Abstract
Hydroxycinnamoylamides are specialised metabolites widely distributed in the plant kingdom. These are phenolic moieties covalently linked to mono- or polyamines through amide bonds. Their oxidative coupling (dimerisation) leads to neolignanamides, a group of compounds showing high chemical, structural and functional diversity. Typical to barley, dehydro dimers of hydroxycinnamoylagmatines, hordatines are primarily found in germinated seeds and at the seedling stage. The first step in the biosynthesis of hordatines is catalysed by acyl-coenzyme A-dependent N-hydroxycinnamoyltransferases, and lead to the formation of hydroxycinnamoylagmatines (HCAgms). The oxidative homo- or hetero-dimerisation of the latter results in different hordatines (A, B, C or D). Hordatines can also undergo various types of conjugation and form hydroxylated, methylated or glycosylated derivatives. Although the research on the bioactivities of the hordatines is still nascent, the in planta antifungal properties have long been recognised. While hordatines are naturally and uniquely synthesised in barley plants, these molecules or lead compounds derived therefrom, also exhibit medicinal and pharmaceutical uses important for human health, stimulating research into the utilisation of biotechnology in alternative production hosts and to enhance agricultural yields and value-added production. This review summarises the older and recent knowledge about hordatines and derivatives and may serve as a springboard for future research on this intriguing class of secondary plant metabolites.
Similar content being viewed by others
Introduction
As multicellular eukaryotic organisms, plants produce a vast array of structurally and functionally diverse metabolites. These compounds can be synthesised for the plant’s primary needs or in response to environmental factors. Central metabolism results in the biosynthesis of primary metabolites or macromolecules such as carbohydrates, lipids, proteins, nucleic acids and hormones; highly conserved in all living organisms (Fernie and Pichersky 2015; Erb and Kliebenstein 2020). Primary metabolites are vital for growth, reproduction and signal transduction, and play a crucial role in photosynthesis, transport, respiration and nutrient assimilation processes. Secondary metabolism, which stems from primary metabolism, results in the biosynthesis of a large number of specialised and structurally diverse metabolites. These are often grouped as phenolic acids, flavonoids, terpenes, nitrogen- and sulphur-containing molecules. They are required for plant survival in the environment and are associated with phenotypic characteristics (Erb and Kliebenstein 2020); hence their biosynthesis varies from plant to plant and from one external pressure to the other. Moreover, the in vivo production of specialised metabolites can be genus- and species-specific and involves the activation of intertwined pathways, supported by the central metabolism, and forming complex metabolic networks. Some plant-specific metabolites include the glycoalkaloid tomatin/lycopersicin in tomato (Kozukue et al. 2023) and avenanthranamides in oat (Pretorius and Dubery 2023). This review focuses on distinctive hydroxycinnamoylagmatine conjugates of barley, Hordeum vulgare L., the hordatines. Their biosynthetic pathway, structural characteristics and – diversity as well as mass spectral identification and bioactivities are explored to provide an in-depth knowledge on hordatines.
Hordatines, unique phytochemicals of barley
Barley is the fourth most important grain crop in the world, with numerous uses and has great importance in the animal and human food and brewing industries. The sequenced genome of barley, its diploid genetics (seven pairs of nuclear chromosomes), and the close connection among Triticeae tribe members, make it easier to apply the knowledge learned from barley studies to other important cereals. Additionally, the high resistance of barley to several environmental stresses support the use of the plant as a model in crop research (Munns and Tester 2008; Kant et al. 2016; Wiegmann et al. 2019).
Barley is capable of producing phytochemicals possessing a wide range of activities such as anti-oxidant, anti-inflammatory and anti-proliferative properties. These include flavonoids, phenolics, alkaloids, lignins and terpenoids (Shewry 2014). Often categorised as phenolamides or hydroxycinnamic acid amides dimers, hordatines are benzofurans renowned for their anti-fungal properties. Among the 31 species of the Hordeum genus, the cultivated barley (H. vulgare spp. vulgare) and wild barley (H. vulgare ssp. spontaneum) are the only two that synthesise hordatines (Batchu et al. 2006; Ube et al. 2017). Hordatines have thus been regarded as signature metabolites in cultivated barley (Hamany Djande et al. 2022). It is worth mentioning that other species are also capable of forming dimers of hydroxycinnamic acid amides (Leonard et al. 2021). However, the linkage type is different to that observed in hordatines (Section “Diversity of hordatine structures and associated nomenclature”).
Biosynthesis of hordatines
Hydroxycinnamic acid amides or phenolamides
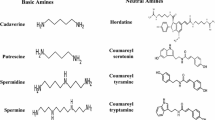
Hydroxycinnamic acid amides (HCAAs) or phenolamides or phenylamides are metabolites found ubiquitously in the plant kingdom and are recently attracting considerable attention (Zeiss et al. 2021). HCAAs are structurally diverse phenolic compounds and often categorised as phenolic acid conjugates or alkaloids because of a N-containing sub-structure. They arise from phenolic moieties, primarily hydroxycinnamic acids (HCAs) and derivatives, forming covalent amide bonds with either an aromatic or aliphatic amine (Fig. 1) (Bassard et al. 2010; Macoy et al. 2015a; Zeiss et al. 2021; Roumani et al. 2021, 2023). HCAs (p-coumaric, caffeic, ferulic, and sinapic acids) are synthesised in the early phenylpropanoid pathway from L-phenylalanine, with the help of the enzymes phenylalanine ammonia lyase (PAL; EC 4.3.1.5) and cinnamic acid 4-hydrolase (C4H; EC 1.14.13.11) (Gray et al. 2012). To a lesser extent, L-tyrosine can also be a precursor, producing 4-coumaric acid in presence of an ammonia-lyase (Young et al. 1966; Petersen 2016). The activation of HCAs as coenzyme A thioesters necessary for downstream events is performed in the presence of hydroxycinnamate:coenzyme A ligase or 4-coumaroylCoA ligase (4CL; EC 6.2.1.12) in a two-step reaction (Petersen 2016). Polyamines on the other hand, are low molecular weight polycations, resulting from the decarboxylation of amino acids, mainly L-arginine. The amino acid can be converted into the non-proteinogenic amino acid ornithine, which in turn is decarboxylated into putrescine by the mitochondrial enzyme arginase (ARG; EC 3.5.3.1) and ornithine decarboxylase (ODC; EC 4.1.1.17) respectively. Alternatively, L-arginine can be decarboxylated by arginine decarboxylase (ADC; EC 4.1.1.19) to produce agmatine from which several polyamines may result. Plant amines include putrescine, spermidine, spermine, (collectively known as polyamines or low molecular weight aliphatic nitrogenous bases that contain two or more amino groups), agmatine, tyramine, anthranilate, tyramine, tryptamine, etc. (Bagni and Tassoni 2001; Mehta et al. 2002; Takahashi and Kakehi 2010).
Biosynthesis of phenolamides / hydroxycinnamic acid amides in plants. The formation of HCAAs requires the participation acyl-activated coenzyme A thioesters (donors of acylated moieties) and amines (acceptors) from the phenylpropanoid – and amine pathways respectively. The reactions are catalysed by plant acyl-CoA dependent N-acyltransferases. PAL phenylalanine ammonia lyase, C4H cinnamic acid 4-hydrolase, 4CL 4-coumaric acid:coenzyme A ligase, C3H 4-coumaric acid 3-hydroxylase, F5H coniferaldehyde/ferulate5 hydroxylase, COMT caffeic acid/5-hydroxyferulic acid O-methyltransferase, ADC arginine decarboxylase, ARG arginase, ODC ornithine decarboxylase, SPDS spermidine synthase, SPMS spermine synthase. The double arrows indicate the implication of more than one step
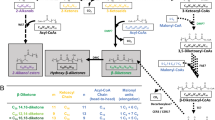
Since the discovery of the first HCAA in the late 1940s, years of phytochemical research have demonstrated a significant structural diversity in the family and a widespread distribution throughout the plant world. The physicochemical diversity of HCAAs is credited to the possible combinations of different HCA and amine moieties, as well as the potential for N-substitution on the aliphatic polyamines (Roumani et al. 2020). More than 800 HCAAs and derivatives were generated in silico with the most encountered building blocks (Li et al. 2018). Chemically, the hordatines are the products of the oxidative dimerisation of two HCAAs derived from the polyamine agmatine and the coenzyme A derivatives of HCAs (Burhenne et al. 2003; Gorzolka et al. 2014; Piasecka et al. 2015) (Fig. 2).
