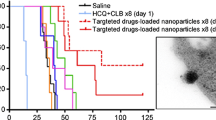
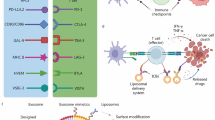
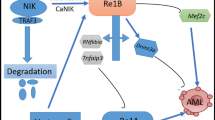
Abstract
Chronic lymphocytic leukemia (CLL) still represents an incurable disorder that may progress to other more aggressive types of cancer despite the available therapy and the development that has been reached in the immunophenotypic and mutational status characterization of CLL. Hence, innovative therapeutics strategies are required together with the advancement in chemo-immunotherapy and targeted treatments. Parallelly, more focus should be put on the drug delivery process to improve the effectiveness/toxicity ratio of both conventional and new drugs and reduce the risk of drug resistance. In the present review, different types of nanocarriers that can be harnessed against CLL, their features, their capabilities in targeting CLL cells, and the latest relevant data are discussed. We provide an integral description of each nanocarrier, including lipidic, polymeric, and inorganic carriers, aiming to offer a constructive resource for the rational design of potential nanomedicines to advance the fight against CLL.
Graphical abstract





Similar content being viewed by others
Abbreviations
- ABC:
-
Accelerated blood clearance
- ACS:
-
American cancer society
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- AMOs:
-
Anti-miRNA oligonucleotides
- APRIL:
-
A proliferation-inducing ligand
- APTES:
-
3-Aminopropyl triethoxysilane
- BAFF:
-
B-cell activating factor
- BAFFR:
-
B-cell activating factor receptor
- BCL-2:
-
B-cell lymphoma 2
- BCMA:
-
B-cell maturation antigen
- BCR:
-
B-cell receptor
- BDM:
-
Bendamustine
- BM:
-
Bone marrow
- BTK:
-
Bruton tyrosine kinase
- CD:
-
Cyclodextrin
- CDC:
-
Complement-dependent cytotoxicity
- cdk6:
-
Cyclin-dependent kinase 6
- Chol:
-
Cholesterol
- CLL:
-
Chronic lymphocytic leukemia
- CPNPs:
-
Calcium phosphate nanoparticles
- CXCL12/CXCL13:
-
C-X-C motif chemokine ligand 12/13
- CXCR4/CXCR5:
-
C-X-C motif chemokine receptor type 4/5
- DDS:
-
Drug delivery system;
- Dlin-MC3-DMA:
-
Dilinoleylmethyl-4-dimethylaminobutyrate
- DMG-PEG:
-
1,2-Dimyristoyl-sn-glycerol, methoxypolyethylene glycol
- DMPG:
-
1,2-Dimyristoyl-sn-glycero-3-phospho-(19-rac-glycerol)
- DMPS:
-
1,2-Dimyristoyl- sn-glycero-3-phospho-L-serine
- DMSA:
-
3-Dimercaptosuccinic acid
- DODAP:
-
1,2-Dioleyl-3-trimethyl ammonium- propane
- DOPC:
-
1,2-Dioleoyl-sn-glycero-3-phosphocholine
- DOPE:
-
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
- Dox:
-
Doxorubicin
- DS:
-
Dense shell
- DSPC:
-
1,2-Distearoyl-sn-glycero-3-phosphocholine
- DSPE-PEG:
-
N-palmitoyl-sphingosine-1- succinylpolyethylene glycol
- DSPG:
-
Distearoylphosphatidylglycerol
- DSPS:
-
Distearoyl phosphatidylserine
- EPR:
-
Enhanced permeability and retention
- FA:
-
Fludarabine
- FDA:
-
US Food and Drug Administration
- G4:
-
4Th generation
- GMS:
-
Glyceryl monostearate
- GNPs:
-
Gold nanoparticles
- GLUTs:
-
Glucose transporters
- HA:
-
Hyaluronic acid
- HDL:
-
High-density lipoprotein
- IBR:
-
Ibrutinib
- IGHV:
-
Immunoglobulin heavy-chain genes
- ILNPs:
-
Ionizable lipid nanoparticles
- ILPs:
-
Immunoliposomes
- IONPs:
-
Iron oxide nanoparticles
- LNs:
-
Lymph nodes
- LPs:
-
Liposomes
- MABs:
-
Monoclonal antibodies
- Mal:
-
Maltose
- Mal-3:
-
Maltotriose
- Mcl-1:
-
Myeloid cell leukemia-1
- miRNA:
-
MicroRNA
- MRI:
-
Magnetic resonance imaging
- MS4A1:
-
Membrane spanning 4-domains A1
- MSNPs:
-
Mesoporous silica nanoparticles
- NLC:
-
Nurse-like cell
- NPs:
-
Nanoparticles
- NSLCs:
-
Nanostructured lipid carriers
- NTs:
-
Nucleoside transporters
- OS:
-
Open shell
- PAMAM:
-
Polyamidoamine
- PBMCs:
-
Peripheral blood mononuclear cells
- PCL:
-
Polycaprolactone
- PDI:
-
Polydispersity index
- PEG:
-
Polyethylene glycol
- PGA:
-
Polyglycolic acid;
- PGMC:
-
Propylene glycol monocaprylate
- PI3Ks:
-
Phosphoinositol-3-kinases
- PLA:
-
Polylactic acid
- PLGA:
-
Poly(lactic acid-co-glycolic acid)
- PPI:
-
Polypropyleneimine
- RES:
-
Reticuloendothelial system
- ROR1:
-
Receptor tyrosine kinase orphan receptor 1
- ROS:
-
Reactive oxygen species
- RTX:
-
Rituximab
- SBE-b-CD:
-
Sulfobutylether-b-cyclodextrin
- SEER:
-
Surveillance, epidemiology, and end result
- sHDL:
-
Synthetic high-density lipoprotein
- siRNA:
-
Small interfering RNA
- SR-B1:
-
Scavenger receptor type B-1
- STAT3:
-
Signal transducer and activator of transcription 3
- TACI:
-
Transmembrane activator and calcium modulator and cyclophin ligand interactor
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- TrxR:
-
Thioredoxin reductase
References
Awan FT, Byrd JC. Chronic Lymphocytic Leukemia. In: Hoffman R, Benz EJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J et al., editors. Hematology: Basic Principles and Practice. Amsterdam: Elsevier 2018. p. 1244–64. https://doi.org/10.1016/B978-0-323-35762-3.00077-9.
