Abstract
Purpose
Green tea is a widely consumed beverage. A recent clinical study reported green tea decreased systemic exposure of raloxifene and its glucuronide metabolites by 34–43%. However, the underlying mechanism(s) remains unknown. This study investigated a change in raloxifene’s solubility as the responsible mechanism.
Methods
The effects of green tea extract, (–)-epigallocatechin gallate (EGCG), and (–)-epigallocatechin (EGC) on raloxifene’s solubility were assessed in fasted state simulated intestinal fluids (FaSSIF) and fed state simulated intestinal fluids (FeSSIF). EGCG and EGC represent green tea’s main bioactive constituents, flavan-3-gallate and flavan-3-ol catechins respectively, and the tested concentrations (mM) match the µg/mg of each compound in the extract. Our mouse study (n = 5/time point) evaluated the effect of green tea extract and EGCG on the systemic exposure of raloxifene.
Results
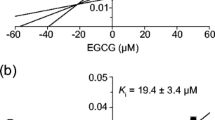
EGCG (1 mM) and EGC (1.27 mM) decreased raloxifene’s solubility in FaSSIF by 78% and 13%, respectively. Micelle size in FaSSIF increased with increasing EGCG concentrations (> 1000% at 1 mM), whereas EGC (1.27 mM) did not change micelle size. We observed 3.4-fold higher raloxifene solubility in FeSSIF compared to FaSSIF, and neither green tea extract nor EGCG significantly affected raloxifene solubility or micelle size in FeSSIF. The mice study showed that green tea extract significantly decreased raloxifene Cmax by 44%, whereas EGCG had no effect. Green tea extract and EGCG did not affect the AUC0-24 h of raloxifene or the metabolite-to-parent AUC ratio.
Conclusions
This study demonstrated flavan-3-gallate catechins may decrease solubility of poorly water-soluble drugs such as raloxifene, particularly in the fasted state.




Similar content being viewed by others
Data availability
All data supporting the findings of this study are included within the paper.
Abbreviations
- AUC:
-
Area under the plasma concentration versus time curve
- BDDCS:
-
Biopharmaceutics drug disposition classification system
- Cg:
-
( +)-Catechin gallate
- Cmax :
-
Maximum plasma concentration
- EGC:
-
(–)-Epigallocatechin
- EGCG:
-
(–) -Epigallocatechin gallate
- FaSSIF:
-
Fasted state simulated intestinal fluid
- FeSSIF:
-
Fed state simulated intestinal fluid
- Gcg:
-
(–)-Gallocatechin gallate
- tmax :
-
Time to Cmax
- UHPLC:
-
Ultra-high performance liquid chromatography
References
Harbowy ME, Balentine DA, Davies AP, Cai Y. Tea Chemistry. CRC Crit Rev Plant Sci. 1997;16:415–80. https://doi.org/10.1080/07352689709701956.
Tea Association of the USA Inc. Tea Fact Sheet n.d. https://www.teausa.com/14655/tea-fact-sheet. Accessed 6 Mar 2023.
UN Comtrade. Leading green tea importing countries worldwide in 2021 (in million U.S. dollars). 2022.
Lin Y-S, Tsai Y-J, Tsay J-S, Lin J-K. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J Agric Food Chem. 2003;51:1864–73. https://doi.org/10.1021/jf021066b.
Chaturvedula VSP, Prakash I. The aroma, taste, color and bioactive constituents of tea. J Med Plants Res. 2011;5:2110–24. https://doi.org/10.5897/JMPR.9001187.
Yang CS, Pan E. The effects of green tea polyphenols on drug metabolism. Expert Opin Drug Metab Toxicol. 2012;8:677–89. https://doi.org/10.1517/17425255.2012.681375.
Wang Z-M, Chen B, Zhou B, Zhao D, Wang L-S. Green tea consumption and the risk of stroke: A systematic review and meta-analysis of cohort studies. Nutrition. 2023;107:111936. https://doi.org/10.1016/j.nut.2022.111936.
Mhatre S, Srivastava T, Naik S, Patravale V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: A review. Phytomedicine. 2021;85:153286. https://doi.org/10.1016/j.phymed.2020.153286.
Wada K, Oba S, Tsuji M, Goto Y, Mizuta F, Koda S, et al. Green tea intake and colorectal cancer risk in Japan: the Takayama study. Jpn J Clin Oncol. 2019;49:515–20. https://doi.org/10.1093/jjco/hyz030.
