geobotany. Academy, London
Coutinho LM (1990) Fire in the ecology of the Brazilian cerrado. In: Goldammer JG (ed) Fire in the tropical biota. Springer, Berlin, pp 82–105
Craine JM (2009) Resource strategies of wild plants. Princeton University Press, Princeton
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107:315–321
Development Core Team R (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Durigan G, Ratter JA (2006) Successional changes in cerrado and cerrado/forest ecotonal vegetation in western São Paulo State, Brazil, 1962–2000. Edinb J Bot 633:119–130
Embrapa (2012) Manual de métodos de análise de solos. Embrapa, Brasília
Epstein E, Bloom AJ (2005) Mineral nutrition of plants: principles and perspectives. Sinauer, Sunderland
Fujita Y, Bodegom PM, Witte JPM (2013) Relationships between nutrient-related plant traits and combinations of soil N and P fertility measures. PloS One 8, e83735
Furley PA (1999) The nature and diversity of neotropical savanna vegetation with particular reference to the Brazilian cerrados. Glob Ecol Biogeogr 8:223–241
Furley PA, Ratter JA (1988) Soil resources and plant communities of the central Brazilian Cerrado and their development. J Biogeogr 15:97–108
Garnier E, Cortez J, Billès G, Navas ML, Roumet C, Debussche M, Laurent G, Blanchard A, Aubry D, Bellmann A, Neill C, Toussaint JP (2004) Plant functional markers capture ecosystem properties during secondary succession. Ecology 85:2630–2637
Goodland R, Pollard R (1973) The Brazilian cerrado vegetation: a fertility gradient. J Ecol 61:219–224
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 982:1169–1194
Grimshaw HM (1987) The determination of total phosphorus in soils by acid digestion. In: Rowland AP (ed) Chemical analysis in environmental research. Natural Environment Research Council, Abbotts Ripton, pp 92–95
Güsewell S (2004) N:P ratios in in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Harrell FE Jr, Dupont C (2014) Hmisc: Harrell miscellaneous. R Foundation for Statistical Computing, Vienna, URL: http://CRAN.R-project.org/package=Hmisc
Hayes P, Turner BL, Lambers H, Laliberté E (2014) Foliar nutrient concentrations and resorption efficiency in plants in contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102:396–410
Hoffmann WA, Franco AC (2003) Comparative growth analysis of tropical forest and savanna woody plants using phylogenetically independent contrasts. J Ecol 91:475–484
Hoffmann WA, Orthen B, Franco AC (2004) Constraints to seedling success of savanna and forest trees across the savanna-forest boundary. Oecologia 140:252–260
Hoffmann WA, Franco AC, Moreira MZ, Haridasan M (2005) Specific leaf area explains differences in leaf traits between congeneric savanna and forest trees. Funct Ecol 19:932–940
Hoffmann WA, Geiger EL, Gotsch SG, Rossatto DR, Silva LCR, Lau OL, Haridasan M, Franco AC (2012) Ecological thresholds at the savanna-forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes. Ecol Lett 15:759–768
Hunke P, Mueller EN, Schröder B, Zeilofer P (2015) The Brazilian Cerrado: assessment of water and soil degradation in catchments under intensive agricultural use. Ecohydrology 8:1154–1180
Ibanez T, Hély C, Gaucherel C (2013) Sharp transitions in microclimatic conditions between savanna and forest in New Caledonia: Insights into the vulnerability of forest edges to fire. Aust Ecol 38:680–687
Jongman RHG, Braak CJF, Tongeren OFR (1995) Data analysis in community and landscape ecology. Cambridge University, Cambridge
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption efficiency. Ecology 77:1716–1727
Köppen W (1931) Grundriss der Klimakunde. Gruyter, Berlin
Krebs CJ (1998) Ecological methodology. Harper Collins, New York
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305
Lambers H, Raven JA, Shaver GR, Smith SE (2008a) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103