Nisticò N, Maisano D, Iaccino E, Vecchio E, Fiume G, Rotundo S, et al. Role of chronic lymphocytic leukemia (CLL)-derived exosomes in tumor progression and survival. Pharmaceuticals. 2020;13:244.
Bosch F, Dalla-Favera R. Chronic lymphocytic leukaemia: from genetics to treatment. Nat Rev Clin Oncol. 2019;16:684–701.
Rawstron AC, Kreuzer KA, Soosapilla A, Spacek M, Stehlikova O, Gambell P, et al. Reproducible diagnosis of chronic lymphocytic leukemia by flow cytometry: An European Research Initiative on CLL (ERIC) & European Society for Clinical Cell Analysis (ESCCA) Harmonisation project. Cytom Part B - Clin Cytom. 2018;94:121–8.
Rawstron AC, Tute RM, Owen RG, Hillmen P. Laboratory diagnosis of chronic lymphocytic leukaemia. In: Hallek M, Eichhorst B, Catovsky D, editors. Chronic Lymphocytic Leukemia. Cham: Springer; 2019. p. 21–35. https://doi.org/10.1007/978-3-030-11392-6_2.
National Cancer Institute .Surveillance epidemiology and end results program. Cancer Stat Facts: Leukemia- Chronic Lymphocytic Leukemia CLL). Available from: https://seer.cancer.gov/statfacts/html/clyl.html. Accessed 12 Jan 2022.
Burger JA, O’Brien S. Evolution of CLL treatmentfrom chemoimmunotherapy to targeted and individualized therapy. Nat Rev Clin Oncol. 2018;15:510–27.
Moia R, Patriarca A, Schipani M, Ferri V, Favini C, Sagiraju S, et al. Precision medicine management of chronic lymphocytic leukemia. Cancers (Basel). 2020;12:642.
Fabbri G, Dalla-favera R. The molecular pathogenesis of chronic lymphocytic leukaemia. Nat Rev Cancer. 2016;16:145–162.
Sajid MI, Moazzam M, Cho Y, Kato S, Xu A, Way JJ, et al. siRNA Therapeutics for the Therapy of COVID-19 and Other Coronaviruses. Mol Pharm. 2021;18:2105–21.
Burger JA. Treatment of Chronic Lymphocytic Leukemia. N Engl J Med. 2020;383:460–73.
Scarfò L, Ghia P. Chronic lymphocytic leukemia: Who, How, and Where? In: Hallek M, Eichhorst B, Catovsky D, editors. Hematologic malignancies. Cham: Springer Nature Switzerland; 2019. https://doi.org/10.1007/978-3-030-11392-6_1.
Darwiche W, Gubler B, Marolleau JP, Ghamlouch H. Chronic lymphocytic leukemia B-cell normal cellular counterpart: Clues from a functional perspective. Front Immunol. 2018:683.
Yosifov DY, Wolf C, Stilgenbauer S, Mertens D. From biology to therapy: The CLL success story. Hemasphere. 2019;3:e175.
Griggio V, Perutelli F, Salvetti C, Boccellato E, Boccadoro M, Vitale C, et al. Immune Dysfunctions and Immune- Based Therapeutic Interventions in Chronic Lymphocytic Leukemia. Front Immunol. 2020;11:954556.
Nabhan C, Rosen ST. Chronic Lymphocytic Leukemia: A clinical review. JAMA. 2014;312:2265–76.
Dennie TW, Kolesar JM. Bendamustine for the treatment of chronic lymphocytic leukemia and rituximab-refractory, indolent B-cell non-hodgkin lymphoma. Clin Ther. 2009;31:2290–311.
Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21:1188–200.
Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–42.
Bharti AC, Vishnoi K, Singh SM, Aggarwal BB. Pathways linked to cancer chemoresistance and their targeting by nutraceuticals. In: Bharti AC, Aggarwal BB, editors. Role of Nutraceuticals in Chemoresistance to Cancer. Amsterdam: Elsevier; 2018. p. 1–30. https://doi.org/10.1016/B978-0-12-812373-7.00001-2.
Chu CS, Rubin SC. Basic principles of chemotherapy. In: DiSaia PJ, Creasman WT, Mannel RS, McMeekin DS, Mutch DG, editors. Clinical Gynecologic Oncology. Amsterdam: Elsevier; 2018. p. 449–469.e2. https://doi.org/10.1016/B978-0-323-40067-1.00017-6.
Kwok KK, Vincent EC, Gibson JN. Antineoplastic Drugs. In: Dowd FJ, Johnson BS, Mariotti AJ, editors. Pharmacology and Therapeutics for Dentistry. Amsterdam: Elsevier; 2017. p. 530–62. https://doi.org/10.1016/B978-0-323-39307-2.00036-9.
Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am J Hematol. 2019;94:1266–87.
Sharma S, Rai KR. Chronic lymphocytic leukemia (CLL) treatment: So many choices, such great options. Cancer. 2019;125:1432–40.
Rogers A, Woyach JA. BTK inhibitors and anti-CD20 monoclonal antibodies for treatment-naïve elderly patients with CLL. Ther Adv Hematol. 2020;11:204062072091299.
Geethakumari PR, Awan F. An evaluation of zanubrutinib, a BTK inhibitor, for the treatment of chronic lymphocytic leukemia. Expert Rev Hematol. 2020;13:1039–46.
