Abstract
Alcohol acyltransferases (AAT) catalyze the esterification reaction of alcohols and acyl-CoA into esters in fruits and flowers. Despite the high divergence between AAT enzymes, two important and conserved motifs are shared: the catalytic HxxxD motif, and the DFGWG motif. The latter is proposed to play a structural role; however, its function remains unclear. The DFGWG motif is located in loop 21 and stabilized by a hydrogen bond between residues Y52 and D381. Also, this motif is distant from the HxxxD motif, and most probably without a direct role in the substrate interaction. To evaluate the role of the DFGWG motif, in silico analysis was performed in the VpAAT1 protein. Three mutants (Y52F, D381A and D381E) were evaluated. Major changes (size and shape) in the solvent channels were found, although no differences were revealed in the entire 3D structure. Molecular dynamics simulations and docking studies described unfavorable energies for interaction of the mutant proteins with different substrates, as well as unfavored ligand orientations in the solvent channel. Additionally, we examined the contribution of different energetic parameters to the total free energy of protein–ligand complexes by the MM-GBSA method. The complexes differed mainly in their van der Waals contributions and have unfavorable electrostatic interactions. VpAAT1, Y52F and D381A mutants showed a dramatic reduction in the binding capacity to several substrates, which is related to differences in electrostatic potential on the protein surfaces, suggesting that D381 from the DFGWG motif and residue Y52 play a crucial role in maintenance of the adequate solvent channel structure required for catalysis.

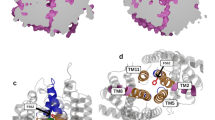
Molecular docking, molecular dynamics (MD) simulations and MM-GBSA free energy calculations were employed to obtain quantitative estimates for the binding free energies of wild type Vasconcellea pubescens alcohol acyltransferase (VpAAT1-WT) and the protein mutants. Left VpAAT1 model structure in cartoon representation showing the solvent channel in the middle of the structure. Center, right Changes in shape and structure in the solvent channel of Y52F and D381A mutant proteins, respectively, compared to WT. The results obtained reveal that the interaction between D381 and Y52 residues is important for the maintenance of solvent channel structure







Similar content being viewed by others
References
Balbontín C, Gaete-Eastman C, Vergara M, Herrera R, Moya-León MA (2007) Treatment with 1-MCP and the role of ethylene in aroma development of mountain papaya fruit. Postharvest Biol Technol 43:67–77
Beekwilder J, Alvarez-Huerta M, Neef E, Verstappen FWA, Bouwmeester HJ, Aharoni A (2004) Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol 135:1865–1878
Dudareva N, Pichersky E, Gershenzon J (2004) Biochemistry of plant volatiles. Plant Physiol 135:1893–1902
Balbontín C, Gaete-Eastman C, Fuentes L, Figueroa CR, Herrera R, Manriquez D, Latche A, Pech JC, Moya-León MA (2010) VpAAT1, a gene encoding an alcohol acyltransferase, is involved in ester biosynthesis during ripening of mountain papaya fruit. J Agric Food Chem 58:5114–5121
St Pierre B, De Luca V (2000) Evolution of acyltransferase genes: origin and diversification of the BAHD superfamily of acyltransferases involved in secondary metabolism. In: Romeo JT, Ibrahim R, Varin L, De Luca V (eds) Recent advances in phytochemistry. Evolution of metabolic pathways, vol 34. Elsevier, Oxford, pp 285–315
El-Sharkawy I, Manriquez D, Flores FB, Regad F, Bouzayen M, Latche A, Pech JC (2005) Functional characterization of a melon alcohol acyl-transferase gene family involved in the biosynthesis of ester volatiles. Identification of the crucial role of a threonine residue for enzyme activity. Plant Mol Biol 59:345–362
Echeverría G, Graell J, López ML, Lara I (2004) Volatile production, quality and aroma-related enzyme activities during maturation of ‘Fuji’ apples. Postharvest Biol Technol 31:217–227
Souleyre EJ, Greenwood DR, Friel EN, Karunairetnam S, Newcomb RD (2005) An alcohol acyltransferase from apple (cv. Royal Gala), MpAAT1, produces esters involved in apple fruit flavor. FEBS J 272:3132–3144
Li D, Xu Y, Xu G, Gu L, Li D, Shu H (2006) Molecular cloning and expression of a gene encoding alcohol acyltransferase (MdAAT2) from apple (cv. Golden Delicious). Phytochemistry 67:658–667
D'Auria JC (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9:331–340
Morales-Quintana L, Nuñez-Tobar MX, Moya-León MA, Herrera R (2013) Molecular dynamics simulation and site-directed mutagenesis of alcohol acyltransferase: a proposed mechanism of catalysis. J Chem Inf Model 53:2689–2700
Bayer A, Ma XY, Stockigt J (2004) Acetyltransfer in natural product biosynthesis—functional cloning and molecular analysis of vinorine synthase. Bioorg Med Chem 12:2787–2795