Lambers H, Chapin FS, Pons TL (2008b) Plant physiological ecology. Springer, New York
Lambers H, Brundrett MC, Ravel JA, Hopper SD (2010) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 334:11–31
Lambers H, Ahmedi I, Berkowitz O, Dunne C, Finnegan PM, Hardy GESJ, Jost R, Laliberté E, Pearse SJ, Teste FP (2013) Phosphorus nutrition of phosphorus-sensitive Australian native plants: threats to plant communities in a global biodiversity hotspot. Conserv Physiol 1:1–21. doi:10.1093/conphys/cot010
Lehmann CER, Archibald SA, Hoffmann WA, Bond WJ (2011) Deciphering the distribution of the savanna biome. New Phytol 191:197–209
Loveless AR (1961) A nutritional interpretations of sclerophylly based on differences in the chemical composition of sclerophyllous and mesophytic leaves. Ann Bot 25:168–184
Maire V, Wright IJ, Prentice IC, Baties NH, Bhaskar R, Bodegon PM, Cornwell WK, Ellsworth D, Niinemets Ü, Ordonez A, Reich PB, Santiago LS (2015) Global effects of soil and climate on leaf photosynthetic traits and rates. Glob Ecol Biogeogr 6:706–717
Mao R, Song CC, Zhang XH, Wang XW, Zhang ZH (2013) Response of leaf, sheath and stem nutrient resorption to 7 years of N addition in freshwater wetland of Northeast China. Plant Soil 346:385–394
Menge DNL, Hedin LO, Pacala SW (2012) Nitrogen and phosphorus limitation over long-term ecosystem development in terrestrial ecosystems. PloS One 7, e42045
Murphy BP, Bowman DMJS (2012) What controls the distribution of tropical forest and savanna? Ecol Lett 15:748–758
Näsholm T, Kielland K, Ganated U (2009) Uptake of organic nitrogen by plants. New Phytol 182:31–48
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) Vegan: community ecology package. R Foundation for Statistical Computing, Vienna, URL: http://CRAN.R-project.org/package=vegan
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, Vos AC, Buchmann N, Funes G, Quétier F, Hodgson CJG, Thompson K, Morgan HD, Steege H, Heijden MGA, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234
Pivello VR, Bitencourt MD, Mantovani W, Mesquita-Junior HN, Batalha MA, Shida CN (1998) Proposta de zoneamento ecológico para a Reserva de Cerrado Pé-de-Gigante (Santa Rita do Passa Quatro, SP). Braz J Ecol 2:108–118
Puyravaud JP, Pascal JP, Dufour C (1994) Ecotone structure as an indicator of changing forest-savanna boundaries (Linganamakki region, southern India). J Biogeogr 21:581–593
Rasband WS (2014) ImageJ. U. S. National Institutes of Health, Bethesda
Reich PB, Wright IJ, Cavender-Bares J, Craine JM, Oleksyn J, Westoby M, Walters MB (2003) The evolution of plant functional variation: traits, spectra, and strategies. Int J Plant Sci 164:S143–S164
Rossatto DR, Hoffmann WA, Franco AC (2009) Differences in growth patterns between co-occurring forest and savanna trees affect the forest–savanna boundary. Funct Ecol 23:689–698
Ruggiero PGC, Batalha MA, Pivello VR, Meirelles ST (2002) Soil-vegetation relationships in cerrado (Brazilian savanna) and semi-deciduous forest, Southeastern Brazil. Plant Ecol 160:1–16
Sarmiento G (1984) The ecology of neotropical Savannas. Harvard University, Cambridge
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Scholes RJ, Hall DO (1996) The carbon budget of tropical savannas, woodlands, and grasslands. In: Breymeyer AK, Hall DO, Melillo JM, Agren GI (eds) Global change: effects on coniferous forests and grasslands. SCOPE, Vol. 56. Wiley, Chichester, pp. 69–100
Schwartz D, Foresta H, Mariotti A, Balesdent J, Massimba JP, Girardin C (1996) Present dynamics of the savanna-forest boundary in the Congolese Mayombe: a pedological, botanical and isotopic (13C and 14C) study. Oecologia 106:516–524
See CR, Yanai RD, Fisk MC, Vadeboncoeur MA, Quintero BA, Fahey TJ (2015) Soil nitrogen affects phosphorus recycling: foliar resorption and plant-soil feedbacks in a northern hardwood forest. Ecology 96:2488–2498
Silva DM, Batalha MA (2008) Soil–vegetation relationships in cerrados under different fire frequencies. Plant Soil 311:87–96