Farooqui AA, Ashraf A, Farooq T Bin, Anjum A, Rehman S ur, Akbar A, et al. Novel targeted therapies for chronic lymphocytic leukemia in elderly patients: A systematic review. Clin Lymphoma Myeloma Leuk. 2020;20:e414–26.
Blair HA. Duvelisib: First Global Approval. Drugs. 2018;78:1847–53.
Shah A, Mangaonkar A. Idelalisib: A novel PI3Kδ inhibitor for chronic lymphocytic leukemia. Ann Pharmacother. 2015;49:1162–70.
Held L, Siu C, Shadman M. Venetoclax as a therapeutic option for the treatment of chronic lymphocytic leukemia: the evidence so far. Expert Opin Pharmacother. 2021;22:655–65.
Schiattone L, Ghia P, Scarfò L. The evolving treatment landscape of chronic lymphocytic leukemia. Curr Opin Oncol. 2019;31:568–73.
Gribben JG. How and when I do allogeneic transplant in CLL. Blood. 2018;132:31–9.
Huang L, Huang J, Huang J, Xue H, Liang Z, Wu J, et al. Nanomedicine-a promising therapy for hematological malignancies. Biomater Sci. 2020;8:2376–93.
Vinhas R, Mendes R, Fernandes AR, Baptista PV. Nanoparticles-Emerging Potential for Managing Leukemia and Lymphoma. Front Bioeng Biotechnol. 2017;5:79.
Shen J, Lu Z, Wang J, Zhang T, Yang J, Li Y, et al. Advances of Nanoparticles for Leukemia Treatment. ACS Biomater Sci Eng. 2020;6:6478–89.
Soni G, Yadav KS. Applications of nanoparticles in treatment and diagnosis of leukemia. Mater Sci Eng C. 2015;47:156–64.
Narum SM, Le T, Le DP, Lee JC, Donahue ND, Yang W, et al. Passive targeting in nanomedicine: fundamental concepts, body interactions, and clinical potential. In: Chung EJ, Leon L, Rinaldi C, editors. Nanoparticles for Biomedical Applications. Amsterdam: Elsevier; 2019. p. 37–53. https://doi.org/10.1016/B978-0-12-816662-8.00004-7.
Kukkar D, Kukkar P, Kumar V, Hong J, Kim K-H, Deep A. Recent advances in nanoscale materials for antibody-based cancer theranostics. Biosens Bioelectron. 2020;173:112787.
Hayden RE, Pratt G, Roberts C, Drayson MT, Bunce CM. Treatment of chronic lymphocytic leukemia requires targeting of the protective lymph node environment with novel therapeutic approaches. Leuk Lymphoma. 2012;53:537–49.
Houshmand M, Garello F, Circosta P, Stefania R, Aime S, Saglio G, et al. Nanocarriers as magic bullets in the treatment of leukemia. Nanomaterials. 2020;10:276.
Liu R, Hu C, Yang Y, Zhang J, Gao H. Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharm Sin B. 2019;9:410–20.
Li J, Sun CK. In vitro analysis of microRNA-26a in chronic lymphocytic leukemia cells. Int J Mol Med. 2018;42:3364–70.
McCallion C, Peters AD, Booth A, Rees-Unwin K, Adams J, Rahi R, et al. Dual-action CXCR4-targeting liposomes in leukemia: Function blocking and drug delivery. Blood Adv. 2019;3:2069–81.
D’Abundo L, Callegari E, Bresin A, Chillemi A, Elamin BK, Guerriero P, et al. Anti-leukemic activity of microRNA-26a in a chronic lymphocytic leukemia mouse model. Oncogene. 2017;36:6617–26.
Chiang CL, Goswami S, Frissora FW, Xie Z, Yan PS, Bundschuh R, et al. ROR1-targeted delivery of miR-29b induces cell cycle arrest and therapeutic benefit in vivo in a CLL mouse model. Blood. 2019;134:432–44.
Kon E, Hazan-Halevy I, Rosenblum D, Cohen N, Chatterjee S, Veiga N, et al. Resveratrol enhances mrna and sirna lipid nanoparticles primary CLL cell transfection. Pharmaceutics. 2020;12:1–14.
Rangaraj N, Pailla SR, Shah S, Prajapati S, Sampathi S. QbD aided development of ibrutinib-loaded nanostructured lipid carriers aimed for lymphatic targeting: evaluation using chylomicron flow blocking approach. Drug Deliv Transl Res. 2020;10:1476–94.
Gothwal A, Khan I, Kumar P, Raza K, Kaul A, Mishra AK, et al. Bendamustine-PAMAM Conjugates for Improved Apoptosis, Efficacy, and in Vivo Pharmacokinetics: A Sustainable Delivery Tactic. Mol Pharm. 2018;15:2084–97.
Gorzkiewicz M, Jatczak-Pawlik I, Studzian M, Pułaski Ł, Appelhans D, Voit B, et al. Glycodendrimer Nanocarriers for Direct Delivery of Fludarabine Triphosphate to Leukemic Cells: Improved Pharmacokinetics and Pharmacodynamics of Fludarabine. Biomacromol. 2018;19:531–43.
Franiak-Pietryga I, Ziemba B, Sikorska H, Jander M, Kuncman W, Danilewicz M, et al. Maltotriose-modified poly(propylene imine) Glycodendrimers as a potential novel platform in the treatment of chronic lymphocytic Leukemia. A proof-of-concept pilot study in the animal model of CLL. Toxicol Appl Pharmacol. 2020;403:115139.
Choi KY, Correa S, Min J, Li J, Roy S, Laccetti KH, et al. Binary Targeting of siRNA to Hematologic Cancer Cells In Vivo Using Layer-by-Layer Nanoparticles. Adv Funct Mater. 2019;29:1900018.
Khan I, Gothwal A, Kaul A, Mathur R, Mishra AK, Gupta U. Radiolabeled PLGA Nanoparticles for Effective Targeting of Bendamustine in Tumor Bearing Mice. Pharm Res. 2018;35:1–11.