Biosynthesis of hordatines in barley. Hydroxycinnamoyl-CoA and agmatine react in the first step, catalysed by agmatine coumaroyltransferase (ACT), to generate a hydroxycinnamoylagmatine (HCAgm). For p-coumaroylagmatine: R1, R2 and R4 = H; feruloylagmatine: R1 = OCH3, R2 and R4 = H; sinapoylagmatine: R1, R2 = OCH3 and R4 = H. The oxidative coupling of two hydroxycinnamoylagmatine molecules in the presence of an oxidase or peroxidase (PER) or laccase (LAC) constitute the second stage.The diversity of the hordatine structures as a result of different R groups and associated nomenclature are described in Section “Diversity of hordatine structures and associated nomenclature”
Hordatines A and B were the first barley specific benzofurans to be characterised (Stoessl 1965, 1967) and their biosynthesis involves the participation of two enzymes: H. vulgare agmatine coumaroyltransferase (HvACT, E.C. 2.3.1.64) and an oxidase. HvACT is a CoA-dependent transacylation system responsible for the formation of hydroxycinnamoylagmatines (HCAgms, e.g. p-coumaroylagmatine) from the corresponding coenzyme A derivatives of the HCAs with agmatine. The enzyme was first purified from barley (GenBank: AY228552) and its cDNA cloned (Burhenne et al. 2003). Since then, ACTs have also been identified in Arabidopsis thaliana (AtACT), resulting in p-coumaroylagmatine biosynthesis as the major product (Muroi et al. 2009). An oxidase, long believed to be a peroxidase, is responsible of the oxidative phenol coupling of HCAAs to produce hordatines (Fig. 2) (Burhenne et al. 2003).
N-hydroxycinnamoyltransferases, members of the BAHD acyltransferase family
Plant acyl-CoA dependent acyltransferases are members of the BAHD superfamily that catalyse reactions in which acylated moieties (RC(O)R’) of acyl-activated coenzyme A thioesters are transferred to an acceptor molecule (St-Pierre and De Lucas 2000; D’auria 2006; Yuan et al. 2022). In these reactions, the phenolic acyl donor is transferred to an amine acceptor compound (Luo et al. 2009), and produce secondary metabolites which act functionally as phytoanticipins and/or phytoalexins. The term BAHD was coined from the first letter of the first four biochemically characterised group of enzymes in the family (St-Pierre and De Lucas 2000; D’auria 2006). These were benzylalcohol O-acetyltransferase (BEAT) (Dudareva et al. 1998), anthocyanin O-hydroxycinnamoyltransferase (AHCT) (Fujiwara et al. 1998), anthranilate N-hydroxycinnamoyl/benzoyltransferase (HCBT) (Yang et al. 1997), and deacetylvindoline 4-O-acetyltransferase (DAT) (St-Pierre et al. 1999). In vitro, the substracte specificity of some family members varies from narrow to broad, and the products generated in planta are thus often dictated by the relative availability of substrates. In addition, motif enrichment analysis and molecular dynamics simulations performed on these BAHD acyltransferases put forward the fact that the specialisation may result from variations in amino acid sequences in specific motifs instead of those uniformly distributed throughout the enzyme structure (Weng et al. 2021).
Several protein sequence alignments demonstrated that BAHD superfamily proteins share high sequence identity (up to 34%) (D’Auria 2006). The BAHD superfamily is characterised by two conserved amino acid motifs. Found in the central region of the enzyme, the first motif comprises a consecutive amino acids string that includes histidine and aspartic acid (HXXXD), where the former plays a function as a catalytic residue and the latter associated with a structural role. The catalytic activity of histidine consists of removing a proton (deprotonation) from the acyl acceptor (the nucleophile), leading to a nucleophilic attack on the carbonyl carbon of the Co-A thioester (donor) to form a tetrahedral intermediate. Sequentially, the protonation of the intermediate results in the formation of an acylated amide and free CoA (Ma et al. 2005; D’Auria 2006; Kong et al. 2020). The second conserved motif DFGWG (Asp, Phe, Gly, Try, Gly), is located next to the C-terminal end, away from the active site of the enzyme (D’Auria et al. 2007; Morales-Quintana et al. 2015). Unlike the first motif, DFGWG is not involved in the catalytic mechanism; instead, it is assumed to play a structural role such as involvement in CoA binding (El-Sharkawy et al. 2005; Molina and Kosma 2015; Petersen 2016). The conserved motifs in the BAHD superfamily allowed the discovery of several acyltransferase genes in different plants notably A. thaliana and Oryza sativa (D’Auria et al. 2007; Yu et al. 2009). In a recent study conducted by Yuan et al. (2022), 116 putative HvBAHD acyltransferase genes were found in the barley genome with 113 mapped across seven chromosome groups.
Regarding the biosynthesis of hordatines from barley, HvACT is one of the phylogenetically unique enzymes among the BAHD acyltransferases, responsible of the biosynthesis of p-coumaroylagmatine and feruloylagmatine. Building on the classification scheme of D’auria (2006), Kruse et al. (2022) grouped BAHD acyltransferases into 8 (from 0–7) clades based on structural similarities between acceptor substrates. N-Hydroxycinnamoyltransferases (N-HCTs) are found in all the clades, except in clades 2 and 7. The acceptors used by clade 4 N-HCTs are agmatine, putrescine, tryptamine, tyramine, and serotonin. Among the acyltransferase in barley, HvACT was the first amine N-HCT characterised and was placed in clade 4 (Yuan et al. 2022; Moghe et al. 2023). In that clade, only enzymes from the Gramineae/Poaceae family (monocot flowering plants / grasses) have been characterised thus far.
In a recent study by Yamane et al. (2020), HvACT was crystallised for the first time, and the apo form structure was elucidated. The overall folding of HvACT was comparable to that of other known members of the BAHD superfamily (Walker et al. 2013; Levsh et al. 2016; Yamane et al. 2020). The enzyme contains 13 α-helices and 18 β-strands, and similarly to other enzymes in the BAHD superfamily, the structure can be divided in two domains (I and II). Both domains are connected by lengthy crossover, interacting with α11 and β14 in domain II. β16 takes part in the core β-sheet of domain I which is stringed over by the loop between β15 and β16. According to the class, architecture, topology and homologous superfamily (CATH) classification, these domains adopt a two-layer αβ-sandwich architecture. In barley, one of the two highly conserved motifs (described above) corresponds to 152HIVSD156 (His, Ileu, Val, Ser, Asp), with 152His located on the solvent channel surface. The other conserved region 385DFGWG390 is located between β15 and β16 (Burhenne et al. 2003; Yamane et al. 2020).