Suzuki H, Nakayama T, Nishino T (2003) Proposed mechanism and functional amino acid residues of malonyl-CoA:anthocyanin 5-O-Glucoside-6”’-O-malonyltransferase from flowers of Salvia splendens, a member of the versatile plant acyltransferase family. Biochemistry 42:1764–1771
Ma X, Koep J, Panjikar S, Fritzch G, Stockigt J (2005) Crystal structure of vinorine synthase, the first representative of the BAHD superfamily. J Biol Chem 280:13576–13583
Morales-Quintana L, Fuentes L, Gaete-Eastman C, Herrera R, Moya-León MA (2011) Structural characterization and substrate specificity of VpAAT1 protein related to ester biosynthesis in mountain papaya fruit. J Mol Graph Model 29:635–642
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802
Abagyan R, Totrov M, Kuznetsov D (1994) ICM- A new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J Comput Chem 15:488–506
MacKerell AD Jr, Bashford D, Bellott M, Dunbrack RL Jr, Evanseck J, Field M, Fischer JS, Gao J, Guo H, Ha S, Joseph D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Roux B, Schlenkrich M, Smith J, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M (1992) Self-consistent parameterization of biomolecules for molecular modeling and condensed phase simulations. FASEB J 6:A143
Schlenkrich M, Brickmann J, MacKerell AD Jr, Karplus M (1996) An empirical potential energy function for phospholipids: criteria for parameter optimization and applications. In: Merz KM, Roux B (eds) Biological membranes: a molecular perspective from computation and experiment. Birkhauser, Boston, pp 31–81
Jorgensen WL, Chandresekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Galaz S, Morales-Quintana L, Moya-León MA, Herrera R (2013) Structural analysis of the alcohol acyltransferase protein family from Cucumis melo shows that enzyme activity depends on an essential solvent channel. FEBS J 280:1344–1357
Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Guvench O, Lopes P, Vorobyov I, MacKerell AD Jr (2010) CHARMM General Force Field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force field. J Comput Chem 31:671–690
Humphrey W, Dalke A, Schulten K (1996) VMD Visual molecular dynamics. J Mol Graph 14:33–38
Homeyer N, Gohlke H (2012) Free energy calculations by the Molecular Mechanics Poisson-Boltzmann surface area method. Mol Inf 31:114–122
Vergara-Jaque A, Comer J, Monsalve L, González-Nilo FD, Sandoval C (2013) Computationally efficient methodology for atomic-level characterization of dendrimer−drug complexes: a comparison of amine- and acetyl-terminated PAMAM. J Phys Chem B 117:6801–6813
Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA 98:10037–10041
Morales-Quintana L, Moya-León MA, Herrera R (2012) Molecular docking simulation analysis of alcohol acyltransferases from two related fruit species explains their different substrate selectivities. Mol Simul 38:912–921
Walker AM, Hayes RP, Youn B, Vermerris W, Sattler SE, Kang CH (2013) Elucidation of the structure and reaction mechanism of sorghum hydroxycinnamoyl transferase and its structural relationship to other coenzyme A-dependent transferases and synthases. Plant Physiol 162:640–651
Wu D, Govindasamy L, Lian W, Gu Y, Kukar T, Agbandje-McKenna M, McKenna R (2003) Structure of human carnitine acetyltransferase: molecular basis for fatty acyl transfer. J Biol Chem 278:13159–13165
Mattevi A, Obmolova G, Schulze E, Kalk KH, Westphal AH, de Kok A, Hol WG (1992) Atomic structure of the cubic core of the pyruvate dehydrogenase multienzyme complex. Science 255:1544–1550
Stoica I, Sadig SK, Coverney PV (2007) Rapid and accurate prediction of binding free energies for saquinavir-bound HIV-1 proteases. J Am Chem Soc 130:2639–2648
Li T, Froeyen M, Herdewijn P (2010) Insight into ligand selectivity in HCV NS5B polymerase: molecular dynamics simulations, free energy decomposition and docking. J Mol Model 16:49–59
Zoete V, Michielin O (2007) Comparison between computational alanine scanning and per-residue binding free energy decomposition for protein–protein association using MM-GBSA: application to the TCR-p-MHC complex. Proteins 67:1026–1047
Li T, Froeyen M, Herdewijn P (2008) Computational alanine scanning and free energy decomposition for E. coli type I signal peptidase with lipopeptide inhibitor complex. J Mol Graph Model 26:813–823
Acknowledgments
We thank the Center of Bioinformatics and Molecular Simulations (CBSM) of the University of Talca for providing ICM programs. Anillo ACT-1110 project and CONICYT PAI/ACADEMIA #79140027 supported this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
(DOCX 1607 kb)
Rights and permissions
About this article
Cite this article
Morales-Quintana, L., Moya-León, M.A. & Herrera, R. Computational study enlightens the structural role of the alcohol acyltransferase DFGWG motif. J Mol Model 21, 216 (2015). https://doi.org/10.1007/s00894-015-2762-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2762-6