Khan N, Mukhtar H. Tea and health: studies in humans. Curr Pharm Des. 2013;19:6141–7. https://doi.org/10.2174/1381612811319340008.
Organic green tea retail sales value in the United States 2021 | Statista n.d. https://www.statista.com/statistics/1378291/retail-sales-value-of-organic-green-tea-in-the-united-states/. Accessed 16 Sep 2023.
Zeng W, Lao S, Guo Y, Wu Y, Huang M, Tomlinson B, et al. The Influence of EGCG on the Pharmacokinetics and Pharmacodynamics of Bisoprolol and a New Method for Simultaneous Determination of EGCG and Bisoprolol in Rat Plasma. Front Nutr. 2022;9:907986. https://doi.org/10.3389/fnut.2022.907986.
Misaka S, Yatabe J, Müller F, Takano K, Kawabe K, Glaeser H, et al. Green tea ingestion greatly reduces plasma concentrations of nadolol in healthy subjects. Clin Pharmacol Ther. 2014;95:432–8. https://doi.org/10.1038/clpt.2013.241.
Ge J, Tan B-X, Chen Y, Yang L, Peng X-C, Li H-Z, et al. Interaction of green tea polyphenol epigallocatechin-3-gallate with sunitinib: potential risk of diminished sunitinib bioavailability. J Mol Med (Berl). 2011;89:595–602. https://doi.org/10.1007/s00109-011-0737-3.
Clarke JD, Judson SM, Tian D, Kirby TO, Tanna RS, Matula-Péntek A, et al. Co-consuming green tea with raloxifene decreases raloxifene systemic exposure in healthy adult participants. Clin Transl Sci. 2023. https://doi.org/10.1111/cts.13578.
Dahan A, Miller JM. The solubility-permeability interplay and its implications in formulation design and development for poorly soluble drugs. AAPS J. 2012;14:244–51. https://doi.org/10.1208/s12248-012-9337-6.
Holm R, Müllertz A, Mu H. Bile salts and their importance for drug absorption. Int J Pharm. 2013;453:44–55. https://doi.org/10.1016/j.ijpharm.2013.04.003.
Porat D, Dahan A. Active intestinal drug absorption and the solubility-permeability interplay. Int J Pharm. 2018;537:84–93. https://doi.org/10.1016/j.ijpharm.2017.10.058.
Bocci G, Oprea TI, Benet LZ. State of the Art and Uses for the Biopharmaceutics Drug Disposition Classification System (BDDCS): New Additions, Revisions, and Citation References. AAPS J. 2022;24:37. https://doi.org/10.1208/s12248-022-00687-0.
Du T, Sun R, Li L, Ebuzoeme C, Bui D, Zheng Z, et al. Development and validation of ultra-high-performance liquid chromatography-mass spectrometry method for the determination of raloxifene and its phase II metabolites in plasma: Application to pharmacokinetic studies in rats. J Sep Sci. 2020;43:4414–23. https://doi.org/10.1002/jssc.202000835.
Hochner-Celnikier D. Pharmacokinetics of raloxifene and its clinical application. Eur J Obstet Gynecol Reprod Biol. 1999;85:23–9. https://doi.org/10.1016/S0301-2115(98)00278-4.
Wang Z, Li Y. Raloxifene/SBE-β-CD Inclusion Complexes Formulated into Nanoparticles with Chitosan to Overcome the Absorption Barrier for Bioavailability Enhancement. Pharmaceutics. 2018;10:76. https://doi.org/10.3390/pharmaceutics10030076.
Murthy A, Rao Ravi P, Kathuria H, Malekar S. Oral Bioavailability Enhancement of Raloxifene with Nanostructured Lipid Carriers. Nanomaterials. 2020;10:1085. https://doi.org/10.3390/nano10061085.
Hosny KM, Bahmdan RH, Alhakamy NA, Alfaleh MA, Ahmed OA, Elkomy MH. Physically Optimized Nano-Lipid Carriers Augment Raloxifene and Vitamin D Oral Bioavailability in Healthy Humans for Management of Osteoporosis. J Pharm Sci. 2020;109:2145–55. https://doi.org/10.1016/j.xphs.2020.03.009.
Sakakibara T, Sawada Y, Wang J, Nagaoka S, Yanase E. Molecular Mechanism by Which Tea Catechins Decrease the Micellar Solubility of Cholesterol. J Agric Food Chem. 2019;67:7128–35. https://doi.org/10.1021/acs.jafc.9b02265.