Dimerisation of hydroxycinnamoylagmatine conjugates and isomerisation of hordatines
The dimerisation of secondary metabolites in planta occurs as an effective biochemical mechanism, to increase the diversity of compounds through the creation of new carbon skeletons, and potentially resulting in stronger biological activities (Pretorius and Dubery 2023). Considering the precursors and the type of linkage occurring, a wide variety of phytochemical dimers can hypothetically be formed; this is without taking into consideration stereoisomers and larger oligomers. These dimeric products of HCAAs also often carry names deriving from the plant in which they were first identified (van Zadelhoff et al. 2021). Accordingly, hordatines are neolignanamides found in H. vulgare. As part of the reaction leading to the biosynthesis of hordatines, a peroxidase enzyme was suggested to catalyse the oxidative phenol coupling or dimerisation of agmatine conjugates, e.g. by linking coumaroylagmatine and feruloylagmatine. The implication of the peroxidase-encoding barley gene Prc7 in the biosynthesis of hordatines was highlighted by their synchronised upregulation following a powdery mildew (Blumeria [syn. Erysiphe] graminis f.sp. hordei) infection (Kristensen et al. 1999). Unfortunately, little is known about the dimerisation step and the characteristics of the peroxidase involved, except that a radical coupling reaction in presence of the enzyme led to the formation of hordatines in vitro and that the catalysis involves an oxidative phenol coupling mechanism (Stoessl 1966, 1967; Burhenne et al. 2003; Ube et al. 2017; van Zadelhoff et al. 2022). In the in vitro reactions using peroxidase in presence of H2O2, not only hordatines were produced, but also compounds with different linkage types (van Zadelhoff et al. 2022). Unlike hordatines synthesised in vivo, the laboratory-produced compounds were optically inactive. Hence, in recent studies, Ube et al. (2023) pointed out to another oxidase as the catalyst of the oxidative coupling of p-coumaroylagmatine. The proposed enzyme was laccase, encoded by HvLAC1 and HvLAC2 genes. Contrary to peroxidase, HvLAC1 and HvLAC2 were shown to catalyse the stereo-specific formation of the enantiopure hordatine A, and may not require a dirigent protein (Ube et al. 2023). Laccases are multicopper glycoprotein oxidases implicated in the biosynthesis of phenolic compound dimers such as the polymerisation of HCA derivatives (Li et al. 2020). Their participation in the oxidative coupling of p-coumaroylagmatine for the formation of hordatines is therefore not unexpected. These HvLAC1 and HvLAC2 were identified in the apoplast and the vacuole (Ube et al. 2023). It was then suggested that the precursor p-coumaroylagmatine, synthesised in the cytosol by HvACT enzymes (Yuan et al. 2022), would be transported to the vacuole and the extracellular space (apoplast) where the formation of hordatines will occur (Burhenne et al. 2003; Ube et al. 2023). So far, the peroxidase route remains the most investigated one regarding the dimerisation of two HCAgms, but the revelation of laccases as oxidative enzymes in the biosynthesis of hordatines opens new fields of study.
Diversity of hordatine structures and associated nomenclature
In a recent study by van Zadelhoff et al. (2022), the oxidative coupling of HCAgms by horseradish peroxidase was investigated and different linkage types, not only found in barley but also in related Hordeum species, or other (neo)lignanamides were revealed. Five linkage types were reported to lead to HCAgm dimers: 4-O-7′/3-8′, 2-7′/8-8′, 8-8′/9-N-7′, 8-8′ and 4-O-8′; three of which were previously reported (Stoessl 1967; Ube et al. 2017). These linkage types descriptors were based on the systematic nomenclature for (neo)lignanamides proposed by van Zadelhoff et al. in (2021) and the only linkage type detected in barley was 4-O-7′/3-8′ (Ube et al. 2017; van Zadelhoff et al. 2021, 2022). The position of the linkage has previously been reported although the numbering system was different (Kageyama et al. 2011; Pihlava 2014; Hamany Djande et al. 2022). In Kageyama et al. (2011), the corresponding linkage was 3-3a/2-O-7′ (Fig. 3A).
Linkage bond types and nomenclature of homodimers of feruloylagmatines. (A) and (B): Hordatine C following the nomenclatures of Kageyama et al. (2011) and van Zadelhoff et al. (2022) respectively; (C) and (D): Murinamides A and B respectively, following van Zadelhoff et al. (2022). The linkage bond type is highlighted in yellow
As explained earlier, HCAs are important constituents that contributes to the respective hordatine structures. When exposed to UV radiation, cinnamic acids have been known to undergo trans (E) to cis (Z) isomerisation due to the presence of a double bond (Salum et al. 2013). A case in hand is transgenic Torenia hybrida plants transformed with agmatine coumaroyltransferase from Arabidopsis, which accumulated substantial amounts of p-coumaroylagmatine, that isomerised from the trans-form to the cis-form in planta (Muroi et al. 2012). A similar photo-isomerisation occurs in barley, leading to the formation of isomeric pairs of the corresponding hordatines (van Zadelhoff et al. 2022). Several authors previously proposed (Stoessl 1966; Yamaji et al. 2007; Kageyama et al. 2011) that the cis and trans isomerisation of hordatines occur at the two chiral centres on carbons 2 and 3, or positions 7′ and 8′ of the dihydrobenzofuran residue found in their core structure (Kageyama et al. 2011). Similarly to HCAs, an isomerisation site was also reported at positions 1′′ and 2′′ (Kageyama et al. 2011, 2012; Hamany Djande et al. 2022) or positions 7 and 8 (van Zadelhoff et al. 2022 nomenclature) (Fig. 3A and B) and the chemical structures of the cis isomers of the 1′′– 2′′ double bond of the cinnamic acid moiety of hordatines A and B were elucidated by mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy, and reported as minor components in barley malt (Kageyama et al. 2012).
Following van Zadelhoff et al. (2021) nomenclature which is more descriptive, hordatine A and B are CouAgm-4-O-7′/3-8′-DCouAgm and FerAgm-4-O-7′/3-8′-DCouAgm respectively, showing the precursors involved and the linkage type. The former results in the dimerisation of two molecules of p-coumaroylagmatine, and the latter in a heterodimer of feruloylagmatine and p-coumaroylagmatine (Gorzolka et al. 2014, 2016; Pihlava et al. 2016). Hordatine C, consisting of two feruloylagmatines (FerAgm-4-O-7′/3-8′-DFerAgm) and hordatine D (FerAgm-4-O-7′/3-8′-DSinAgm), a dimer of feruloylagmatine and sinapoylagmatine, were also reported by Gorzolka et al. (2014) and Hamany Djande et al. (2022). Up to now, caffeoylagmatine has not been reported as a component of a hordatine, and while homodimers of p-coumaroylagmatine and feruloylagmatine have been reported (hordatine A and hordatine C respectively), it does not appear to be the case with sinapoylagmatine.
Of special interest is a collection of compounds resulting from the dimerisation of HCAgms but with different linkage types to that of the hordatines (2-7′/8-8′, and 8-8′/9-N-7′, as in Fig. 3C and D). Murinamides A and B were discovered during a study of evolutionary changes in specialised defensive metabolism in the genus Hordeum. Murinamides consist of two molecules of feruloylagmatine (similarly to hordatine C) and were identified in the shoot and root tissues of related Hordeum species, H. murinum and H. bulbosum (Ube et al. 2017; van Zadelhoff et al. 2021). Their absence in the cultivated and wild barley, which are close relatives, led to the speculation that murinamides were replaced by hordatines during speciation (Ube et al. 2017). It was suggested that the activation of precursor supply, the expression of the coupling enzyme, as well as a collection of factors regulating the stereo- and regioselectivity of cyclisation reactions occurring subsequent to the coupling reaction, are responsible for the differences in the accumulation of dehydrodimers of agmatine HCAAs.
The complexity of hordatines thus derives not only from the origin of the carbon skeletons from two major metabolic pathways, but also the hydroxylation level, distinct conjugations, substitutions and polymerisation level. Figure 4 and Table 1 shows the core structure of hordatines and derivatives. The hydroxylation of hordatines was reported by Gorzolka et al. (2014); Heuberger et al. (2014) and Pihlava et al. (2016) and characterised by the presence of one or two molecules of hydroxyagmatine (OHAgm). Hordatine A1 or A2 for example, corresponded to the compound with only one or two OHAgm(s) respectively (CouOHAgm-CouAgm or CouOHAgm–CouOHAgm). In the context of conjugation, hordatines (A-D) can occur attached to one or more hexosyl group (up to 9 units) (Heuberger et al. 2014; Gorzolka et al. 2014; Pihlava 2014; Piasecka et al. 2015; Gorzolka et al. 2016). Glycosylation might exhibit a level of tissue and developmental specificity. For example, although higher in germinated seed, hordatine A β-D-glucopyranoside was also found in ungerminated mature seeds of barley. Hordatines can be stored in the glycosylated form in mature grains and partially synthesised or generated from the hydrolysis of the glycosides during/following germination (Kohyama and Ono 2013). Gorzolka et al. (2016) also reported hordatines that occur in both hydroxylated and glycosylated forms. In addition, monomethylated hordatines A, B, and C were found in barley samples (Pihlava 2014). Older literature mentions hordatine M, which is a mixture of the glycosylated versions of hordatines A and B (Smith and Best 1978).
