Abstract
Purpose
The objective of this study is to assess the effects of green tea and its major catechin component, (−)-epigallocatechin gallate (EGCG), on CYP2C9-mediated substrate metabolism in vitro, and the pharmacokinetics of fluvastatin in healthy volunteers.
Methods
The metabolism of diclofenac and fluvastatin in human recombinant CYP2C9 was investigated in the presence of EGCG. In a randomized three-phase crossover study, 11 healthy volunteers ingested a single 20-mg dose of fluvastatin with green tea extract (GTE), containing 150 mg of EGCG, along with water (300 mL), brewed green tea (300 mL), or water (300 mL) after overnight fasting. Plasma concentrations of fluvastatin and EGCG were measured by ultra-performance liquid chromatography with fluorescence detection and a single mass spectrometer.
Results
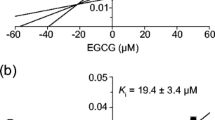
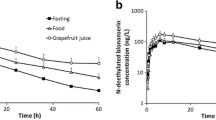
EGCG inhibited diclofenac 4′-hydroxylation and fluvastatin degradation with IC50 of 2.23 and 48.04 μM, respectively. Brewed green tea used in the clinical study also dose-dependently inhibited the metabolism of diclofenac and fluvastatin in vitro. However, no significant effects of GTE and brewed green tea were observed in plasma concentrations of fluvastatin. The geometric mean ratios with 90% CI for area under the plasma concentration–time curve (AUC0–∞) of fluvastatin were 0.993 (0.963–1.024, vs. brewed green tea) and 0.977 (0.935–1.020, vs. GTE).
Conclusions
Although in vitro studies indicated that EGCG and brewed green tea produce significant inhibitory effects on CYP2C9 activity, the concomitant administration of green tea and fluvastatin in healthy volunteers did not influence the pharmacokinetics of fluvastatin.




Similar content being viewed by others
References
Khan N, Mukhtar H (2007) Tea polyphenols for health promotion. Life Sci 81(7):519–533. https://doi.org/10.1016/j.lfs.2007.06.011
Muto S, Fujita K, Yamazaki Y, Kamataki T (2001) Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat Res 479(1-2):197–206. https://doi.org/10.1016/S0027-5107(01)00204-4
Misaka S, Kawabe K, Onoue S, Werba JP, Giroli M, Tamaki S, Kan T, Kimura J, Watanabe H, Yamada S (2013) Effects of green tea catechins on cytochrome P450 2B6, 2C8, 2C19, 2D6 and 3A activities in human liver and intestinal microsomes. Drug Metab Pharmacokinet 28(3):244–249. https://doi.org/10.2133/dmpk.DMPK-12-RG-101
Satoh T, Fujisawa H, Nakamura A, Takahashi N, Watanabe K (2016) Inhibitory effects of eight green tea catechins on cytochrome P450 1A2, 2C9, 2D6, and 3A4 activities. J Pharm Pharm Sci 19(2):188–197. https://doi.org/10.18433/J3MS5C
Jodoin J, Demeule M, Beliveau R (2002) Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim Biophys Acta 1542(1-3):149–159. https://doi.org/10.1016/S0167-4889(01)00175-6
Sugihara N, Tsutsui Y, Tagashira T, Choshi T, Hibino S, Kamishikiryou J, Furuno K (2011) The ability of gallate and pyrogallol moieties of catechins to inhibit P-glycoprotein function. J Funct Foods 3(4):298–304. https://doi.org/10.1016/j.jff.2011.05.005
Kitagawa S, Nabekura T, Kamiyama S (2004) Inhibition of P-glycoprotein function by tea catechins in KB-C2 cells. J Pharm Pharmacol 56(8):1001–1005. https://doi.org/10.1211/0022357044003
Qian F, Wei D, Zhang Q, Yang S (2005) Modulation of P-glycoprotein function and reversal of multidrug resistance by (−)-epigallocatechin gallate in human cancer cells. Biomed Pharmacother 59(3):64–69. https://doi.org/10.1016/j.biopha.2005.01.002
Kawasaki T, Ito H, Omote H (2014) Components of foods inhibit a drug exporter, human multidrug and toxin extrusion transporter 1. Biol Pharm Bull 37(2):292–297. https://doi.org/10.1248/bpb.b13-00815
Knop J, Misaka S, Singer K, Hoier E, Müller F, Glaeser H, König J, Fromm MF (2015) Inhibitory effects of green tea and (−)-epigallocatechin gallate on transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-glycoprotein. PLoS One 10(10):e0139370. https://doi.org/10.1371/journal.pone.0139370
Roth M, Timmermann BN, Hagenbuch B (2011) Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab Dispos 39(5):920–926. https://doi.org/10.1124/dmd.110.036640