Ogawa K, Hirose S, Nagaoka S, Yanase E. Interaction between Tea Polyphenols and Bile Acid Inhibits Micellar Cholesterol Solubility. J Agric Food Chem. 2016;64:204–9. https://doi.org/10.1021/acs.jafc.5b05088.
Kellogg JJ, Graf TN, Paine MF, McCune JS, Kvalheim OM, Oberlies NH, et al. Comparison of Metabolomics Approaches for Evaluating the Variability of Complex Botanical Preparations: Green Tea (Camellia sinensis) as a Case Study. J Nat Prod. 2017;80:1457–66. https://doi.org/10.1021/acs.jnatprod.6b01156.
McFarland JW, Avdeef A, Berger CM, Raevsky OA. Estimating the Water Solubilities of Crystalline Compounds from Their Chemical Structures Alone. J Chem Inf Comput Sci. 2001;41:1355–9. https://doi.org/10.1021/ci0102822.
Biorelevant.com. Media prep tool n.d. https://biorelevant.com/#media_prep_tool_tab. Accessed 6 Mar 2023.
Klumpp L, Leigh M, Dressman J. Dissolution behavior of various drugs in different FaSSIF versions. Eur J Pharm Sci. 2020;142:105138. https://doi.org/10.1016/J.EJPS.2019.105138.
Kalam A, Talegaonkar S, Vohora D. Effects of raloxifene against letrozole-induced bone loss in chemically-induced model of menopause in mice. Mol Cell Endocrinol. 2017;440:34–43. https://doi.org/10.1016/j.mce.2016.11.005.
Yang ZhY, Zhang ZhF, He XB, Zhao GY, Zhang YQ. Validation of a Novel HPLC Method for the Determination of Raloxifene and Its Pharmacokinetics in Rat Plasma. Chromatographia. 2007;65:197–201. https://doi.org/10.1365/s10337-006-0123-4.
Trontelj J, Bogataj M, Marc J, Mrhar A. Development and validation of a liquid chromatography–tandem mass spectrometry assay for determination of raloxifene and its metabolites in human plasma. J Chromatogr B. 2007;855:220–7. https://doi.org/10.1016/j.jchromb.2007.05.004.
Rajagopaludu P, Saritha N, Devanna N, Srinivas M. Method development and validation of anabasine and nornicotine in human plasma by LC-MS/MS. J Pharm Res Int. 2021:7–17. https://doi.org/10.9734/jpri/2021/v33i1731301.
Zhang J, Shao B, Yin J, Wu Y, Duan H. Simultaneous detection of residues of β-adrenergic receptor blockers and sedatives in animal tissues by high-performance liquid chromatography/tandem mass spectrometry. J Chromatogr B. 2009;877:1915–22. https://doi.org/10.1016/j.jchromb.2009.05.025.
Teleki A, Nylander O, Bergström CAS. Intrinsic Dissolution Rate Profiling of Poorly Water-Soluble Compounds in Biorelevant Dissolution Media. Pharmaceutics. 2020;12:493. https://doi.org/10.3390/pharmaceutics12060493.
Riethorst D, Baatsen P, Remijn C, Mitra A, Tack J, Brouwers J, et al. An In-Depth View into Human Intestinal Fluid Colloids: Intersubject Variability in Relation to Composition. Mol Pharm. 2016;13:3484–93. https://doi.org/10.1021/acs.molpharmaceut.6b00496.
Jamil R, Polli JE. Prediction of In Vitro Drug Dissolution into Fed-state Biorelevant Media: Contributions of Solubility Enhancement and Relatively Low Colloid Diffusivity. Eur J Pharm Sci. 2022;173:106179. https://doi.org/10.1016/j.ejps.2022.106179.
Albassam AA, Markowitz JS. An Appraisal of Drug-Drug Interactions with Green Tea (Camellia sinensis). Planta Med. 2017;83:496–508. https://doi.org/10.1055/s-0043-100934.
Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem. 2003;14:326–32. https://doi.org/10.1016/s0955-2863(03)00054-8.
Ikeda H, Yamanaka M, Takahashi S, Ohata T, Yukawa M, Nakashima R, et al. Drug-Tea Polyphenol Interaction (III) Incompatibility between Aripiprazole Oral Solution and Green Tea. Chem Pharm Bull (Tokyo). 2022;70:230–4. https://doi.org/10.1248/cpb.c21-00746.