Examples of the chemical structures of hordatine and derivatives. (Adapted from Pihlava (2014), Pihlava et al. (2016)). The various substitutions are listed in Table 1. The positions of X1 and X2 were suggested based on MS analysis alone and are thus not conclusive as yet. X3 can occur on either of the two guanidino moieties Further NMR analyses may be required to provide more information regarding these positions
Mass spectrometric analysis of hordatines and diagnostic ions
Such structural diversification as described above supports functionalisation and increases the ‘chemical space’ occupied by hordatines in the barley plant. Assessing the functional characteristics of bound phenolic acids often requires an understanding of their stereoisomeric configuration. Furthermore, the complexity of profiling hordatines found in barley extracts is influenced by differences in substitution patterns as well as geometric isomerisation that results in differences in retention times in the course of reverse phase chromatographic separation procedures.
As described above, hordatines are structurally complex polar molecules within the class of HCAAs dimers. Their extraction has mostly been done with 70–80% methanol or 80% 2-propanol solutions, and solid–liquid extraction (SLE) with 60–75% of acetone, sometimes followed by purification and fractionation steps. Different analytical platforms have been employed to elucidate the structural diversity of these classes of compounds. These include ultra-high performance liquid chromatography (UHPLC) for separation, coupled to MS for detection and structural elucidation and NMR for final structural verification. In addition, matrix-assisted laser desorption ionisation time-of-flight MS (MALDI-TOF-MS)-profiling and imaging mass spectrometry (MSI) have been used (Kohyama and Ono 2013; Pihlava 2014; Piasecka et al. 2015; Sarabia et al. 2018; Spreng and Hofmann 2018; Becker et al. 2022; Hamany Djande et al. 2021, 2022; Laupheimer et al. 2023). Both hordatines and precursors have been identified and characterised using these protocols. Due to their structural similarities, all four hordatine aglycones cluster closely to each other during chromatographic separation, and poor baseline resolution of peaks frequently does not allow the generation of clear spectra that only feature the compound of interest (Hamany Djande et al. 2022). A similar observation was made by Pihlava et al. (2016) who noted co- or close eluting compounds in addition to several precursor ions being very low. Hence, the necessity to improve chromatographic methods capable of clearly separating the hordatines, related derivatives and isomers (Gorzolka et al. 2014; Pihlava 2014; Piasecka et al. 2015; Hamany Djande et al. 2022).
In general, the hordatine precursors p-coumaroylagmatine (MW = 276 g/mol), feruloylagmatine (MW = 306 g/mol) and sinapoylagmatine (MW = 336 g/mol) are characterised by a neutral loss of m/z 130 corresponding to agmatine and the presence of a dehydroxylated hydroxycinnamoyl moiety (Fig. 5) (Gorzolka et al. 2014; Piasecka et al. 2015; Pihlava et al. 2016). For p-coumaroylagmatine for example, in the positive ionisation mode, the protonated parent ion has a m/z of 277 and the fragment ion m/z 147 resulting from the loss of a residue of agmatine (130) and corresponding to the dehydroxylated p-coumaric acid (164–18 = 147). The similar pattern is observed with ferulic acid and sinapic acid. Gorzolka et al. (2014) used MALDI coupled with Fourier transform ion cyclotron resonance (FT–ICR) MS (MALDI–FT–ICR) imaging, or with TOF (time-of-flight) MS and UHPLC-electrospray ionisation (ESI) tandem MS (MS/MS) to elucidate the chemical structures of the HCAgms and derivatives. Hordatines A (MW = 550 g/mol) and B (MW = 580 g/mol) as well as two glycosylated derivatives were identified in the ‘Metlin’ (Metabolite and Chemical Entity, https://massconsortium.com/; Smith et al. 2005) database. In addition, two non-identified peaks with a similar fragmentation pattern as hordatines A and B were identified and named hordatine C (MW = 610 g/mol) and D (MW = 640 g/mol). The neutral losses and the in silico fragmentation of known compounds were assessed using ‘Mass Frontier’ spectral interpretation software from ThermoFischer (www.thermofisher.com). Neutral losses to keep in mind during the interpretation of mass fragmentation data of hordatines are m/z 130, corresponding to one agmatine moiety, 260 corresponding to the loss of two agmatine side chains, 276 (130 + 146) matching the elimination of one agmatine and a modified agmatine via hydroxylation (130 + 16 = 146), 42 for the head group (NH–C = NH) of the side chains, 17 for the loss of one of the four amines and 162 for the loss of a hexoside. A mass difference of 30 corresponding to the methoxyl group could be noted between hordatine A, B, C and D (Gorzolka et al. 2014; Hamany Djande et al. 2022). This observation was also evident with the precursors p-coumaroylagmatine, feruloylagmatine and sinapoylagmatine (Gorzolka et al. 2014; Piasecka et al. 2015; Pihlava et al. 2016; Hamany Djande et al. 2022).

(Modified from Hamany Djande et al. 2022)
Illustration of the fragmentation patterns (ESI +) of p-coumaroylagmatine (m/z 277), feruloylagmatine (m/z 307) and sinapoylagmatine (m/z 337) and their corresponding structures showing the fragmentation site. The grey side of the structure correspond to the neutral loss as indicated with the red arrows. The dotted lines are just an extension of the fragment helping to illustrate the neutral loss.
Using a quadrupole time-of-flight (qTOF) MS instrument, following a reverse phase chromatographic separation of 30 min, Hamany Djande et al. (2022), also identified hordatine A, B, C and D, and glycosylated derivatives, with two isomers of hordatine B and hordatine C hexose. Similarly to Gorzolka et al. (2014), no glycosylated derivative of hordatine D was detected. The structural information was interpreted using a chemically intelligent structure elucidation tool, ‘Massfragment’ within the MassLynx MS™ software from Waters (www.waters.com) (Hamany Djande et al. 2022). In negative ESI mode, deprotonated ([M-H]−) hordatine A (m/z 549), B (m/z 579) and C (m/z 609), yielded fragment ions with m/z 237, 267 and 297, respectively, corresponding to their acidic cores (Piasecka et al. 2015; Hamany Djande et al. 2022). Here the presence of formic acid adducts in the negative ionisation mode can add to the characteristic ions and also interfere with the structural identification (Piasecka et al. 2015; Hamany Djande et al. 2022). In the positive ESI mode ([M + H]+), hordatine A (m/z 551), B (m/z 581), C (m/z 611) and D (m/z 641) were characterised by the presence of diagnostic fragment ions m/z 157 (C6H13N4O), 131 (C5H15N4), 114 (C5H12N3) deriving from agmatine molecules. In addition, double protonated ([M + 2H]2+) base peaks with m/z 276, 291 and 306 for hordatine A, B and C respectively, are usually the main ions detected (Fig. 6). The double charge observed is due to the easy protonation of the two guanidino moieties. As a consequence, the positive ionisation of these compounds is more favourable as opposed the negative ionisation.

(Modified from Hamany Djande et al. 2022)
Illustration of the fragmentation patterns (ESI +) of hordatine A, B and C, and some of the diagnostic fragments. Left: fragments characteristics of the molecule of agmatine, centre: the double protonated fragments of hordatine with m/z 276, 291 and 306 characteristic of hordatine A (m/z 551), B (m/z 581) and C (m/z 611), respectively, right.
Similar signature fragment ions were noted with hordatine hexosides. In addition, a mass difference of m/z 81 was observed between adjacent hexosides due to the double charges (Gorzolka et al. 2014; Pihlava 2014; Piasecka et al. 2015; Hamany Djande et al. 2022). In the case of hydroxylated hordatines (either as aglycones or hexosylated), the compounds were characterised by fragment ions with m/z 129 (C5H15N4), 147 (C5H15N4O) and 173 (C6H13N4O2), verifying the presence of a hydroxyagmatine residue (Gorzolka et al. 2014; Pihlava 2014; Becker et al. 2022). As for the methylated agmatines, ions with m/z 145 (C6H17N4), 171 (C7H15N4O) and 128 (C6H14N3) were characteristic fragments (Pihlava 2014).