Fuchikami H, Satoh H, Tsujimoto M, Ohdo S, Ohtani H, Sawada Y (2006) Effects of herbal extracts on the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos 34(4):577–582. https://doi.org/10.1124/dmd.105.007872
Misaka S, Yatabe J, Müller F, Takano K, Kawabe K, Glaeser H, Yatabe MS, Onoue S, Werba JP, Watanabe H, Yamada S, Fromm MF, Kimura J (2014) Green tea ingestion greatly reduces plasma concentrations of nadolol in healthy subjects. Clin Pharmacol Ther 95(4):432–438. https://doi.org/10.1038/clpt.2013.241
An G, Wang X, Morris ME (2014) Flavonoids are inhibitors of human organic anion transporter 1 (OAT1)-mediated transport. Drug Metab Dispos 42(9):1357–1366. https://doi.org/10.1124/dmd.114.059337
Zhang S, Yang X, Morris ME (2004) Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol 65(5):1208–1216. https://doi.org/10.1124/mol.65.5.1208
Netsch MI, Gutmann H, Luescher S, Brill S, Schmidlin CB, Kreuter MH, Drewe J (2005) Inhibitory activity of a green tea extract and some of its constituents on multidrug resistance-associated protein 2 functionality. Planta Med 71(2):135–141. https://doi.org/10.1055/s-2005-837780
Fleisher B, Unum J, Shao J, An G (2015) Ingredients in fruit juices interact with dasatinib through inhibition of BCRP: a new mechanism of beverage-drug interaction. J Pharm Sci 104(1):266–275. https://doi.org/10.1002/jps.24289
Feng WY (2006) Metabolism of green tea catechins: an overview. Curr Drug Metab 7(7):755–809. https://doi.org/10.2174/138920006778520552
Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC (2006) The human intestinal cytochrome P450 “pie”. Drug Metab Dispos 34(5):880–886. https://doi.org/10.1124/dmd.105.008672
Werba JP, Misaka S, Giroli MG, Yamada S, Cavalca V, Kawabe K, Squellerio I, Laguzzi F, Onoue S, Veglia F, Myasoedova V, Takeuchi K, Adachi E, Inui N, Tremoli E, Watanabe H (2015) Overview of green tea interaction with cardiovascular drugs. Curr Pharm Des 21(9):1213–1219. https://doi.org/10.2174/1381612820666141013135045
Chow HH, Hakim IA, Vining DR, Crowell JA, Cordova CA, Chew WM, Xu MJ, Hsu CH, Ranger-Moore J, Alberts DS (2006) Effects of repeated green tea catechin administration on human cytochrome P450 activity. Cancer Epidemiol Biomark Prev 15(12):2473–2476. https://doi.org/10.1158/1055-9965.EPI-06-0365
Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS (2005) Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res 11(12):4627–4633. https://doi.org/10.1158/1078-0432.CCR-04-2549
Tse FL, Jaffe JM, Troendle A (1992) Pharmacokinetics of fluvastatin after single and multiple doses in normal volunteers. J Clin Pharmacol 32(7):630–638. https://doi.org/10.1002/j.1552-4604.1992.tb05773.x
Fischer V, Johanson L, Heitz F, Tullman R, Graham E, Baldeck JP, Robinson WT (1999) The 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor fluvastatin: effect on human cytochrome P-450 and implications for metabolic drug interactions. Drug Metab Dispos 27(3):410–416
Toda T, Eliasson E, Ask B, Inotsume N, Rane A (2009) Roles of different CYP enzymes in the formation of specific fluvastatin metabolites by human liver microsomes. Basic Clin Pharmacol Toxicol 105(5):327–332. https://doi.org/10.1111/j.1742-7843.2009.00453.x
Kantola T, Backman JT, Niemi M, Kivistö KT, Neuvonen PJ (2000) Effect of fluconazole on plasma fluvastatin and pravastatin concentrations. Eur J Clin Pharmacol 56(3):225–229. https://doi.org/10.1007/s002280000127
Kivistö KT, Kantola T, Neuvonen PJ (1998) Different effects of itraconazole on the pharmacokinetics of fluvastatin and lovastatin. Br J Clin Pharmacol 46(1):49–53. https://doi.org/10.1046/j.1365-2125.1998.00034.x
Kirchheiner J, Kudlicz D, Meisel C, Bauer S, Meineke I, Roots I, Brockmöller J (2003) Influence of CYP2C9 polymorphisms on the pharmacokinetics and cholesterol-lowering activity of (−)-3S,5R-fluvastatin and (+)-3R,5S-fluvastatin in healthy volunteers. Clin Pharmacol Ther 74(2):186–194. https://doi.org/10.1016/S0009-9236(03)00121-8
Keskitalo JE, Pasanen MK, Neuvonen PJ, Niemi M (2009) Different effects of the ABCG2 c.421C>A SNP on the pharmacokinetics of fluvastatin, pravastatin and simvastatin. Pharmacogenomics 10(10):1617–1624. https://doi.org/10.2217/pgs.09.85
Hirano M, Maeda K, Matsushima S, Nozaki Y, Kusuhara H, Sugiyama Y (2005) Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol 68:800–807. https://doi.org/10.1124/mol.105.014019