Riethorst D, Mols R, Duchateau G, Tack J, Brouwers J, Augustijns P. Characterization of Human Duodenal Fluids in Fasted and Fed State Conditions. J Pharm Sci. 2016;105:673–81. https://doi.org/10.1002/jps.24603.
Flanagan T, Van Peer A, Lindahl A. Use of physiologically relevant biopharmaceutics tools within the pharmaceutical industry and in regulatory sciences: Where are we now and what are the gaps? Eur J Pharm Sci. 2016;91:84–90. https://doi.org/10.1016/j.ejps.2016.06.006.
Dahlgren D, Venczel M, Ridoux J-P, Skjöld C, Müllertz A, Holm R, et al. Fasted and fed state human duodenal fluids: Characterization, drug solubility, and comparison to simulated fluids and with human bioavailability. Eur J Pharm Biopharm. 2021;163:240–51. https://doi.org/10.1016/j.ejpb.2021.04.005.
Wu C, Benet LZ. Predicting drug disposition via application of BCS: Transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. https://doi.org/10.1007/s11095-004-9004-4.
Golden EB, Lam PY, Kardosh A, Gaffney KJ, Cadenas E, Louie SG, et al. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009;113:5927–37. https://doi.org/10.1182/blood-2008-07-171389.
Ohata T, Ikeda H, Inenaga M, Mizobe T, Yukawa M, Fujisawa M, et al. Drug-tea polyphenol interaction (II) complexation of piperazine derivatives with green tea polyphenol. Thermochim Acta. 2017;653:1–7. https://doi.org/10.1016/j.tca.2017.03.023.
Ikeda H, Tsuji E, Matsubara T, Yukawa M, Fujisawa M, Yukawa E, et al. Incompatibility between propericiazine oral solution and tea-based drink. Chem Pharm Bull (Tokyo). 2012;60:1207–11. https://doi.org/10.1248/cpb.c12-00116.
Kobayashi M, Nishizawa M, Inoue N, Hosoya T, Yoshida M, Ukawa Y, et al. Epigallocatechin gallate decreases the micellar solubility of cholesterol via specific interaction with phosphatidylcholine. J Agric Food Chem. 2014;62:2881–90. https://doi.org/10.1021/jf405591g.
Seedher N, Kanojia M. Micellar solubilization of some poorly soluble antidiabetic drugs: a technical note. AAPS PharmSciTech. 2008;9:431–6. https://doi.org/10.1208/s12249-008-9057-5.
Fagerberg JH, Bergström CAS. Intestinal solubility and absorption of poorly water soluble compounds: predictions, challenges and solutions. Ther Deliv. 2015;6:935–59. https://doi.org/10.4155/tde.15.45.
Misaka S, Abe O, Ono T, Ono Y, Ogata H, Miura I, et al. Effects of single green tea ingestion on pharmacokinetics of nadolol in healthy volunteers. Br J Clin Pharmacol. 2020;86:2314–8. https://doi.org/10.1111/bcp.14315.
Abe O, Ono T, Sato H, Müller F, Ogata H, Miura I, et al. Role of (-)-epigallocatechin gallate in the pharmacokinetic interaction between nadolol and green tea in healthy volunteers. Eur J Clin Pharmacol. 2018;74:775–83. https://doi.org/10.1007/s00228-018-2436-2.
Muramatsu K, Fukuyo M, Hara Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J Nutr Sci Vitaminol (Tokyo). 1986;32:613–22. https://doi.org/10.3177/jnsv.32.613.
Funding
This work was supported by the National Institutes of Health National Center for Complementary and Integrative Health grants R21 AT011101 and U54 AT008909.
Author information
Authors and Affiliations
Contributions
Participated in research design: Victoria Oyanna and John Clarke
Conducted experiments: Victoria Oyanna, Baron Bechtold, Katherine Lynch, M. Ridge Call, and John Clarke
Contributed new reagents or analytic tools: Tyler Graf and Nicholas Oberlies
Performed data analysis: Victoria Oyanna and John Clarke
Wrote or contributed to the writing of the manuscript: Victoria Oyanna and John Clarke
Corresponding author
Ethics declarations
Conflicts of Interest
None
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oyanna, V.O., Bechtold, B.J., Lynch, K.D. et al. Green Tea Catechins Decrease Solubility of Raloxifene In Vitro and Its Systemic Exposure in Mice. Pharm Res 41, 557–566 (2024). https://doi.org/10.1007/s11095-024-03662-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-024-03662-w