In planta bioactivities of hordatines
Many specialised secondary metabolites have important adaptive significances in protection against adverse conditions. In this context, the biosynthesis of secondary metabolites, and the regulation thereof, play a complex role in the ability of plants to overcome unfavourable growth conditions and to adapt to environmental stressors. Below- and above-ground tissues experience very different environmental conditions and encounter different pathogens. Therefore, the differential composition and tissue-specific synthesis of hordatines and precursor metabolites warrants further investigation to determine their functional significance. Here, the blend of specific hordatines and tissue-specific post-synthesis modifications can support their functional diversification through changes in stability, hydrophobicity, subcellular localization, and thus bioactivity. Likewise, the presence of different hordatines in the cell wall might alter the structural properties of roots and shoots during growth and defence (Gorzolka et al. 2014).
Of late, renewed interest has been directed at the hordatine class of compounds because of their growing importance in diverse areas (Gorzolka et al. 2014; van Zadelhoff et al. 2022; Becker et al. 2022; Ube et al. 2023; Laupheimer et al. 2023). Although the mechanisms behind the biological implication of hordatines are still to be fully uncovered (e.g. germination vs. grain maturation), the most alluring quality of these barley-specific metabolites for plant science and agricultural development research remains their remarkable antifungal properties (Stoessl 1967). As dimerised HCAgms, the hordatines are important and understudied compounds with reported antimicrobial properties. HvACT and other enzymes (e.g. ADC) were found to be associated with barley resistance against fungal pathogens (Yuan et al. 2022; Moghe et al. 2023) and it has been reported that the concentration of hordatines in barley leaves increase after infection, e.g. by powdery mildew (Erysiphe graminis f. sp. hordei) (Smith and Best 1978; Von Röpenack et al. 1998). Synthesis of hordatines in seedlings in response to fungal infection result in concentrations of hordatines A/B and M greatly in excess of that required to inhibit spore germination of Erysiphe graminis (Smith and Best 1978). Hordatines have thus been regarded as signature compounds in cultivated barley, abundant in the seeds of mature plants and the shoots of barley seedlings (Hamany Djande et al. 2021, 2022, 2023a).
Studies conducted in vitro have revealed that hordatines and p-coumaroylagmatine prevent spore germination in a variety of fungal infections (Stoessl 1967; Stoessl and Unwin 1969; Smith and Best 1978; Macoy et al. 2015b). In our most recent study on the defence response of barley to the necrotrophic fungal pathogen Pyrenophora teres f. teres, hordatines A and B were identified as discriminant metabolites; while downregulated in shoot tissue of a susceptible cultivar, their involvement in plant defence was reiterated (Hamany Djande et al. 2023a). In another study, barley leaf tissue was treated with dichlorinated derivatives of anthranilic acid, salicylic acid and isonicotinic acid as priming agents or inducers of plant immunity and a positive correlation of coumaroylagmatine and hordatines A, B and C to the treatments were reported (Hamany Djande et al. 2023b). In addition, their functional significance in plant–pathogen interactions has been observed at an early developmental stage, after germination, and a role as phytoanticipins/phytoalexins has been inferred (Hamany Djande et al. 2021, 2022). Another function ascribed to HCAmgs is cell wall fortification, also associated with hordatines during plant-pathogen interactions (Von Röpenack et al. 1998), perhaps as nucleating agents for the deployment of lignification as a structural defence.
A possibility of involvement of hordatines in plant abiotic stress was previously postulated (Sarabia et al. 2018) and Piasecka et al. (2020) reported the role of hordatine B and its hexoside in the barley response to water deficit. Various internal signals modify metabolism, growth, and development in plants and enhanced biosynthesis of hordatines in response to multiple stresses or combined abiotic and biotic stresses might be due to common upstream response regulators such as reactive oxygen/nitrogen species (ROS/RNS) (Cortese-Krott et al. 2017) and shared signaling circuits to transduce diverse biotic and abiotic signals into the same physiological response.
Hordatines as pharmacological agents and health beneficial compounds
Extracts from plants that contain important sources of various biologically active compounds are becoming increasingly important in various fields other than agriculture, such as environmental protection, pharmacology and medicine. Barley is known to be rich in recognised neutraceuticals such as glucans and tocols (Idehen et al. 2020; Raj et al. 2023). Due to their biological activity as radical scavenging agents / antioxidants (Spreng and Hofmann 2018), hordatines have attracted attention for their possible use in human nutrition. This group of compounds specific to barley possess health beneficial attributes and may have a role as disease preventative agents, supporting their consumption as a component of functional foods (Dahab et al. 2020).
The investigation of hordatines against α-glucosidase, a carbohydrate hydrolase, revealed them as potent inhibitors of the enzyme (Becker et al. 2021, 2022). α-Glucosidase catalyses the hydrolysis of starch to simple sugars (Zhai et al. 2022) and inhibitors of the enzyme may function to regulate or slow the release of glucose and to avoid hyperglycemia (Bhatnagar and Mishra 2022). Relatedly, hordatine A was found to exhibit muscarinic M3 receptor-binding activity (Yamaji et al. 2007), suggesting a potential role in the regulation of insulin secretion and glucose homeostasis (Gautam et al. 2006) and regulating gastro intestinal smooth muscle function (Yamaji et al. 2007; Kohyama and Ono 2013; Tanahashi et al. 2021). In addition, the compounds also have an antagonistic effect on α1-adrenergic receptors, located in the peripheral and central nervous system and involved in regulating the contraction of smooth muscle tissue in the lower urinary tract and cardiovascular system (Wakimoto et al. 2009).
Using in silico molecular docking studies, hordatine scaffold structures were identified as potential inhibitors of the Severe Acute Respiratory Syndrome Coronavirus 2 that causes coronavirus disease 2019 (COVID-19). Hordatine A and B showed the highest binding affinity to the protease and RNA polymerase targets. This was demonstrated by the strong hydrogen bonds created with the catalytic residues, in addition to noteworthy interactions with other receptor-binding residues. These findings indicated that hordatines could be significant lead candidates for the development of COVID-19 medicines (Dahab et al. 2020).
The guanidine moieties of hordatines can mimic lysine and arginine residues and interact with negatively charged groups or with π-electron-rich aromatic moieties in potential binding sites. The presence of these functional head groups on hordatines may enable their conjugation and derivatisation leading to the development of rationally designed compounds and the investigation of guanidine-based libraries useful in the search of potential pharmacophores. Currently, the hordatines and some structural analogues are being investigated as activating and/or anchoring groups in bioactive compounds and a wide range of related biological activities show the potential to regulate corresponding pathways and control several diseases (Yamaji et al. 2007; Wakimoto et al. 2009; Dahab et al. 2020). However, little is known about the bioavailability, pharmacokinetics and metabolism of hordatines.
Biotechnological approaches for hordatine synthesis
The manipulation of the biosynthesis of bioactive compounds in plants by conventional and biotechnological approaches can provide many opportunities for enhancing concentration levels and yields. Accordingly, the synthesis of valuable secondary plant metabolites in production hosts, such as cultured plant cells and microbial cells, has generated significant interest.
In an early study by Nomura et al. (1999), biosynthesis of hordatines was observed in wheat (Triticum aestivum) addition lines carrying the short arm chromosome 2H (2HS) of barley. This revealed the chromosome as responsible of the biosynthesis of hordatines. In a follow up study, the chromosomal locations of HvACT and HvPrx7 stipulated to be responsible for the formation of HCAgms and hordatines respectively, were further investigated. These genes were found located on the long arm of the chromosome 2H (2HL) and not on 2HS. In addition, while HCAgms were detected after addition of both 2HL and 2HS, no hordatines were found in 2HL containing wheat. These results suggested the presence of another HvACT (probably an isoform) located on the HS and that another barley peroxidase is responsible for the formation of hordatines (Nomura et al. 2007).