Li J, Volpe DA, Wang Y, Zhang W, Bode C, Owen A, Hidalgo IJ (2011) Use of transporter knockdown Caco-2 cells to investigate the in vitro efflux of statin drugs. Drug Metab Dispos 39(7):1196–1202. https://doi.org/10.1124/dmd.111.038075
Noé J, Portmann R, Brun ME, Funk C (2007) Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos 35(8):1308–1314. https://doi.org/10.1124/dmd.106.012930
Varma MV, Rotter CJ, Chupka J, Whalen KM, Duignan DB, Feng B, Litchfield J, Goosen TC, El-Kattan AF (2011) pH-sensitive interaction of HMG-CoA reductase inhibitors (statins) with organic anion transporting polypeptide 2B1. Mol Pharm 8(4):1303–1313. https://doi.org/10.1021/mp200103h
Kopplow K, Letschert K, König J, Walter B, Keppler D (2005) Human hepatobiliary transport of organic anions analyzed by quadruple-transfected cells. Mol Pharmacol 68(4):1031–1038. https://doi.org/10.1124/mol.105.014605
Niemi M, Pasanen MK, Neuvonen PJ (2006) SLCO1B1 polymorphism and sex affect the pharmacokinetics of pravastatin but not fluvastatin. Clin Pharmacol Ther 80(4):356–366. https://doi.org/10.1016/j.clpt.2006.06.010
Mizukami Y, Sawai Y, Yamaguchi Y (2007) Simultaneous analysis of catechins, gallic acid, strictinin, and purine alkaloids in green tea by using catechol as an internal standard. J Agric Food Chem 55(13):4957–4964. https://doi.org/10.1021/jf070323f
Misaka S, Kawabe K, Onoue S, Werba JP, Giroli M, Kimura J, Watanabe H, Yamada S (2013) Development of rapid and simultaneous quantitative method for green tea catechins on the bioanalytical study using UPLC/ESI-MS. Biomed Chromatogr 27(1):1–6. https://doi.org/10.1002/bmc.2740
Masuda N, Akasaka I, Suzuki J, Fujino A, Ohtawa M, Nakaya N, Goto Y, Takahashi N, Sekino H, Amamoto T, Urae A, Maeda A, Irie S (1995) Phase I study of fluvastatin, a new synthetic HMG-CoA reductase inhibitor—pharmacokinetics of fluvastatin in healthy male volunteers after single and multiple oral administration. Rinsho Iyaku 11:65–81
Mooiman KD, Goey AK, Huijbregts TJ, Maas-Bakker RF, Beijnen JH, Schellens JH, Meijerman I (2014) The in-vitro effect of complementary and alternative medicines on cytochrome P450 2C9 activity. J Pharm Pharmacol 66(9):1339–1346. https://doi.org/10.1111/jphp.12259
Zhang L, Zheng Y, Chow MS, Zuo Z (2004) Investigation of intestinal absorption and disposition of green tea catechins by Caco-2 monolayer model. Int J Pharm 287(1-2):1–12. https://doi.org/10.1016/j.ijpharm.2004.08.020
Zhou Q, Ruan ZR, Yuan H, Zeng S (2012) CYP2C9*3(1075A>C), MDR1 G2677T/A and MDR1 C3435T are determinants of inter-subject variability in fluvastatin pharmacokinetics in healthy Chinese volunteers. Arzneimittelforschung 62(11):519–524. https://doi.org/10.1055/s-0032-1323696
Birmingham BK, Bujac SR, Elsby R, Azumaya CT, Wei C, Chen Y, Mosqueda-Garcia R, Ambrose HJ (2015) Impact of ABCG2 and SLCO1B1 polymorphisms on pharmacokinetics of rosuvastatin, atorvastatin and simvastatin acid in Caucasian and Asian subjects: a class effect? Eur J Clin Pharmacol 71(3):341–355. https://doi.org/10.1007/s00228-014-1801-z
Kimura M, Ieiri I, Mamiya K, Urae A, Higuchi S (1998) Genetic polymorphism of cytochrome P450s, CYP2C19, and CYP2C9 in a Japanese population. Ther Drug Monit 20:243–247
Acknowledgements
We thank Dr. Yoshinori Tanino, Dr. Xintao Wang, Ms. Ayaka Ushigome, and Mr. Rintaro Miyo for their excellent technical support. This work was supported partly by the Honjo International Scholarship Foundation.
Author information
Authors and Affiliations
Contributions
Misaka, Abe, Shikama, Onoue, Yabe, and Kimura participated in the research design. Misaka, Abe, Sato, and Ono conducted the experiments and clinical study. Misaka, Abe, Sato, Onoue, and Kimura performed the data analysis. Misaka, Abe, Shikama, Onoue, Yabe, and Kimura wrote or contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declared that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Misaka, S., Abe, O., Sato, H. et al. Lack of pharmacokinetic interaction between fluvastatin and green tea in healthy volunteers. Eur J Clin Pharmacol 74, 601–609 (2018). https://doi.org/10.1007/s00228-018-2420-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2420-x