Alshetaili AS, Ansari MJ, Anwer MK, Ganaie MA, Iqbal M, Alshahrani SM, et al. Enhanced Oral Bioavailability of Ibrutinib Encapsulated Poly (Lactic-co- Glycolic Acid) Nanoparticles: Pharmacokinetic Evaluation in Rats. Curr Pharm Anal. 2019;15:661–8.
Capolla S, Mezzaroba N, Zorzet S, Tripodo C, Mendoza-Maldonado R, Granzotto M, et al. A new approach for the treatment of CLL using chlorambucil/hydroxychloroquine-loaded anti-CD20 nanoparticles. Nano Res. 2016;9:537–48.
Zhao L, Tang B, Tang P, Sun Q, Suo Z, Zhang M, et al. Chitosan/Sulfobutylether-β-Cyclodextrin Nanoparticles for Ibrutinib Delivery: A Potential Nanoformulation of Novel Kinase Inhibitor. J Pharm Sci. 2020;109:1136–44.
Boca S, Lucan C, Frinc I, Petrushev B, Simon T, Berce C, et al. Gold nanoparticles conjugated with rituximab for the treatment of chronic lymphocytic leukaemia. Farmacia. 2016;64:688–98.
Song S, Hao Y, Yang X, Patra P, Chen J. Using gold nanoparticles as delivery vehicles for targeted delivery of chemotherapy drug fludarabine phosphate to treat hematological cancers. J Nanosci Nanotechnol. 2016;16:2582–6.
Arib C, Spadavecchia J. Lenalidomide (LENA) Hybrid Gold Complex Nanoparticles: Synthesis, Physicochemical Evaluation, and Perspectives in Nanomedicine. ACS Omega. 2020;5:28483–92.
McMahon KM, Scielzo C, Angeloni NL, Deiss-Yehiely E, Scarfo L, Ranghetti P, et al. Synthetic high-density lipoproteins as targeted monotherapy for chronic lymphocytic leukemia. Oncotarget. 2017;8:11219–27.
Li Q, Yuan Q, Zhao M, Yao Y, Gao L, Liu R, et al. Au nanoclusters suppress chronic lymphocytic leukaemia cells by inhibiting thioredoxin reductase 1 to induce intracellular oxidative stress and apoptosis. Sci Bull. 2017;62:537–45.
Yao Y, Lu C, Gao L, Cao K, Yuan H, Zhang X, et al. Gold Cluster Capped with a BCL-2 Antagonistic Peptide Exerts Synergistic Antitumor Activity in Chronic Lymphocytic Leukemia Cells. ACS Appl Mater Interfaces. 2021;13:21108–18.
Song L, Zhang W, Chen H, Zhang X, Wu H, Ma M, et al. Apoptosis-promoting effect of rituximab-conjugated magnetic nanoprobes on malignant lymphoma cells with CD20 overexpression. Int J Nanomedicine. 2019;14:921–36.
Song L, Chen Y, Ding J, Wu H, Zhang W, Ma M, et al. Rituximab conjugated iron oxide nanoparticles for targeted imaging and enhanced treatment against CD20-positive lymphoma. J Mater Chem B. 2020;8:895–907.
Kickham LC. Development of a novel CD52 functionalised nanoparticle for the targeting of Chronic Lymphocytic Leukaemia [Doctoral dissertation]. [Dublin, Irland]: University of Dublin; 2019.
Zhou S, Wu D, Yin X, Jin X, Zhang X, Zheng S, et al. Intracellular pH-responsive and rituximab-conjugated mesoporous silica nanoparticles for targeted drug delivery to lymphoma B cells. J Exp Clin Cancer Res. 2017;36:1–14.
Thomas SC, Madaan T, Iqbal Z, Talegaonkar S. Box-Behnken Design of Experiment Assisted Development and Optimization of Bendamustine HCl loaded Hydroxyapatite Nanoparticles. Curr Drug Deliv. 2018;15:1230–44.
Thi TTH, Suys EJA, Lee JS, Nguyen DH, Park KD, Truong NP. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines. 2021;9:359.
Yang C, Merlin D. Lipid-based drug delivery nanoplatforms for colorectal cancer therapy. Nanomater (Basel, Switzerland). 2020;10:1424.
García-Pinel B, Porras-Alcalá C, Ortega-Rodríguez A, Sarabia F, Prados J, Melguizo C, et al. Lipid-based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials. 2019;9:638.
Cao J, Huang D, Peppas NA. Advanced engineered nanoparticulate platforms to address key biological barriers for delivering chemotherapeutic agents to target sites. Adv Drug Deliv Rev. 2020;167:170–88.
Eroğlu İ, İbrahim M. Liposome-ligand conjugates: a review on the current state of art. J Drug Target. 2020;28:225–44.
Wang X, Song Y, Su Y, Tian Q, Li B, Quan J, et al. Are PEGylated liposomes better than conventional liposomes? A special case for vincristine. 2015;23:1092–100. https://doi.org/10.3109/1071754420151027015.
Krauss AC, Gao X, Li L, Manning ML, Patel P, Fu W, et al. FDA Approval Summary: (Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid Leukemia. Clin Cancer Res. 2019;25:2685–90.
Mayer LD, Tardi P, Louie AC. CPX-351: a nanoscale liposomal co-formulation of daunorubicin and cytarabine with unique biodistribution and tumor cell uptake properties. Int J Nanomedicine. 2019;14:3819–30.
Tzogani K, Penttilä K, Lapveteläinen T, Hemmings R, Koenig J, Freire J, et al. EMA Review of Daunorubicin and Cytarabine Encapsulated in Liposomes (Vyxeos, CPX-351) for the Treatment of Adults with Newly Diagnosed, Therapy-Related Acute Myeloid Leukemia or Acute Myeloid Leukemia with Myelodysplasia-Related Changes. Oncologist. 2020;25:e1414–20.