Using a transgenic approach, a ‘rational metabolic-flow switching strategy’ for the production of exogenous metabolites in suspension cultured bamboo cells as production hosts was proposed as a hordatine-producing system (Nomura et al. 2018). Stable transformants with the HvACT gene exhibited metabolic-flow switching, from HCAAs of putrescine to those of the desired agmatine. Relatedly, Torenia hybrida plants transformed with agmatine coumaroyltransferase from Arabidopsis (AtACT), accumulated significant amounts of p-coumaroylagmatine (Muroi et al. 2012). Although high levels of p-coumaroylagmatine were found in both cases, no further dimerisation to hordatines was reported, indicating the requirement of additional barley-specific enzymes for the synthesis of hordatines.
In a comparative study of five cultivars of barley, hordatines A and B as well as the hexosylated derivatives were identified as signatory or discriminant metabolites. These compounds may be considered as potential biomarkers in plant breeding practices for barley cultivar differentiation (Hamany Djande et al. 2021).
Biotechnological advances can also act in support of agroprocessing applications. Originating from barley, hordatines are found in beer and spent grains from the brewing process (Kageyama et al. 2011; Pihlava 2014; Becker et al. 2022). The latter has been investigated as a potential source for agroprocessing purposes, valorisation of waste streams through extraction of hordatines and economic exploitation (Becker et al. 2022).
Concluding remarks and future perspectives
Hordatines play a crucial role as specialised secondary metabolites in barley, especially as multipurpose plant-protective phytochemicals. These phenolamides are the focus of several investigations aiming to elucidate their contribution to plant defence and crop protection. Despite their significance to date, the breadth of hordatine studies is limited and gaps in the literature have created many opportunities for further explorative studies. Precisely, research on the biosynthesis of hordatines in response to pathogens beyond fungi, has been largely unexplored. This presents a potential avenue for gaining deeper insights into their implication in plant defence and crop protection. Thus, hordatines and associated precursors may be used as prospective biomarkers for desirable performance characteristics in crop improvement programmes.
Further investigation of hordatines under various environmental conditions and in response to eliciting agents, is worthwhile being conducted. By adopting a systems biology approach, such studies provide insights into the understanding of interrelationships between genotype-environment-phenotype systems. In this case, thorough genomic and integrated multi-omic data can shed light on the developmental and environmental regulation of hordatine metabolism. The biosynthesis of hordatines exhibit unique features where carbon skeletons derived from the early phenylpropanoid pathway combine with polyamines to generate the co-substrates for the conjugation reaction with hordatines as neolignanamide products. There is limited knowledge reagarding the inducibility of the ACT enzyme(s) and regulation of the flux of carbon through pathways supporting the biosynthesis of hordatine precursors. Filling up those gaps will help to manipulate these pathways to improve crops during contemporary breeding and biotechnological approaches.
Most importantly, as the foundation of future investigations, it is essential to improve analytical platforms and methods for better profiling, characterisation, identification and quantification of the hordatines, precursors and derivatives, as well as for their tissue-specific localisation. The identification and adequate structural elucidation of this complex and diverse class of metabolites and their hydroxylated, methylated or hexosylated derivatives is crucial to elucidate their bioactivities, given the paucity of authentic standards.
Moreover, research into the neutraceutical and beneficial properties related to human health through the consumption of barley and barley-derived products is becoming increasingly relevant. Here, further studies are required to fully address the claimed beneficial effects of hordatines as plant-derived bioactives. Growing interest has been given to these benzofurans which are offering great potential in diverse research areas. Natural products such as hordatines may serve as drug leads in in silico molecular docking studies to screen and identify potential therapeutic drugs. This includes characterisation of structural characteristics and mechanisms mediating the pleiotropic effects of hordatines in different models of human diseases.
Data availability
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Abbreviations
- 4CL:
-
4-Coumaric acid:coenzyme A ligase
- ADC:
-
Arginine decarboxylase
- AHCT:
-
Anthocyanin O-hydroxycinnamoyltransferase
- ARG:
-
Arginase
- Asp:
-
Aspartic acid
- AtACT:
-
Arabidopsis thaliana agmatine coumaroyltransferase
- BEAT:
-
Benzylalcohol O-acetyltransferase
- C3H:
-
4-Coumaric acid 3-hydroxylase
- C4H:
-
Cinnamic acid 4-hydrolase
- COMT:
-
Caffeic acid/5-hydroxyferulic acid O-methyltransferase
- CouAgm-4-O-7′/3-8′-DCouAgm:
-
Hordatine A
- CouOHAgm-CouAgm:
-
Hordatine A1
- CouOHAgm–CouOHAgm:
-
Hordatine A2
- DAT:
-
Deacetylvindoline 4-O-acetyltransferase
- F5H:
-
Ferulate 5-hydroxylase
- FerAgm-4-O-7′/3-8′-DCouAgm:
-
Hordatine B
- FerAgm-4-O-7′/3-8′-DFerAgm:
-
Hordatine C
- FerAgm-4-O-7′/3-8′-DSinAgm:
-
Hordatine D
- FT–ICR:
-
Fourier transform ion cyclotron resonance
- HCAAs:
-
Hydroxycinnamic acid amides
- HCAgms:
-
Hydroxycinnamoylagmatines
- HCAs:
-
Hydroxycinnamic acids
- HCBT:
-
Anthranilate N-hydroxycinnamoyl/benzoyltransferase
- His:
-
Histidine
- HvACT:
-
Hordeum vulgare Agmatine coumaroyltransferase
- HvLAC1 and HvLAC2:
-
Hordeum vulgare Laccase 1 and 2
- Ileu:
-
Isoleucine
- MALDI-TOF-MS:
-
Matrix-assisted laser desorption ionisation time-of-flight MS
- MS:
-
Mass spectrometry
- MSI:
-
Imaging mass spectrometry
- N-HCTs:
-
N-hydroxycinnamoyltransferases
- NMR:
-
Nuclear magnetic resonance
- ODC:
-
Ornithine decarboxylase
- OHAgm:
-
Hydroxyagmatine
- PAL:
-
Phenylalanine ammonia lyase
- Ser:
-
Serine
- SLE:
-
Solid–liquid extraction
- SPDS:
-
Spermidine synthase
- SPMS:
-
Spermine synthase
- UHPLC:
-
Ultra-high performance liquid chromatography
- Val:
-
Valine
References
Bagni N, Tassoni A (2001) Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids 20:301–317
Bassard JE, Ullmann P, Bernier F et al (2010) Phenolamides: bridging polyamines to the phenolic metabolism. Phytochemistry 71:1808–1824
Batchu AK, Zimmermann D, Schulze-Lefert P et al (2006) Correlation between hordatine accumulation, environmental factors and genetic diversity in wild barley (Hordeum spontaneum C. Koch) accessions from the Near East Fertile Crescent. Genetica 127:87–99
Becker D, Bakuradze T, Hensel M et al (2021) Influence of brewer’s spent grain compounds on glucose metabolism enzymes. Nutrients 13:2696
Becker D, Permann S, Bakuradze T et al (2022) Isolation and characterisation of hordatine-rich fractions from brewer’s spent grain and their biological activity on α-glucosidase and glycogen phosphorylase α. Sustainability 14:8421
Bhatnagar A, Mishra A (2022) α-Glucosidase inhibitors for diabetes/blood sugar regulation. In: Maheshwari VL, Patil RH (eds) Natural products as enzyme inhibitors: an industrial perspective. Springer Nature, Singapore
Burhenne K, Kristensen BK, Rasmussen SK (2003) A new class of N-hydroxycinnamoyltransferases: purification, cloning, and expression of a barley agmatine coumaroyltransferase (EC 2.3. 1.64). J Biol Chem 278:13919–13927