SMA. Secretaria de Estado do Meio Ambiente (1997) Cerrado: bases para conservação e uso sustentável das áreas de cerrado do estado de São Paulo. SMA, São Paulo
Soil Survey Staff (2014) Keys to soil taxonomy. USDA, Washington
Souza AF, Martins FR (2004) Microsite specialization and spatial distribution of Geonoma brevispata, a clonal palm in south-eastern Brazil. Ecol Res 19:521–532
Staver AC, Archibald S, Levin SA (2011) The global extent and determinants of Savanna and forest as alternative biome states. Science 334:230–232
Sutherland WJ (2006) Ecological census techniques. Cambridge University, Cambridge
Turner IM (1994) Sclerophylly: primarily protective? Funct Ecol 8:669–675
Van Heerwaarden LM, Toet S, Aerts R (2003) Current measures of nutrient resorption efficiency lead to a substantial underestimation of real resorption efficiency: facts and solutions. Oikos 101:664–669
Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82:205–220
Viani RAC, Rodrigues RR, Dawson TE, Oliveira RS (2011a) Functional differences between woodland savannas and seasonally dry forests from south-eastern Brazil: Evidence from 15N natural abundance studies. Aust Ecol 36:974–982
Viani RAG, Rodrigues RR, Dawson TE, Oliveira RS (2011b) Savanna soil fertility limits growth but not survival of tropical forest tree seedlings. Plant Soil 349:341–353
Viani RA, Rodrigues RR, Dawson TE, Lambers H, Oliveira RS (2014) Soil pH accounts for differences in species distribution and leaf nutrient concentrations of Brazilian woodland savannah and seasonally dry forest species. Perspect Plant Ecol Evol Syst 16:64–74
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitations: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15
Vourlitis GL, Lobo FA, Lawrence S, Holt K, Zappia A, Pinto OB Jr, Nogueira JS (2014) Nutrient resorption in tropical savanna forests and woodlands of central Brazil. Plant Ecol 215:963–975. doi:10.1007/s11258-014-0348-5
Warman L, Bradford MG, Moles AT (2013) A broad approach to abrupt boundaries: looking beyond the boundary at soil attributes within and across tropical vegetation types. PloS One 8, e60789
Wright IJ, Westoby M (2003) Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophyll species. Funct Ecol 17:10–19
Wright IJ, Westoby M, Reich PB (2002) Convergence towards higher leaf mass per area in dry and nutrient-poor habitats has different consequences for leaf lifespan. J Ecol 90:534–543
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Yuan ZY, Chen HYH (2015) Negative effects of fertilization on plant nutrient resorption. Ecology 96:373–380
Acknowledgments
We are grateful to São Paulo Research Foundation (FAPESP, grant 2012/18295-4) and to Coordination for the Improvement of Higher Education Personnel (CAPES, grant BEX 12105/13-9) for the scholarships granted to RCM; to the National Council for Scientific and Technological Development for financial support and scholarship granted to MAB (CNPq, grant 305912/2013-5); to São Paulo Forestry Institute, for the research permit; to the Vaçununga State Park staff, for logistical assistance; to M Groppo and WM Mantovani, for helping us with species identification; to N Abe, ALS Albino, KR Coelho, P Dodonov, JR Freitas, CS Gonçalves, DT Gregolin, LA Joaquim, MB Leite, PP Loiola, WB Nascimento, CG Netto, LV Nóbrega, BA Severian, A Viscardi, and CB Zanelli, for valuable help with field work; and to GH Carvalho for revising a previous version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Appendix
Appendix
Table 3
Rights and permissions
About this article
Cite this article
Miatto, R.C., Wright, I.J. & Batalha, M.A. Relationships between soil nutrient status and nutrient-related leaf traits in Brazilian cerrado and seasonal forest communities. Plant Soil 404, 13–33 (2016). https://doi.org/10.1007/s11104-016-2796-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2796-2