Vu MN, Kelly HG, Wheatley AK, Peng S, Pilkington EH, Veldhuis NA, et al. Cellular Interactions of Liposomes and PISA Nanoparticles during Human Blood Flow in a Microvascular Network. Small. 2020;16:e2002861.
Nakamura T, Kawai M, Sato Y, Maeki M, Tokeshi M, Harashima H. The Effect of Size and Charge of Lipid Nanoparticles Prepared by Microfluidic Mixing on Their Lymph Node Transitivity and Distribution. Mol Pharm. 2020;17:944–53.
Xu Y, Michalowski CB, Beloqui A. Advances in lipid carriers for drug delivery to the gastrointestinal tract. Curr Opin Colloid Interface Sci. 2021;52:101414.
Alavi M, Hamidi M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab Pers Ther. 2019;34:10.
Shen Z, Fisher A, Liu WK, Li Y. PEGylated “stealth” nanoparticles and liposomes. In: Parambath A, editor. Engineering of Biomaterials for Drug Delivery Systems. Cambridge: Woodhead Publishing; 2018. p. 1–26. https://doi.org/10.1016/B978-0-08-101750-0.00001-5.
Abu Lila AS, Shimizu T, Ishida T. PEGylation and anti-PEG antibodies. In: Parambath A, editor. Engineering of Biomaterials for Drug Delivery Systems, Woodhead Publishing; 2018, p. 51–68.
Di J, Xie F, Xu Y. When liposomes met antibodies: Drug delivery and beyond. Adv Drug Deliv Rev 2020;154–155:151–62.<br>97. Estanqueiro M, Vasconcelos H, Lobo JMS, Amaral H. Delivering miRNA modulators for cancer treatment. In: Grumezescu AM, editor. Drug Targeting and Stimuli Sensitive Drug Delivery Systems. Norwich: William Andrew Publishing; 2018. p. 517–65. https://doi.org/10.1016/B978-0-12-813689-8.00014-8.
Mohamed M, Lila ASA, Shimizu T, Alaaeldin E, Hussein A, Sarhan HA, et al. PEGylated liposomes: immunological responses. Sci Technol Adv Mater. 2019;20:710–24.
Chena S, Zaifmana J, Kulkarnia JA, Zhigaltseva IV, Tama YK, Ciufolinic MA, et al. Dexamethasone prodrugs as potent suppressors of the immunostimulatory effects of lipid nanoparticle formulations of nucleic acids. J Control Release. 2018;286:46–54.
Riaz MK, Riaz MA, Zhang X, Lin C, Wong KH, Chen X, et al. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int J Mol Sci. 2018;19:195.
Eloya JO, Petrillib R, Trevizana LNF, Chorilli M. Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids Surf B Biointerfaces. 2017;159:454–67.
Yonezawa S, Koide H, Asai T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv Drug Deliv Rev. 2020;154–155:64–78.
Sato Y, Nakamura T, Yamada Y, Harashima H. The nanomedicine rush: New strategies for unmet medical needs based on innovative nano DDS. J Control Release. 2021;330:305–16.
Hoy SM. Patisiran: First Global Approval. Drugs. 2018;78:1625–31.
Kim M, Jeong M, Hur S, Cho Y, Park J, Jung H, et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci Adv. 2021;7:eabf4398.
Schlich M, Palomba R, Costabile G, Mizrahy S, Pannuzzo M, Peer D, et al. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng Transl Med. 2021;6:e10213.
Billingsley MM, Singh N, Ravikumar P, Zhang R, June CH, Mitchell MJ. Ionizable Lipid Nanoparticle-Mediated mRNA Delivery for Human CAR T Cell Engineering. Nano Lett. 2020;20:1578–89.
Abdel-Magid AF. Myeloid Cell Leukemia-1 Inhibitors as Emerging Cancer Treatment. ACS Med Chem Lett. 2021;12:334–6.
Gutjahr JC, Greil R, Hartmann TN. The role of CD44 in the pathophysiology of chronic lymphocytic leukemia. Front Immunol. 2015;6:1–7.
Shi Y, Zhang Z, Qu X, Zhu X, Zhao L, Wei R, et al. Roles of STAT3 in Leukemia (Review). Int J Oncol. 2018;53:7–20.
Chauhan I, Yasir M, Verma M, Singh AP. Nanostructured Lipid Carriers : A Groundbreaking Approach for Transdermal Drug Delivery. Adv Pharm Bull. 2020;10:150–65.
Estanqueiro M, Vasconcelos H, Lobo JMS, Amaral H. Delivering miRNA modulators for cancer treatment. Drug Targeting and Stimuli Sensitive Drug Delivery Systems: Elsevier; 2018. p. 517–65.
Shekunov B. Physicochemical properties of respiratory particles and formulations. In: Hickey AJ, Mansour HM, editors. Inhalation Aerosols. Boca Raton: CRC Press; 2019. p. 3–30. https://doi.org/10.1201/9781315159768-1.
De Claro RA, McGinn KM, Verdun N, Lee SL, Chiu HJ, Saber H, et al. FDA approval: Ibrutinib for patients with previously treated mantle cell lymphoma and previously treated chronic lymphocytic leukemia. Clin Cancer Res. 2015;21:3586–90.
Lee CS, Rattu MA, Kim SS. A review of a novel, Bruton’s tyrosine kinase inhibitor, ibrutinib. J Oncol Pharm Pract. 2016;22:92–104.
Shi X, Song S, Ding Z, Fan B, Huang W, Xu T. Improving the Solubility, Dissolution, and Bioavailability of Ibrutinib by Preparing It in a Coamorphous State With Saccharin. J Pharm Sci. 2019;108:3020–8.
Qiu Q, Lu M, Li C, Luo X, Liu X, Hu L, et al. Novel Self-Assembled Ibrutinib-Phospholipid Complex for Potently Peroral Delivery of Poorly Soluble Drugs with pH-Dependent Solubility. AAPS PharmSciTech. 2018;19:3571–83.