Cortese-Krott MM, Koning A, Kuhnle GGC et al (2017) The reactive species interactome: Evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid Redox Signal 27:684–712. https://doi.org/10.1089/ars.2017.7083
D’Auria JC (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9:331–340
D’Auria JC, Pichersky E, Schaub A et al (2007) Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J 49:194–207
Dahab MA, Hegazy MM, Abbass HS (2020) Hordatines as a potential inhibitor of COVID-19 main protease and RNA polymerase: an in silico approach. Nat Prod Bioprospect 10:453–462
Dudareva N, D’auria JC, Nam KH et al (1998) Acetyl-CoA: benzylalcohol acetyltransferase–an enzyme involved in floral scent production in Clarkia breweri. Plant J 14:297–304
El-Sharkawy I, Manríquez D, Flores FB et al (2005) Functional characterization of a melon alcohol acyl-transferase gene family involved in the biosynthesis of ester volatiles. Identification of the crucial role of a threonine residue for enzyme activity. Plant Mol Biol 59:345–362
Erb M, Kliebenstein DJ (2020) Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol 184:39–52
Fernie AR, Pichersky E (2015) Focus issue on metabolism: metabolites, metabolites everywhere. Plant Physiol 169:1421–1423
Fujiwara H, Tanaka Y, Yonekura-Sakakibara K et al (1998) cDNA cloning, gene expression and subcellular localization of anthocyanin 5-aromatic acyltransferase from Gentiana triflora. Plant J 16:421–431
Gautam D, Han SJ, Hamdan FF et al (2006) A critical role for β cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab 3:449–461
Gorzolka K, Bednarz H, Niehaus K (2014) Detection and localization of novel hordatine-like compounds and glycosylated derivates of hordatines by imaging mass spectrometry of barley seeds. Planta 239:1321–1335
Gorzolka K, Kölling J, Nattkemper TW et al (2016) Spatio-temporal metabolite profiling of the barley germination process by MALDI MS imaging. PLoS ONE 11:e0150208
Gray J, Caparrós-Ruiz D, Grotewold E (2012) Grass phenylpropanoids: regulate before using! Plant Sci 184:112–120
Hamany Djande CY, Piater LA, Steenkamp PA et al (2021) A metabolomics approach and chemometric tools for differentiation of barley cultivars and biomarker discovery. Metabolites 11:578
Hamany Djande CY, Steenkamp PA, Piater LA et al (2022) Hordatines and associated precursors dominate metabolite profiles of barley (Hordeum vulgare L.) seedlings: a metabolomics study of five cultivars. Metabolites 12:310
Hamany Djande CY, Piater LA, Steenkamp PA et al (2023a) Metabolomic reconfiguration in primed barley (Hordeum vulgare) plants in response to Pyrenophora teres f. teres infection. Metabolites 13:997
Hamany Djande CY, Piater LA, Steenkamp PA et al (2023b) Metabolic reprogramming of barley in response to foliar application of dichlorinated functional analogues of salicylic acid as priming agents and inducers of plant defence. Metabolites 13:666
Heuberger AL, Broeckling CD, Kirkpatrick KR (2014) Application of nontargeted metabolite profiling to discover novel markers of quality traits in an advanced population of malting barley. Plant Biotechnol J 12:147–160
Idehen E, Wang W, Sang S (2020) Health benefits of barley for diabetes. J Food Bioact 12. https://doi.org/10.31665/JFB.2020.12246
Kageyama N, Inui T, Fukami H et al (2011) Elucidation of chemical structures of components responsible for beer aftertaste. J Am Soc Brew Chem 69:255–259
Kageyama N, Inui T, Fukami H et al (2012) Structures in the hordatine family with cis-cinnamoyl moieties. J Am Soc Brew Chem 70:133–136
Kant L, Amrapali S, Babu BK (2016) Barley. In: Singh M, Upadhyaya HD (eds) Genetic and genomic resources for grain cereals improvement. Academic Press, London, UK
Kohyama N, Ono H (2013) Hordatine A β-D-glucopyranoside from ungerminated barley grains. J Agric Food Chem 61:1112–1116
Kong D, Li S, Smolke CD (2020) Discovery of a previously unknown biosynthetic capacity of naringenin chalcone synthase by heterologous expression of a tomato gene cluster in yeast. Sci Adv 6:eabd1143
Kozukue N, Kim DS, Choi S-H et al (2023) Isomers of the tomato glycoalkaloids α-tomatine and dehydrotomatine: relationship to health benefits. Molecules 28:3621
Kristensen BK, Bloch H, Rasmussen SK (1999) Barley coleoptile peroxidases. Purification, molecular cloning, and induction by pathogens. Plant Physiol 120:501–512
Kruse LH, Weigle AT, Irfan M et al (2022) Orthology-based analysis helps map evolutionary diversification and predict substrate class use of BAHD acyltransferases. Plant J 111:1453–1468
Laupheimer S, Kurzweil L, Proels R et al (2023) Volatile-mediated signalling in barley induces metabolic reprogramming and resistance against the biotrophic fungus Blumeria hordei. Plant Biol 25:72–84
Leonard W, Zhanga P, Ying D, Fang Z (2021) Lignanamides: sources, biosynthesis and potential health benefits – a minireview. Crit Rev Food Sci Nutr 61:1404–1414
Levsh O, Chiang YC, Tung CF et al (2016) Dynamic conformational states dictate selectivity toward the native substrate in a substrate-permissive acyltransferase. Biochemistry 55:6314–6326
Li Z, Zhao C, Zhao X et al (2018) Deep annotation of hydroxycinnamic acid amides in plants based on ultra-high-performance liquid chromatography–high-resolution mass spectrometry and its in silico database. Anal Chem 90:14321–14330
Li Z, Chen Z, Zhu Q et al (2020) Improved performance of immobilized laccase on Fe3O4@ C-Cu2+ nanoparticles and its application for biodegradation of dyes. J Hazard Mater 399:123088
Luo J, Fuell C, Parr A et al (2009) A novel polyamine acyltransferase responsible for the accumulation of spermidine conjugates in Arabidopsis seed. Plant Cell 21:18–333
Ma X, Koepke J, Panjikar S et al (2005) Crystal structure of vinorine synthase, the first representative of the BAHD superfamily. J Biol Chem 280:13576–13583
Macoy DM, Kim WY, Lee SY et al (2015a) Biosynthesis, physiology, and functions of hydroxycinnamic acid amides in plants. Plant Biotechnol Rep 9:269–278
Macoy DM, Kim WY, Lee SY et al (2015b) Biotic stress related functions of hydroxycinnamic acid amide in plants. J Plant Biol 58:156–163
Mehta RA, Cassol T, Li N (2002) Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat Biotechnol 20:613–618
Moghe G, Kruse LH, Petersen M et al (2023) BAHD company: the ever-expanding roles of the BAHD acyltransferase gene family in plants. Annu Rev Plant Biol 74:165–194
Molina I, Kosma D (2015) Role of HXXXD-motif/BAHD acyltransferases in the biosynthesis of extracellular lipids. Plant Cell Rep 34:587–601
Morales-Quintana L, Moya-León MA, Herrera R (2015) Computational study enlightens the structural role of the alcohol acyltransferase DFGWG motif. J Mol Model 21:1–10
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Muroi A, Ishihara A, Tanaka C et al (2009) Accumulation of hydroxycinnamic acid amides induced by pathogen infection and identification of agmatine coumaroyltransferase in Arabidopsis thaliana. Planta 230:517–527
Muroi A, Matsui K, Shimoda T (2012) Acquired immunity of transgenic torenia plants overexpressing agmatine coumaroyltransferase to pathogens and herbivore pests. Sci Rep 2:689
Nomura T, Sue M, Horikoshi R (1999) Occurrence of hordatines, the barley antifungal compounds, in a wheat-barley chromosome addition line. Genes Genet Syst 74:99–103
Nomura T, Ishizuka A, Kishida K et al (2007) Chromosome arm location of the genes for the biosynthesis of hordatines in barley. Genes Genet Syst 82:455–464
Nomura T, Ogita S, Kato Y (2018) Rational metabolic-flow switching for the production of exogenous secondary metabolites in bamboo suspension cells. Sci Rep 8:13203
Petersen M (2016) Hydroxycinnamoyltransferases in plant metabolism. Phytochem Rev 15:699–727
Piasecka A, Sawikowska A, Krajewski P et al (2015) Combined mass spectrometric and chromatographic methods for in-depth analysis of phenolic secondary metabolites in barley leaves. J Mass Spectrom 50:513–532
Piasecka A, Sawikowska A, Kuczyńska A et al (2020) Phenolic metabolites from barley in contribution to phenome in soil moisture deficit. Int J Mol Sci 21:6032
Pihlava JM (2014) Identification of hordatines and other phenolamides in barley (Hordeum vulgare) and beer by UPLC-QTOF-MS. J Cereal Sci 60:645–652
Pihlava JM, Kurtelius T, Hurme T (2016) Total hordatine content in different types of beers. J Inst Brewing 122:212–217
Pretorius CJ, Dubery IA (2023) Avenanthramides, distinctive hydroxycinnamoyl conjugates of oat, Avena sativa l.: an update on the biosynthesis, chemistry, and bioactivities. Plants 12:1388
Raj R, Shams R, Pandey VK et al (2023) Barley phytochemicals and health promoting benefits: a comprehensive review. J Agric Food Res 2023:100677
Roumani M, Duval RE, Ropars A et al (2020) Phenolamides: plant specialized metabolites with a wide range of promising pharmacological and health-promoting interests. Biomed Pharmacother 131:110762
Roumani M, Besseau S, Gagneul D et al (2021) Phenolamides in plants: an update on their function, regulation, and origin of their biosynthetic enzymes. J Exp Bot 72:2334–2355
Roumani M, Ropars A, Robin C et al (2023) Characterization of biological properties of individual phenolamides and phenolamide-enriched leaf tomato extracts. Molecules 28:552
Salum ML, Itovich LM, Erra-Balsells R (2013) Z-Sinapinic acid: the change of the stereochemistry of cinnamic acids as rational synthesis of a new matrix for carbohydrate MALDI-MS analysis. J Mass Spectrom 48:1160–1169
Sarabia LD, Boughton BA, Rupasinghe T et al (2018) High-mass-resolution MALDI mass spectrometry imaging reveals detailed spatial distribution of metabolites and lipids in roots of barley seedlings in response to salinity stress. Metabolomics 14:1–16
Shewry PR (2014) Minor component of the barley grain: minerals, lipids, terpenoids, phenolics and vitamins. In: Shewry PR, Ullrich SE (eds) Barley: chemistry and technology, 2nd edn. AACC International, Elsevier Inc
Smith TA, Best GR (1978) Distribution of the hordatines in barley. Phytochemistry 17:1093–1098
Smith CA, O’Maille G, Want EJ et al (2005) METLIN: a metabolite mass spectral database. Ther Drug Monit 27:747–751
Spreng S, Hofmann T (2018) Activity-guided identification of in vitro antioxidants in beer. J Agric Food Chem 66:720–731
St-Pierre B, De Luca V (2000) Origin and diversification of the BAHD superfamily of acyltransferases involved in secondary metabolism. Recent Adv Phytochem 34:285–315
St-Pierre B, Vazquez-Flota FA, De Luca V (1999) Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell 11:887–900
Stoessl A (1965) The antifungal factors in barley-III.: isolation of p-coumaroylagmatine. Phytochemistry 4:973–976
Stoessl A (1966) The antifungal factors in barley-the constitutions of hordatines A and B. Tetrahedron Lett 7:2287–2292
Stoessl A (1967) The antifungal factors in barley. IV. Isolation, structure, and synthesis of the hordatines. Can J Chem 45:1745–1760
Stoessl A, Unwin CH (1969) The antifungal factors in barley. V. Antifungal activity of the hordatines. Can J Bot 48:465–470
Takahashi T, Kakehi JI (2010) Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Ann Bot 105:1–6
Tanahashi Y, Komori S, Matsuyama H et al (2021) Functions of muscarinic receptor subtypes in gastrointestinal smooth muscle: a review of studies with receptor-knockout mice. Int J Mol Sci 22:926
Ube N, Nishizaka M, Ichiyanagi T et al (2017) Evolutionary changes in defensive specialized metabolism in the genus Hordeum. Phytochemistry 141:1–10
Ube N, Ishihara A, Yabuta Y et al (2023) Molecular identification of a laccase that catalyzes the oxidative coupling of a hydroxycinnamic acid amide for hordatine biosynthesis in barley. Plant J 115:1037–1050
van Zadelhoff A, de Bruijn WJ, Fang Z et al (2021) Toward a systematic nomenclature for (neo) lignanamides. J Nat Prod 84:956–963
Van Zadelhoff A, Meijvogel L, Seelen AM et al (2022) Biomimetic enzymatic oxidative coupling of barley phenolamides: hydroxycinnamoylagmatines. J Agric Food Chem 70:16241–16252
von Röpenack E, Parr A, Schulze-Lefert P (1998) Structural analyses and dynamics of soluble and cell wall-bound phenolics in a broad spectrum resistance to the powdery mildew fungus in barley. J Biol Chem 273:9013–9022
Wakimoto T, Nitta M, Kasahara K et al (2009) Structure–activity relationship study on α1 adrenergic receptor antagonists from beer. Bioorg Med Chem Lett 19:5905–5908
Walker AM, Hayes RP, Youn B et al (2013) Elucidation of the structure and reaction mechanism of sorghum hydroxycinnamoyltransferase and its structural relationship to other coenzyme A-dependent transferases and synthases. Plant Physiol 162:640–651
Weng JK, Lynch JH, Matos JO et al (2021) Adaptive mechanisms of plant specialized metabolism connecting chemistry to function. Nat Chem Biol 17:1037–1045
Wiegmann M, Maurer A, Pham A et al (2019) Barley yield formation under abiotic stress depends on the interplay between flowering time genes and environmental cues. Sci Rep 9:6397
Yamaji N, Yokoo Y, Iwashita T et al (2007) Structural determination of two active compounds that bind to the muscarinic M3 receptor in beer. Alcohol Clin Exp Res 31:S9–S14
Yamane M, Takenoya M, Yajima S et al (2020) Crystal structure of barley agmatine coumaroyltransferase, an N-acyltransferase from the BAHD superfamily. Acta Cryst F 76:590–596
Yang Q, Reinhard K, Schiltz E et al (1997) Characterization and heterologous expression of hydroxycinnamoyl/benzoyl-CoA: anthranilate N-hydroxycinnamoyl/benzoyltransferase from elicited cell cultures of carnation, Dianthus caryophyllus L. Plant Mol Biol 35:777–789
Young MR, Towers GHN, Neish AC (1966) Taxonomic distribution of ammonia-lyases for L-phenylalanine and L-tyrosine in relation to lignification. Canad J Bot 44:341–349
Yu XH, Gou JY, Liu CJ (2009) BAHD superfamily of acyl-CoA dependent acyltransferases in Populus and Arabidopsis: bioinformatics and gene expression. Plant Mol Biol 70:421–442
Yuan Z, Yang H, Pan L et al (2022) Systematic identification and expression profiles of the BAHD superfamily acyltransferases in barley (Hordeum vulgare). Sci Rep 12:5063
Zeiss DR, Piater LA, Dubery IA (2021) Hydroxycinnamate amides: an intriguing combination of plant protective metabolites. Trends Plant Sci 26:184
Zhai X, Wu K, Ji R, et al (2022) Structure and function insight of the α-glucosidase QsGH13 from Qipengyuania seohaensis sp. SW-135. Front Microbiol 13:849585
Acknowledgements
The University of Johannesburg, South Africa, is thanked for postdoctoral fellowship support to C.Y.H.D.
Funding
Open access funding provided by University of Johannesburg.
Author information
Authors and Affiliations
Contributions
Conceptualisation, CYHD and IAD; writing – original draft preparation, CYHD and IAD; writing – review and editing, CYHD and IAD; supervision, IAD; Both authors agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamany Djande, C.Y., Dubery, I.A. Hordatines, dimerised hydroxycinnamoylagmatine conjugates of barley (Hordeum vulgare L.): an appraisal of the biosynthesis, chemistry, identification and bioactivities. Phytochem Rev (2024). https://doi.org/10.1007/s11101-024-09961-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11101-024-09961-9