Lombardo D, Kiselev MA, Caccamo MT. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater. 2019;2019. https://doi.org/10.1155/2019/3702518.
Zoulikha M, Xiao Q, Boafo GF, Sallam MA, Chen Z, He W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm Sin B. 2022;12:600–20.
Delyanee M, Akbari S, Solouk A. Amine-terminated dendritic polymers as promising nanoplatform for diagnostic and therapeutic agents ’ modi fi cation : A review. Eur J Med Chem. 2021;221:113572.
Nikzamir M, Hanifehpour Y, Akbarzadeh A, Panahi Y. Applications of Dendrimers in Nanomedicine and Drug Delivery : A Review. J Inorg Organomet Polym Mater. 2021;31:2246–61.
Kesharwani P, Jain K, Jain NK. Progress in Polymer Science Dendrimer as nanocarrier for drug delivery. Prog Polym Sci. 2014;39:268–307.
Janaszewska A, Lazniewska J, Trzepinski P, Marcinkowska M, Klajnert-Maculewicz B. Cytotoxicity of dendrimers. Biomolecules. 2019;9:330.
Surekha B, Kommana NS, Dubey SK, Kumar AVP, Shukla R, Kesharwani P. PAMAM dendrimer as a talented multifunctional biomimetic nanocarrier for cancer diagnosis and therapy. Colloids Surfaces B Biointerfaces. 2021;204:111837.
Abedi-Gaballu F, Dehghan G, Ghaffari M, Yekta R, Abbaspour-Ravasjanid S, Baradarana B, et al. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl Mater Today. 2018;12:177–90.
Dunn A, Shi D. Polymeric Vectors for Strategic Delivery of Nucleic Acids. Nano Life. 2017;07:1730003.
Patel V, Rajani C, Paul D, Borisa P, Rajpoot K, Youngren-Ortiz SR, et al. Dendrimers as novel drug-delivery system and its applications. In: Tekade RK, editor. Drug Delivery Systems. London: Academic Press; 2020. p. 333–92. https://doi.org/10.1016/B978-0-12-814487-9.00008-9.
Franiak-Pietryga I, Maciejewski H, Ostrowska K, Appelhans D, Voit B, Misiewicz M, et al. Dendrimer-based nanoparticles for potential personalized therapy in chronic lymphocytic leukemia: Targeting the BCR-signaling pathway. Int J Biol Macromol. 2016;88:156–61.
Szulc A, Pulaski L, Appelhans D, Voit B, Klajnert-Maculewicz B. Sugar-modified poly(propylene imine) dendrimers as drug delivery agents for cytarabine to overcome drug resistance. Int J Pharm. 2016;513:572–83.
Adekola KUA, Dalva Aydemir S, Ma S, Zhou Z, Rosen ST, Shanmugam M. Investigating and targeting chronic lymphocytic leukemia metabolism with the human immunodeficiency virus protease inhibitor ritonavir and metformin. Leuk Lymphoma. 2015;56:450–9.
Sakamaki Y, Ozdemir J, Diaz Perez A, Heidrick Z, Watson O, Tsuji M, et al. Maltotriose Conjugated Metal-Organic Frameworks for Selective Targeting and Photodynamic Therapy of Triple Negative Breast Cancer Cells and Tumor Associated Macrophages. Adv Ther. 2020;3:2000029.
Gorzkiewicz M, Deriu MA, Studzian M, Janaszewska A, Grasso G, Pułaski Ł, et al. Fludarabine-Specific Molecular Interactions with Maltose-Modified Poly(propyleneimine) Dendrimer Enable Effective Cell Entry of the Active Drug Form: Comparison with Clofarabine. Biomacromol. 2019;20:1429–42.
Puy JY, Jordheim LP, Cros-Perrial E, Dumontet C, Peyrottes S, Lefebvre-Tournier I. Determination and quantification of intracellular fludarabine triphosphate, cladribine triphosphate and clofarabine triphosphate by LC–MS/MS in human cancer cells. J Chromatogr B Anal Technol Biomed Life Sci. 2017;1053:101–10.
Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues: Mechanisms of drug resistance and reversal strategies. Leukemia. 2001;15:875–90.
Franiak-Pietryga I, Ostrowska K, Maciejewski H, Appelhans D, Misiewicz M, Ziemba B, et al. PPI-G4 Glycodendrimers Upregulate TRAIL-Induced Apoptosis in Chronic Lymphocytic Leukemia Cells. Macromol Biosci. 2017;17:1600169.
Franiak-Pietryga I, Maciejewski H, Ziemba B, Appelhans D, Voit B, Robak T, et al. Blockage of Wnt/β-Catenin Signaling by Nanoparticles Reduces Survival and Proliferation of CLL Cells In Vitro-Preliminary Study. Macromol Biosci. 2017;17:1700130.
Franiak-pietryga I, Ziemba B, Sikorska H, Jander M, Appelhans D, Bryszewska M, et al. Neurotoxicity of poly(propylene imine) glycodendrimers. Drug Chem Toxicol 2020:1–9. https://doi.org/10.1080/01480545.2020.1843472.
Idrees H, Zohaib S, Zaidi J, Sabir A, Khan RU, Zhang X, et al. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials. 2020;10:1970.
Yadav HK, Almokdad AA, Shaluf SI, Debe MS. Polymer-based nanomaterials for drug delivery carriers. In: Mohapatra SS, Ranjan S, Dasgupta N, Mishra RK, Thomas S, editors. Nanocarriers for Drug Delivery. Amsterdam: Elsevier; 2019. p. 531–56. https://doi.org/10.1016/B978-0-12-814033-8.00017-5.
Bhatt P, Trehan S, Inamdar N, Mourya VK, Misra A. Polymers in drug delivery: An update. In: Misra A, Shahiwala A, editors. Applications of Polymers in Drug Delivery. Amsterdam: Elsevier; 2021. p. 1–42. https://doi.org/10.1016/B978-0-12-819659-5.00001-X.
Piotrowski-Daspit AS, Kauffman AC, Bracaglia LG, Saltzman WM. Polymeric vehicles for nucleic acid delivery. Adv Drug Deliv Rev. 2020;156:119–32.
Kargaard A, Sluijter JPG, Klumperman B. Polymeric siRNA gene delivery – transfection efficiency versus cytotoxicity. J Control Release. 2019;316:263–91.
Yen J, Ying H, Wang H, Yin L, Uckun F, Cheng J. CD44 Mediated Nonviral Gene Delivery into Human Embryonic Stem Cells via Hyaluronic-Acid-Coated Nanoparticles. ACS Biomater Sci Eng. 2016;2:326–35.
Pan C, Zhang T, Li S, Xu Z, Pan B, Xu S, et al. Hybrid nanoparticles modified by hyaluronic acid loading an hsp90 inhibitor as a novel delivery system for subcutaneous and orthotopic colon cancer therapy. Int J Nanomedicine. 2021;16:1743–55.
Pavlasova G, Mraz M. The regulation and function of CD20: An “enigma” of B-cell biology and targeted therapy. Haematologica. 2020;105:1494–506.
Tyler B, Gullotti D, Mangraviti A, Utsuki T, Brem H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv Drug Deliv Rev. 2016;107:163–75.
Da SJ, Jesus S, Bernardi N, Colaço M, Borges O. Poly(D, L-Lactic Acid) Nanoparticle Size Reduction Increases Its Immunotoxicity. Front Bioeng Biotechnol. 2019;7:137.
Łukasiewicz S, Mikołajczyk A, Błasiak E, Fic E, Dziedzicka-Wasylewska M. Polycaprolactone Nanoparticles as Promising Candidates for Nanocarriers in Novel Nanomedicines. Pharmaceutics. 2021;13:191.
Witt S, Scheper T, Walter J-G. Production of polycaprolactone nanoparticles with hydrodynamic diameters below 100 nm. Eng Life Sci. 2019;19:658–65.
Casalini T, Rossi F, Castrovinci A, Perale G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front Bioeng Biotechnol. 2019;7:259.
Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications — A comprehensive review. Adv Drug Deliv Rev. 2016;107:367–92.
Astuti SH, Rahma WA, Budianto E. Biodegradable Microcapsules from D, L-PLA/PCL as Controlled Nifedipine Drug Delivery Carrier. Macromol Symp. 2020;391:1900132.
Ostafinska A, Fortelny I, Nevoralova M, Hodan J, Kredatusova J, Slouf M. Synergistic effects in mechanical properties of PLA/PCL blends with optimized composition, processing, and morphology. RSC Adv. 2015;5:98971–82.
Behl A, Parmar VS, Malhotra S, Chhillar AK. Biodegradable diblock copolymeric PEG-PCL nanoparticles : Synthesis, characterization and applications as anticancer drug delivery agents. Polymer (Guildf). 2020;207:122901.
Garg U, Chauhan S, Nagaich U, Jain N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. 2019;9:195–204.
Yuan Z, Ye Y, Gao F, Yuan H, Lan M, Lou K, et al. Chitosan-graft-B-cyclodextrin nanoparticles as a carrier for controlled drug release. Int J Pharm. 2013;446:191–8.
Mahmoud AA, El-Feky GS, Kamel R, Awad GE. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles as a potential approach for ocular drug delivery. Int J Pharm. 2011;413:229–36.
Yanat M, Schroën K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React Funct Polym. 2021;161:104849.
Gidwani B, Vyas AA. Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed Res Int. 2015;2015:198268.
Huang H, Feng W, Chen Y, Shi J. Inorganic nanoparticles in clinical trials and translations. Nano Today. 2020;35:100972.
Bayda S, Hadla M, Palazzolo S, Riello P, Corona G, Toffoli G, et al. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr Med Chem. 2018;25:4269–303.
Montaseri H, Kruger CA, Abrahamse H. Inorganic Nanoparticles Applied for Active Targeted Photodynamic Therapy of Breast Cancer. Pharmaceutics. 2021;13:296.
Wang C, Zhang W, He Y, Gao Z, Liu L, Yu S, et al. Anti-leukaemia therapeutic effects. Nat Nanotechnol. 2021;16:1413–23.
Kumana CR, Mak R, Kwong YL, Gill H. Resurrection of Oral Arsenic Trioxide for Treating Acute Promyelocytic Leukaemia: A Historical Account From Bedside to Bench to Bedside. Front Oncol. 2020;10:1294.
Gurnari C, De Bellis E, DIvona M, Ottone T, Lavorgna S, Voso MT. When Poisons Cure: The Case of Arsenic in Acute Promyelocytic Leukemia. Chemotherapy. 2019;64:238–47.
Kong F-Y, Zhang J-W, Li R-F, Wang Z-X, Wang W-J, Wang W. Unique Roles of Gold Nanoparticles in Drug Delivery. Targeting and Imaging Applications Molecules. 2017;22:1445.
Elahi N, Kamali M, Baghersad MH. Recent biomedical applications of gold nanoparticles: A review. Talanta. 2018;184:537–56.
Yue S, Luo M, Liu H, Wei S. Recent Advances of Gold Compounds in Anticancer Immunity. Front Chem. 2020;8:543.
Rothan HA, Stone S, Natekar J, Kumari P, Arora K, Kumar M. The FDA- approved gold drug Auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology. 2020;547:7–11.
Boullosa LF, Van Loenhout J, Flieswasser T, De Waele J, Hermans C, Lambrechts H, et al. Auranofin reveals therapeutic anticancer potential by triggering distinct molecular cell death mechanisms and innate immunity in mutant p53 non-small cell lung cancer. Redox Biol. 2021;42:101949.
Onodera T, Momose I, Kawada M. Potential Anticancer Activity of Auranofin. Chem Pharm Bull. 2019;67:186–91.
Fiskus W, Saba N, Shen M, Ghias M, Liu J, Das Gupta S, et al. Auranofin induces lethal oxidative and endoplasmic reticulum stress and exerts potent preclinical activity against chronic lymphocytic leukemia. Cancer Res 2014;74:2520–32.
Itchaki G, Brown JR. Lenalidomide in the treatment of chronic lymphocytic leukemia. Expert Opin Investig Drugs. 2017;26:633–50.
Yuan J, Hou K, Yao Y, Du Z, Lu C, Yuan Q, et al. Gold Clusters Attenuate Inflammation in Rat Mesangial Cells via Inhibiting the Activation of NF-κB Pathway. Nanomaterials. 2020;10:712.
Geppert M, Himly M. Iron Oxide Nanoparticles in Bioimaging – An Immune Perspective. Front Immunol. 2021;12:688927.
Vangijzegem T, Stanicki D, Laurent S. Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics. Expert Opin Drug Deliv. 2019;16:69–78.
Su S, Kang PM. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics. 2020;12:837.
Rueda-Gensini L, Cifuentes J, Castellanos MC, Puentes PR, Serna JA, Muñoz-Camargo C, et al. Tailoring iron oxide nanoparticles for efficient cellular internalization and endosomal escape. Nanomaterials. 2020;10:1–56.
Luther DC, Huang R, Jeon T, Zhang X, Lee Y-W, Nagaraj H, et al. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv Drug Deliv Rev. 2020;156:188–213.
Chee CF, Leo BF, Lai CW. Superparamagnetic iron oxide nanoparticles for drug delivery. In: Inamuddin, Asiri AM, Mohammad A, editors. Applications of Nanocomposite Materials in Drug Delivery. Cambridge: Woodhead Publishing; 2018. p. 861–903. https://doi.org/10.1016/B978-0-12-813741-3.00038-8.
Lorkowski ME, Atukorale PU, Ghaghada KB, Karathanasis E. Stimuli-Responsive Iron Oxide Nanotheranostics : A Versatile and Powerful Approach for Cancer Therapy. Adv Heal Mater. 2021;10:e2001044.
Wang F, Lv H, Zhao B, Zhou L, Wang S, Luo J, et al. Iron and leukemia: new insights for future treatments. J Exp Clin Cancer Res. 2019;38:406.
Yang R, Li Y, Wang X, Yan J, Pan D, Xu Y, et al. Doxorubicin loaded ferritin nanoparticles for ferroptosis enhanced targeted killing of cancer cells. RSC Adv. 2019;9:28548–53.
Luo T, Gao J, Lin N, Wang J. Effects of Two Kinds of Iron Nanoparticles as Reactive Oxygen Species Inducer and Scavenger on the Transcriptomic Profiles of Two Human Leukemia Cells with Different Stemness. Nanomaterials. 2020;10:1951.
Trujillo-Alonso V, Pratt EC, Zong H, Lara-Martinez A, Kaittanis C, Rabie MO, et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat Nanotechnol. 2019;14:616–22.
Dadfar SM, Roemhild K, Drude NI, von Stillfried S, Knüchel R, Kiessling F, et al. Iron Oxide Nanoparticles : Diagnostic, Therapeutic and Theranostic Applications. Adv Drug Deliv Rev. 2019;138:302–25.
Lim CK, Sun L, Feng Q, Law P, Chua WT, Lim SN, et al. Effect of anti-CD52 antibody alemtuzumab on ex-vivo culture of umbilical cord blood stem cells. J Hematol Oncol. 2008;1:19.
Demko S, Summers J, Keegan P, Pazdur R. FDA Drug Approval Summary: Alemtuzumab as Single-Agent Treatment for B-Cell Chronic Lymphocytic Leukemia. Oncologist. 2008;13:167–74.
Eichhorst B, Al-Sawaf O, Hallek M. Initial Therapy of Chronic Lymphocytic Leukemia. In: Hallek M, Eichhorst B, Catovsky D, editors. Chronic Lymphocytic Leukemia.Cham: Springer; 2019. p. 79–96. https://doi.org/10.1007/978-3-030-11392-6_6.
Vallet-Regí M, Colilla M, Izquierdo-Barba I, Manzano M. Mesoporous silica nanoparticles for drug delivery: Current insights. Molecules. 2018;23:47.
Manzano M, Vallet-Regí M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv Funct Mater. 2020;30:1902634.
Hakeem A, Duan R, Zahid F, Dong C, Wang B, Hong F, et al. Dual stimuli-responsive nano-vehicles for controlled drug delivery: Mesoporous silica nanoparticles end-capped with natural chitosan. Chem Commun. 2014;50:13268–71.
Khalifehzadeh R, Arami H. Biodegradable Calcium Phosphate Nanoparticles for Cancer Therapy. Adv Colloid Interface Sci. 2020;279:102157.
Piao Y, Bei HP, Tam A, Yang Y, Zhang Q, Yang M, et al. Calcium phosphatenanoparticle based systems for therapeutic delivery. In: Cui W, Zhao X, editors. Theranostic Bionanomaterials. Amsterdam: Elsevier; 2019. p. 147–64. https://doi.org/10.1016/B978-0-12-815341-3.00006-7.
ACKNOWLEDGMENTS AND DISCLOSURES
This study was supported by the National Natural Science Foundation of China (Nos. 81872823, and 82073782), the Shanghai Science and Technology Committee (No. 19430741500), the Key Laboratory of Modern Chinese Medicine Preparation of Ministry of Education of Jiangxi University of Traditional Chinese Medicine (zdsys-202103, China). The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zoulikha, M., He, W. Targeted Drug Delivery for Chronic Lymphocytic Leukemia. Pharm Res 39, 441–461 (2022). https://doi.org/10.1007/s11095-022-03214-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03214-0